
Bioleaching of low grade nickel sulfide mineral in column reactor
ZHEN Shi-jie(甄世杰)1, 2, QIN Wen-qing(覃文庆)1, YAN Zhong-qiang(闫忠强)2,
ZHANG Yan-sheng(张雁生)1, WANG Jun(王 军)1, REN Liu-yi(任浏祎)1
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Jinchuan Group Ltd. (JNMC), Jinchang 737100, China
Received 20 September 2008; accepted 5 November 2008
Abstract:
Jinchuan low grade nickel (0.4%-0.6% Ni, mass fraction) sulfide mineral ore contains a remarkably high content of magnesia (30%-35% MgO, mass fraction) present in the main gangue minerals. Bioleaching was performed to investigate the feasibility to process the mineral due to its relative simplicity, eco-friendly operation and low capital cost requirements. The mixed mesophiles were enriched from acid mine drainage samples collected from several acid mines in China. Considering that the magnesia is easily extracted by acid solution and the excessive Mg2+ will exceed the tolerance of the mixed mesophiles, three effective means were used to reduce the disadvantage of magnesia during the bioleaching operation. They were adaptation of the mixed mesophiles to improve the tolerance; pre-leaching to remove most leachable magnesia and periodic bleeds of a portion of the pregnant leaching solution to control the level of Mg2+ based on the tolerance of the mixed mesophiles. An extraction of nickel (90.3%) and cobalt (88.6%) was successfully achieved within a 300 d leaching process from the Jinchuan low grade nickel sulfide mineral ore using a column reactor at ambient temperature.
Key words:
column reactor; mixed mesophiles; nickel sulfide mineral; magnesia adaptation; pre-leaching;
1 Introduction
Jinchuan Group Ltd (JNMC) has about 400 Mt of low grade nickel sulfide mineral ore containing a remarkably high content of magnesia (0.4%-0.6% Ni, 30%-35% MgO, mass fraction). Due to the stricter environmental regulations, the existing process for high grade ore is not feasible for the low grade ore. JNMC is increasingly faced with the need to extract nickel and cobalt economically from the low-grade ore.
Significant attention has been focused on the development of bioleaching due to its relative simplicity, eco-friendly operation and low capital cost requirements[1-4]. Many chalcocite heap bioleaching operations have been commissioned since 1980[2-3]. Billion tons of low-grade copper ore could be considered waste without an appropriate heap bioleaching technology[5]. Nowadays, bioleaching is no longer a promising technology but an actual economical alternative for treating specific mineral ores. The recovery of valuable metals from the Jinchuan low-grade nickel sulfide ore by bioleaching process is a particular interest of researchers concerning utilization of nickel resources in China[6-8]. However, studies on bioleaching of nickel sulfide minerals are less frequent [9-10]. Due to a remarkably high content of magnesia, there are some difficulties during the bioleaching process of Jinchuan ore[11-12]. So far, no report is available in the literature for column bioleaching of the ore.
In this work, bioleaching was performed to process Jinchuan low grade sulfide mineral ore using column reactor at ambient temperature. Mixed mesophiles were used in the experiment considering that mixed bacteria were better than pure bacteria[13]. The purpose of this work was to investigate the feasibility of using bioleaching to recover valuable metals from Jinchuan low grade nickel sulfide mineral ore.
2 Materials and methods
2.1 Microorganisms
Microorganisms used as inocula were collected from several acid mine drainages. They were mixed mesophiles composed of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. The medium used to adapt the mixed culture to the ore was composed of (NH4)2SO4 0.20-0.40 g/L, K2HPO4 0.10-0.20 g/L and Jinchuan low grade sulfide mineral 3%-6%. The incubation was performed in shaking Erlenmeyer flask at 30-33 ℃ and pH 1.8-2.0. The adaptation to Mg2+ was performed by gradually increasing the Mg2+ concentration in the medium.
2.2 Mineral ore
The mineral ore used in the test was obtained from Jinchuan Mine. Mineralogical examination showed that the main sulfide minerals were ~3.0% pyrrhotite, ~1.5% pentlandite, ~0.7% chalcopyrite, ~0.7% pyrite, and ~0.2% violarite. The chemical analysis was Ni 0.55%, Co 0.023%, Cu 0.20%, Fe 11.6%, S 2.8%, MgO 33%, CaO 0.60%, SiO2 42% and Al2O3 1.5%. Ore samples used as column charge were crushed and screened to different particle size and the fine ore (particle size<0.5 mm) was controlled less than 10% (mass fraction).
