J. Cent. South Univ. (2017) 24: 2245-2252
DOI: https://doi.org/10.1007/s11771-017-3634-2
Effect of ultraviolet mutagenesis on heterotrophic strain mutation and bioleaching of low grade copper ore
WU Ai-xiang(吴爱祥)1, HU Kai-jian(胡凯建)2, WANG Hong-jiang(王洪江)1,
ZHANG Ai-qing(张爱卿)1, YANG Ying(杨莹)1
1. School of Civil and Environmental Engineering, University of Science and Technology Beijing,Beijing 100083, China;
2. School of Resources and Environmental Engineering, Jiangxi University of Science and Technology,Ganzhou 341000, China
 Central South University Press and Springer-Verlag GmbH Germany 2017
Central South University Press and Springer-Verlag GmbH Germany 2017
Abstract:
The effect of ultraviolet mutagenesis on a heterotrophic strain (Providencia JAT-1) mutation was studied and bioleaching of low grade copper ore with mutant bacteria was investigated. The results show that the activity of bacteria was improved after ultraviolet mutagenesis; the best irradiation time was 120 s. Compared to the original bacteria, the cells density of mutant bacteria at stationary phase increased by 26% and ammonia produced by mutant bacteria increased by 12%. Higher activity of bacteria leads to a higher copper extraction rate. The bioleaching performance of Providencia JAT-1 was improved after UV mutagenesis. The copper extraction rate with mutant bacteria increased by 10.6% compared to the original bacteria. The ore surface was corroded and the fine particles were absent after bioleaching. Free copper oxide and copper silicates could be leached out easily by using JAT-1; a small part of the copper sulfide can also be leached out. Bioleaching using JAT-1 is more effective than ammonia leaching and copper extraction rate with mutant bacteria was 21.1% higher than that by ammonia leaching under the same condition.
Key words:
ultraviolet mutagenesis; bioleaching; heterotrophic strain; ammonia; low grade copper ore;
1 Introduction
Compared to the conventional metal extraction technologies, bioleaching is a promising technology due to its advantages such as simple operation, low operating cost, low energy input and friendly towards the environment [1, 2]. There are many studies related to the bioleaching of sulfide copper ores with autotrophic bacteria such as A. ferrooxidans and L. thiooxidans [3–5]. For the past few years, low grade copper ores, with high contents of oxide ore and carbonate gangue, have gradually accounted for a high proportion in microbial leaching industry [6, 7]. However, acidic leaching becomes ineffective and uneconomical for these ores owing to the excessive acid consumption [8].
In the ammonia leaching system, copper can be dissolved selectively, leaving undesired metals in the residue [9]. Being more selective, less corrosive and lowering reagent consumption for carbonate gangue are the main advantages of ammonia leaching. Though a number of literatures reported the leaching of copper ore containing carbonate gangue in ammonia medium, little attention has been paid to the bioleaching in alkaline environments. In our previous study, a heterotrophic strain, which can grow robustly in the presence of urea and produce ammonia, was obtained and successfully used in bioleaching of low grade copper ore [10].
To improve the bioleaching activity of bacteria, UV-induced mutagenesis breeding method was introduced in this study. UV mutagenesis is the simplest and effective physical mutation method; it can cause the change of DNA structure and lead to the mutation [11–13]. The effective wavelength is mainly around 255 nm, which is the same with the DNA absorption spectrum of general bacteria.
The objective of this work is to improve the copper extraction from low grade ores in alkaline bioleaching environments with mutant ammonia-producing heterotrophic strain. Firstly, the effect of ultraviolet on bacteria mutation was investigated, and then the most effective mutant bacteria were selected and used in bioleaching. Secondly, series of shake flask leaching experiments were carried out and the bioleaching performance of mutant bacteria and its advantage compared to chemical leaching were studied. Moreover,the phase transformation and morphological property of copper ore were analyzed after bioleaching.