2.3 Leaching experiment
2.3.1 Leaching parameter determination
Several small columns (height 50 cm; diameter 10 cm) were used at ambient temperature to investigate leaching parameter on nickel extraction. Considering that magnesia in the mineral is easily extracted by acid solution and the excessively leached magnesia is disadvantageous to microorganisms, the charge should be pre-leached by sulfuric acid to remove most leachable magnesia before inoculation. Irrigation of the pre- leaching was stopped when the pH of the off-solution was stabilized at 1.8-2.0. The solution was removed to perform bioleaching. 10% (volume fraction) inocula was added in each column leaching system. The pH was adjusted to 1.8-2.0. Periodic bleeds of a portion of the pregnant leaching solution were necessary to control the Mg2+ concentration based on the tolerance of the mixed mesophiles. Off-solutions both from pre-leaching and bioleaching were sampled and analyzed to determine metal dissolution and acid balance. When analysis indicated that the bioleaching had ended, irrigation was stopped to prepare for final analysis.
2.3.2 Verification test
When optimized leaching parameters were determined by small column leaching reactors, two big columns (height 2.0 m; diameter 30 cm) were used at ambient temperature to verify these parameters. One column was designed as verification test and the other was designed as abiotic control. The Jinchuan low grade sulfide mineral ore was loaded in each column. The temperature was measured throughout the bioleaching process. One thermocouple was installed to measure temperature within the charge and the other was installed to measure the temperature of the ambient air.
2.4 Analytical techniques
Free bacteria in solution were counted by direct counting using Thoma chamber with an optical microscope. Soluble metal ions in the leached solutions (Ni, Co, Cu Mg, Ca and Fe) were measured by AAS method using an atomic absorption spectrophotometer. The solid residues were air dried and samples were taken for chemical analysis and X-ray diffractometry(XRD). The ferric ion in the solution was determined by 5-sulfosalicylic acid spectroscopy method [14]. The ferrous ion was ascertained by a volumetric method by titration with potassium dichromate. The pH values of the cultural suspension and nickel extractive solutions were monitored at room temperature with a pH meter calibrated with a low pH buffer. The redox potential (Eh) in the leaching solution was measured with a Pt electrode in reference to a saturated Ag/AgCl electrode. For all of the experiments, chemical grade reagents and distilled water were used, with the exception of the chemical analysis in which double distilled water was used.
3 Results and discussion
3.1 Microorganisms adaptation
Metallic ions beyond the range of bacteria regulation are lethal to the mixed mesophiles. Tolerance of the mixed mesophiles to several metallic ions was measured in 9K medium containing 10% inocula (volume fraction). The results show that the mixed mesophiles can endure 10 g/L nickel, 10 g/L cobalt, 20 g/L copper, 40 g/L iron and 10 g/L magnesium. It is impossible for these metallic ions except magnesium to exceed the tolerance in the leaching solution considering that magnesia in the ore is easily extracted by acid solution and its concentration can bring up to 50 g/L. Thereafter, the adaptation of the mixed mesophiles to Mg2+ was performed through serial sub-culturing and gradually increasing Mg2+concentration in the medium. The results show that the tolerance is up to 20 g/L after one year adaptation. The mixed bacteria was eugenic under 20 g/L Mg2+, reproduced very slowly under 22 g/L Mg2+ and died out during 90 d incubation at a concentra- tion of 24 g/L Mg2+.
3.2 Leaching parameter determination
The pre-leaching of the Jinchuan ore at different particle sizes in small columns was investigated at different on-solution pH values. The results are listed in Table 1.
Table 1 Permeability, magnesia and nickel extraction of charge after pre-leaching operation
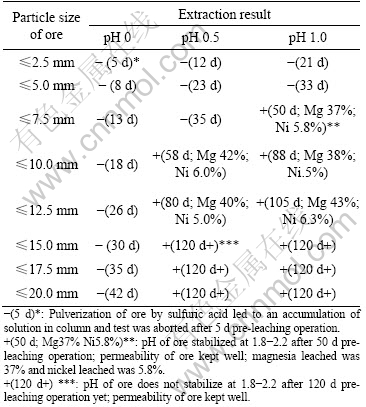
It can be seen from Table 1 that some tests were aborted because of solution accumulation in columns. These columns failed to perform bioleaching at next stage. Table 1 also showed that some tests took more than 120 d to stabilize the pH at 1.8-2.2. These tests were deleted also due to economic reason. Other tests were performed for bioleaching and the results are listed in Table 2.
Table 2 Permeability, magnesia and nickel extraction of charge after bioleaching operation
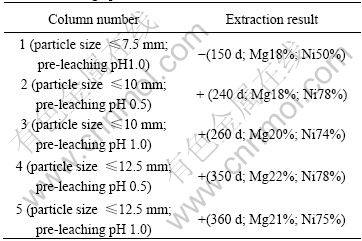
Table 2 shows that the optimized particle size is not larger than 10 mm and the pre-leaching pH should be controlled at 0.5 based on permeability, leaching time and metal extraction comprehensively. The results can be explained that the main gangue minerals of the Jinchuan ore are antigorite, chlorite, talc and tremolite which are easily pulverized by sulfuric acid. Smaller particle size and lower pre-leaching pH require shorter time to stabilize the pH and extract metals however will lead to more rapid pulverization of the ore. Larger particle size and higher pre-leaching pH will lead to a better permeability however requires longer time. The optimized particle size and pre-leaching pH depend on the balance between these leaching parameters.
Irrigation rate and aeration rate were optimized also. The optimized irrigation rates were 60-70 L/(m2?h) and 30-40 L/(m2?h) during pre-leaching and bioleaching, respectively. The optimized aeration rate was 2-5 L/(h?kg) ore.
3.3 Verification test
When the optimized leaching parameters were determined by small column reactors, verification test and abiotic control were performed using big column reactor. The particle size of the Jinchuan ore was not larger than 10 mm.
3.3.1 Pre-leaching
The pre-leaching pH and irrigation rate of on-solution were controlled at pH 0.45-0.55 and 60-70 L/(m2?h), respectively. The off-solution pH and redox potential of the test are shown in Fig.1.
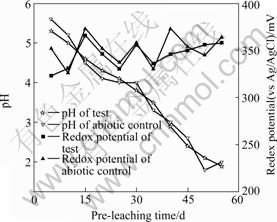
Fig.1 Off-solution pH and redox potential during pre-leaching
It could be seen from Fig.1 that the off-solution redox potential (vs Ag/AgCl) showed no marked difference between the verification test and the abiotic control. They all ranged from 320 to 380 mV. This indicated that the pre-leaching process was a typical acid leaching. Fig.1 also showed that the verification test and the abiotic control stabilized at pH1.8-2.0 after 55 d pre-leaching. The permeability of the charge in columns was checked also. The results showed that no solution accumulation and preferential flow were found in the two columns thus the permeability was good.
3.3.2 Bioleaching
The pH and the irrigation rate of on-solution were controlled at pH 1.8-2.0 and 30-40 L/(m2?h), respectively. The aeration rate was controlled at 2-5 L/(h?kg) ore. Some parameters of the leaching process were investigated. The pH and the redox potential are shown in Fig.1; the iron concentrations are shown in Fig.2; the temperature and the concentrations of the mixed mesophiles are shown in Fig.3.
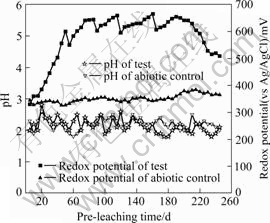
Fig.2 Off-solution pH and redox potential during bio-leaching
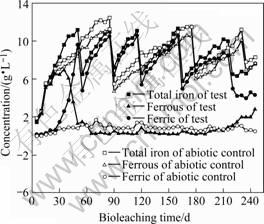
Fig.3 Iron concentrations of off-solution during bioleaching operation
Though the pH of the on-solution was controlled at 1.8-2.0, the pH of the off-solution shown in Fig.2 ranged from 1.8 to 2.8. This indicated that acid was continuously consumed and the pH of the off-solution often exceeded 2.2 above which iron can precipitate. The iron precipitation was confirmed by the dismantling of the column at the termination of the test. Significant quantities of iron precipitates were observed and confirmed to be jarosite by later mineralogical examination.