2 Experimental
2.1 Strain and culture media
The strain was isolated from alkaline soil sample collected from Wushan copper mine in Inner Mongolia, China. The identification result of 16Sr DNA shows that the strain belonged to Providencia Sp. and it was designated as Providencia JAT-1. The strain is heterotrophic and takes organic carbon as energy and carbon source. The urea is utilized as nitrogen source for the growth of strain. Thus, the strain was enriched in urea medium consisting of following compounds: sodium citrate (10 g/L), urea (20 g/L), Na2HPO4 (2.1 g/L), KH2PO4 (1.4 g/L), MgSO4·7H2O (0.02 g/L). The medium was autoclaved at 121 °C for 20 min prior to inoculation; urea was separately sterilized through a 0.2 μm filter and was added aseptically to the urea free medium.
2.2 Copper ores
The copper ore used in this study was obtained from Yunnan province, China. The chemical analysis of the copper ore is shown in Table 1. The content of copper in the ore was too low to be detected by the X-ray diffraction, so the phases of copper ore were identified by the polarizing microscope [14]. The phases mainly were malachite, chrysocolla and chalcopyrite, corresponding to free copper oxide, copper silicates and copper sulfide, respectively (Table 2). The main gangue minerals were quartz, limonite, calcite and dolomite. The samples were crushed into the particle size of 74 μm before bioleaching.
Table 1 Chemical composition of copper ore (mass fraction, %)
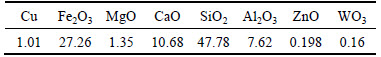
Table 2 Phase composition of copper ore

2.3 Mutations
The bacterial cells in the logarithmic phase were centrifuged for 20 min (5000 r/min) and the solid cells were obtained after removing the supernatant. The solid cells were washed and suspended in sterilized physiologic saline (9 g/L NaCl) and the cells density was adjusted to 1×108 cell/mL.
Ten milliliters of cells solution were added to a petri-dish and irradiated with UV light at a distance of 30 cm for 30, 60, 90, 120, 150, and 180 s, respectively. The power of UV lamp was 15 W and the UV wavelength was 254 nm. The number of mutant cells in the petri-dish was counted under the microscope and the lethality rate of cells can be calculated. The mutants with different radiation time were cultured in the urea medium at optimal growth condition and the growth and ammonia production of bacteria were measured at certain intervals. The mutant bacteria with the best growth rate and ammonia production were used in the bioleaching experiments. The 16S rRNA gene of mutant bacteria was amplified and sequenced, and then compared with the National Center for Biotechnology Information (NCBI) GenBank database using the nucleotide BLAST.
2.4 Bioleaching experiment
To investigate the effects of original and mutant bacteria on bioleaching of low grade copper ore, bioleaching experiments with original and mutant bacteria were carried out in 250 mL flasks. Each flask contained 80 mL urea medium and 20 mL of enriched bacteria and the cells density was 2×107 cell/mL after inoculation. The pulp density of copper ore was 5% (w/v). After inoculation, all flasks were incubated in a rotary shaker at 30°C, initial pH 8.0 and 180 r/min. During the experiments, samples were withdrawn at certain intervals to monitor the growth of bacteria, pH value and concentration of Cu2+ in the leaching solution. Each experiment was carried out in triplicate. Control experiment without bacteria inoculation was also carried out.
To investigate the advantage of bioleaching, ammonia leaching experiments were conducted under the same conditions with bioleaching. The initial ammonia concentration in chemical leaching was determined by the ammonia production in urea medium when the urea was completely hydrolyzed by strain; pulp density was 5% (w/v). The copper extraction rate was monitored during the leaching process.
2.5 Analytical methods
The concentration of dissolved copper ions in the leaching solution was determined by atomic absorption spectrometry, and the percentage of the Cu recovery was evaluated by Eq. (1) as follows:
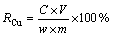 (1)
(1)
where RCu is the percentage of the Cu recovery from the copper ores; C (mg/L) is the concentration of Cu in the leaching solution; V (L) is the volume of the leaching solution; w is the mass fraction of the Cu in the copper ore and m is the mass of copper ore in the leaching solution.
The pH was measured using a digital pH meter. The ammonia concentration was figured out by the concentration of NH4+ in leaching solution, which was measured by ion chromatograph (792 Basic IC). Growth of bacteria was monitored by cell counting using Neubauer counting chamber. The SEM was used in the experiment to observe the morphological characteristics of copper ores before and after bioleaching.