It can be seen from Fig.2, Fig.3 and Fig.4 that the abiotic control was a typical acid leaching process: the redox potential ranged from 300 to 400 mV; the concentration of the ferric ion was below 2.0 g/L and the concentration of the ferrous ion was relatively high; the temperature in the charge was nearly the same as the ambient temperature.
Fig.2, Fig.3 and Fig.4 also show that the verification test was a typical bioleaching process. It was composed of three different leaching phases. At the initial phase (40 d), the concentration of the ferrous ion increased linearly and that of the ferric ion increased very slowly; the redox potential also increased slowly coinciding with a slow increase in column temperature and a low concentration of the mixed mesophiles. This trend indicated that the initial phase was dominated mainly by chemical (acid- leaching) leaching and kept consistent with the delayed phase of the mixed mesophiles. However, at the second phase (150 d), high value of the ferric ion concentration, high value of the redox potential, increased temperature of the charge and high value of the mixed mesophiles concentration indicated that this phase was a typical bioleaching process and kept consistent with the logarithmic phase and the stationary phase of the mixed mesophiles. At the last phase (55 d), the concentration of the ferric ion and the redox potential decreased coinciding with a decrease in column temperature and the concentration of the mixed mesophiles. The trend indicated that the last phase was a regressive bioleaching process and kept consistent with the extinction phase of the mixed mesophiles. This regressive trend also indicated that it was time to stop the leaching operation.
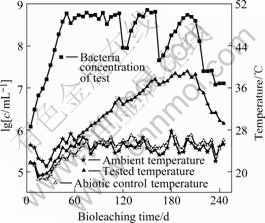
Fig.4 Bacteria concentration and temperature measured during bioleaching operation
The off-solution was bled periodically to control the levels of Mg2+ (not more than 20 g/L) and water with pro rata nutriment was added to make up for the bled solution. This operation kept consistent with the zigzag curves in Fig.2, Fig.3 and Fig.4. The bioleaching process of the Jinchuan low grade sulfide mineral ore proved some equations as follows[15-18].
1) Ferric ion can dissolve these sulfide minerals (MS):
MS+Fe3+![]() Fe2++S0+M2+
Fe2++S0+M2+
2) Bacteria can regenerate ferric iron:
Fe2+-e![]() Fe3+
Fe3+
3) The hydrolyzation of ferric sulfate can form precipitates at suitable pH:
Fe3++M2SO4+H2O![]()
M[Fe3(SO4)2(OH)6](s)+H2SO4
where M is a monovalent cation, e.g., H+, Na+, K+ and NH4+.
3.3.3 Metals extraction
Metals extraction in verification test and abiotic control during the whole leaching operation is listed in Table 3.
Table 3 Metals extraction during whole leaching operation
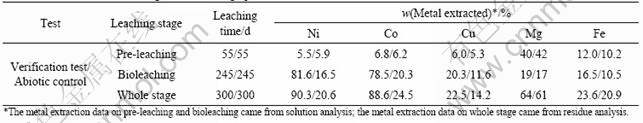
It could be seen from Table 3 that an extraction of nickel (90.3%) and cobalt (88.6%) were achieved within a 300 d leaching process from the Jinchuan low grade nickel sulfide ore. Table 3 also showed that the dissolution of copper extracted very slowly during bioleaching. This can be explained by the electrochemical mechanism that chalcopyrite is protected as cathode in the presence of pentlandite.
Three effective means were performed to reduce the disadvantages of magnesia in the ore. They were pre- leaching to remove most leachable magnesia, adaptation of the mixed mesophiles to improve the tolerance and periodic bleeds of a portion of the pregnant leaching solution to control the Mg2+ concentration based on the tolerance of the mixed microorganisms. It can be seen from the results of the valuable metals extraction that the disadvantages of magnesia were successfully reduced.
4 Conclusions
1) An extraction of nickel (90.3%) and cobalt (88.6%) was achieved within a 300 d leaching process from the Jinchuan low grade nickel sulfide ore.
2) Pre-leaching, adaptation and periodic bleeds were effective means to reduce the disadvantages of the magnesia during bioleaching process of the ore.