3 Results and discussion
3.1 Optimal growth condition of strain
To obtain the optimal growth condition of strain, the effects of temperature, initial pH and shaking rate of rotary shaker were taken into consideration. The physiological action of bacteria was directly influenced by the temperature and initial pH; the oxygen dissolution capacity of culture medium was influenced by the shaking rate of rotary shaker [15].
The results are shown in Fig. 1. According to the Fig. 1(a), the growth of bacteria was influenced obviously when the temperature was lower than 27 °C, whereas the bacteria grew well when the temperature was higher than 30 °C. The density of cells reached a maximum of 4.5×108 cell/mL at the stationary phase when inoculation temperature is 30 °C. According to Fig. 1(b), the growth of bacteria was inhibited obviously when the initial pH was either higher than 10.0 or lower than 6.0. The optimal initial pH was 8.0, at which the cells density reached a maximum of 4.6×108 cell/mL at the stationary phase. In Fig. 1(c), the cell density increased with the increasing of shaking rate until 180 r/min. Therefore, 180 r/min was chosen as the optimal shaking rate. The optimal growth conditions of strain were temperature of 30 °C, initial pH 8.0 and shaking rate of 180 r/min.
3.2 Effect of ultraviolet on strain mutation
Ultraviolet is an effective physical mutation method, which will cause the change of DNA structure and form the thymine dimmers, leading to the mutation of AT to GC during replication and transcription of DNA [16]. However, excessive UV irradiation usually leads to the death of cells. Under this condition, numerous thymine dimmers were formed, which resulted in an obvious genetic variation and generation of a large amount of mutagen, leading to a high lethality rate of cells. Figure 2 shows the relationship between irradiation time and lethality rate of JAT-1 cells. The lethality rate increased with irradiation time. The lethality rate of bacteria was 10% when the irradiation time was 30 s, while it increased to 87% when the irradiation time was 180 s. The results indicated that with a longer irradiation time, large amount of mutagen was produced and thus a high lethality rate was revealed.
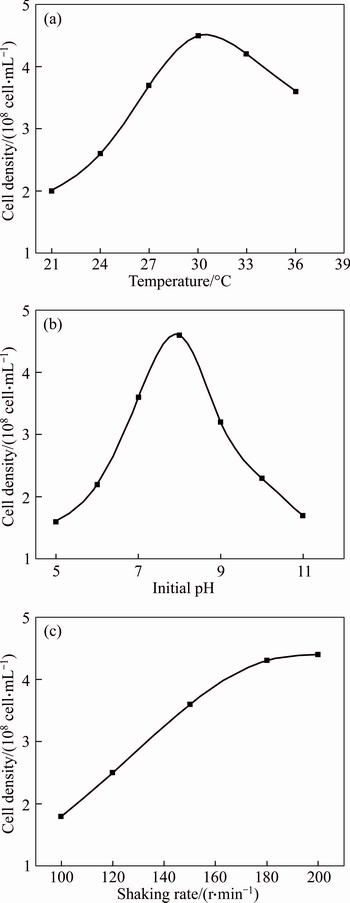
Fig. 1 Effect of temperature (a), initial pH (b) and shaking rate (c) on growth of strain
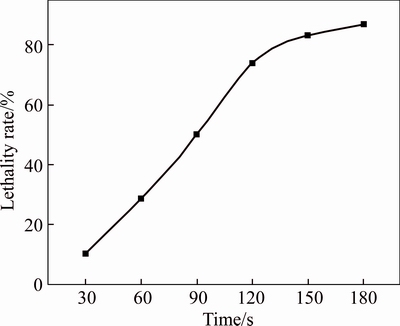
Fig. 2 Effect of UV irradiation time on lethality rate
In order to ensure the stability of mutants, the mutant bacteria were cultured for several generations. And then, the mutant bacteria with different irradiation time were cultured in urea medium at the optimal condition. The strain growth and ammonia production in culture solution are shown in Figs. 3 and 4, respectively.