References
[1] Ehrlich H L. Past, present and future of biometallurgy [J]. Hydrometallurgy, 2001, 59(2/3): 127-134.
[2] Brierley J, Brierley C. Present and future commercial applications of biohydrometallurgy [J]. Hydrometallurgy, 2001, 59(2/3): 233-239.
[3] Watling H R. The bioleaching of sulfide minerals with emphasis on copper sulphides—A review [J]. Hydrometallurgy, 2006, 84(1/2): 81-108.
[4] YANG Song-rong, XIE Ji-yuan, QIU Guan-zhou, HU Yue-hua. Research and application of bioleaching and biooxidation technologies in China [J]. Minerals Engineering, 2002, 22(5): 361-363.
[5] Demergasso C S, Galleguillos P A. Molecular characterization of microbial populations in a low-grade copper ore bioleaching test heap [J]. Hydrometallurgy, 2005, 80(4): 241-253.
[6] Zhang Guang-ji, Fang Zhao-heng. The contribution of direct and indirect actions in bioleaching of pentlandite [J]. Hydrometallurgy, 2005, 80(1/2): 59-66.
[7] Ke Jia-jun, Li Hong-mei. Bacterial leaching of nickel-bearing pyrrhotite [J]. Hydrometallurgy, 2006, 82(3/4): 172-175.
[8] LI Hao-ran, FENG Ya-li. Bioleaching the open stripe nickel ore in Jinchuan Mine of China [J]. Journal of University of Science and Technology Beijing, 2004, 26(6): 584-587. (in Chinese)
[9] Hunter C. Bioheap leaching of a primary nickel-copper sulphide ore [C]// ALTA. Nickel/Cobalt. Perth, WA: ALTA, Melbourne, 2002: 11.
[10] Giaveno A, Donati E. Bioleachhing of heazelwoodite by Thiobacillus spp. [J]. Process Biochemistry, 2001, 36(10): 955-962.
[11] LI Hong-mei, KE Jia-jun. Influence of Ni2+ and Mg2+ on the growth and activity of Cu2+-adapted Thiobacillus ferrooxidans [J]. Hydrometallurgy, 2001, 61(3): 151-156.
[12] LI Hong-mei, KE Jia-jun. Influence of Cu2+ and Mg2+ on the growth and activity of Ni2+-adapted Thiobacillus ferrooxidans [J]. Minerals Engineering, 2001, 14(1): 113-116.
[13] QIU Mu-qing, XIONG Shui-ying, ZHANG Wei-min, WANG Gen-xuan. A comparison of bioleaching of chalcopyrite using pure culture or a mixed culture [J]. Minerals Engineering, 2005, 18(9): 987-990.
[14] Karamanev D G, Nikolov L N, Mamatarkova V. Rapid simultaneous quantitative determination of ferric and ferrous ions in drainage waters and similar solutions [J]. Miner Eng, 2002, 15(5): 341-346.
[15] Watling H R. The bioleaching of nickel-copper sulfides [J]. Hydrometallurgy, 2008, 91(1/4): 70-88.
[16] Pradhan N, Nathsarma K C, Srinivasa R K. Heap bioleaching of chalcopyrite: A review [J]. Minerals Engineering, 2008, 21(5): 355-365.
[17] Nicol M J, Lazaro I. The role of non-oxidative processes in the leaching of chalcopyrite [C]// Riveros P A, Dixon D, Dreisinger D B, Menacho J. Copper 2003—Cobre 2003 (Santiago) Volume VI (Book 1). Montreal: Canadian Institute of Mining, Metallurgy and Petroleum, 2003: 367-381.
[18] Lazaro I, Nicol M J. The mechanism of the dissolution and passivation of chalcopyrite: an electrochemical study [C]// Young C A, Alfantazi A M, Anderson C G, Dreisinger D B, Harris B, James A. Hydrometallurgy 2003. Warrendale: TMS, 2003: 405-417.
Foundation item: Project(50621063) supported by the National Natural Science Foundation of China; Project(2004CB619205) supported by the National Basic Research Program of China; Project(2007AA060902) supported by the High-tech Research and Development Program of China
Corresponding author: ZHEN Shi-jie, Tel: +86-731-8830002; E-mail: zhenshijie369@126.com