As shown in Figs. 3 and 4, the activity of bacteria was improved after ultraviolet mutagenesis. Mutant bacteria with 120 s UV irradiation had a best growth rate and the cells density reached 5.85×108 cell/mL at the stationary phase, which increased by 26% compared to the original bacteria. With the lethality rate of 50%–80%, more positive mutant strains were in survival cells and a few high efficiency mutants will also be there [12, 17]. When the irradiation time was longer than 150 s, the growth rate of bacteria decreased compared to that with 120 s, which indicates that with a lethality rate higher than 80%, more negative mutant strains and fewer positive mutant strains are in survival cells.
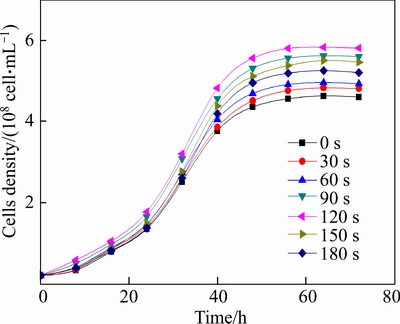
Fig. 3 Growth of bacteria with different irradiation time
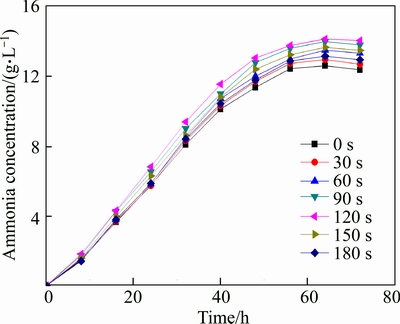
Fig. 4 Ammonia produced by bacteria with different irradiation time
According to Fig. 4, ammonia production increased with the trend of bacterial growth. Mutant bacteria with 120 s UV irradiation had the highest ammonia production, the ammonia concentration in leaching solution reached 14.16 g/L which increased by 12% compared to the original bacteria. The mutant bacteria with 120 s irradiation were selected and used in the bioleaching experiments.
After mutation, the 16S rRNA gene sequence of strain was blasted into the database of GenBank and the similar BLAST hits of 16S rRNA indicated that the strain still belonged to the genus Providencia. Phylogenetic analysis of mutant bacteria with 120 s irradiation was shown in Fig. 5. The result indicates that the strain belonged to Providencia sp. with a bootstrap support of 100% and it was most closely related to Providencia sp. NCCP-604 with a similarity of 99%.
3.3 Bioleaching experiments with mutant strain
3.3.1 Bioleaching experiments
To investigate the bioleaching performance of strain JAT-1 after mutation, bioleaching experiments were carried out with the experiment conditions mentioned in Section 2.4. The results are shown in Fig. 6. Copper extraction rate increased slowly at the beginning of bioleaching and enhanced greatly after 40 h. After bioleaching for 152 h, the copper extraction rates reached 41.09% and 51.69% for original and mutant bacteria, respectively. The bioleaching efficiency was improved by using mutant bacteria; copper extraction rate with mutant bacteria increased by 10.6% compared with that of original bacteria. The results indicated that the UV mutation can improve the bioleaching performance of JAT-1 in processing low grade copper ore.
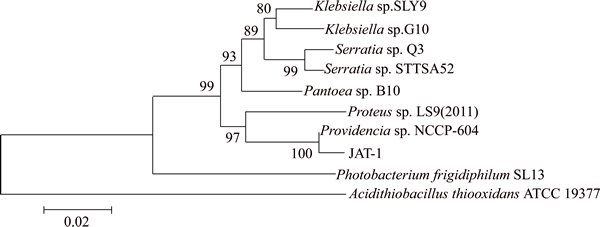
Fig. 5 Phylogenetic tree of JAT-1 after mutation based on 16S rRNA sequences
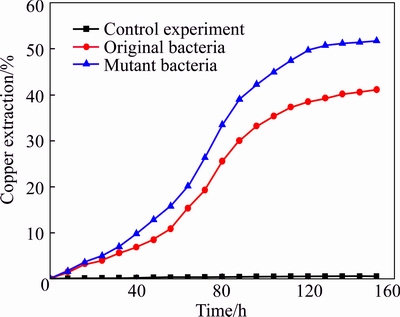
Fig. 6 Bioleaching of copper ore by bacteria before and after mutation
Figure 7 shows the growth of bacteria and variation of ammonia concentration in leaching system. Compared to the original bacteria, the mutant bacteria grew faster and the cells density was higher in leaching solution; ammonia concentration also showed the same trend. Higher cells density and ammonia concentration result in a higher copper extraction rate. It also indicated that the dissolution of copper ore was related to the activity of bacteria. The activity of bacteria was improved after ultraviolet mutagenesis, thus, leading to an improvement of its bioleaching performance. With the decrease of cells density and ammonia concentration at the decline phase, the copper extraction rate increased slowly. To improve the bioleaching efficiency, fresh medium is needed to improve the activity of bacteria at the decline phase of bacteria. After bioleaching, the 16S rRNA gene of bacteria was sequenced and blasted into the database of GenBank. The result indicated that the strain belonged to the genus Providencia. That means that the bacteria after bioleaching were still JAT-1.
3.3.2 Phase transformation of copper ore after bioleaching
The bioleaching process of malachite, chrysocolla and chalcopyrite can be described as Eq. (2)– Eq. (6).
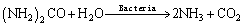 (2)
(2)
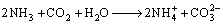 (3)
(3)
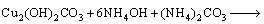
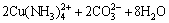 (4)
(4)
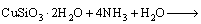
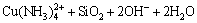 (5)
(5)
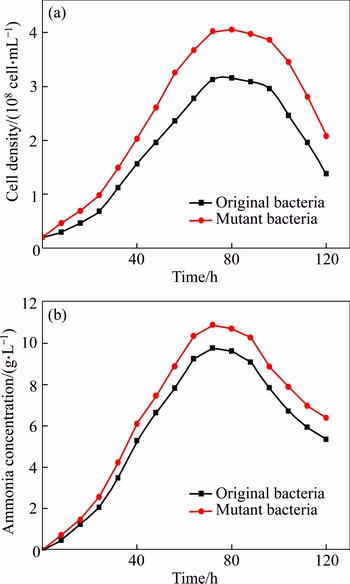
Fig. 7 Bacteria growth (a) and variation of ammonia concentration (b) in bioleaching system

 (6)
(6)
The different phase contents of copper ore before and after bioleaching are shown in Fig. 8. The free copper oxides are easy to be leached out; the dissolution rate of free copper oxide reached 43.18% and 57.38% for original and mutant bacteria, respectively. Copper silicate is very difficult to be leached out owing to dispersion of copper atoms in the crystal lattice of gangue ores [14]. However, most of copper silicate was leached out in this bioleaching system. This phenomenon was also reported by JAIN [18] that the silicon–oxygen bond is disrupted in the high alkalization condition, resulting in the release of silicon from quartz. The dissolution rate of copper silicate reached 51.9% and 65.39% for original and mutant bacteria, respectively. The phase of copper sulfides was leached out partly, with the oxygen dissolved in the solution as an oxidant [14].
Dissolution rates of free copper oxide and copper silicate increased obviously after mutation, while dissolution of copper sulfide showed little difference before and after mutation. Results revealed that the dissolution of free copper oxide and copper silicate mainly depended on the bacterial growth and its metabolic by-products, while dissolution of copper sulfide is related to the oxidant in the solution. LIU et al [19] reported that the copper sulfides can be oxidized and dissolved effectively in ammonia solution with sodium persulfate as an oxidant, so we can infer that a more efficient oxidant will promote the dissolution of copper sulfide in bioleaching system.
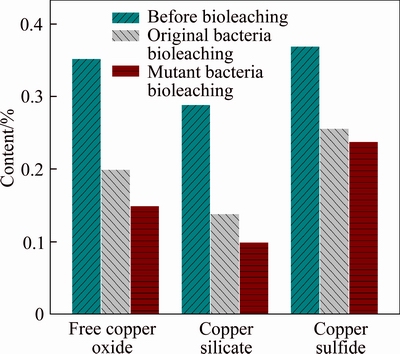
Fig. 8 Phase transformation of copper ore before and after bioleaching
3.4 SEM analysis of bioleached residue
SEM analyses of copper ore surface before and after bioleaching are shown in Figs. 9 and 10, respectively. Figure 10 reveals an absence of fine particles on the surface of bioleached copper ore. The surface of copper ore was corroded after bioleaching; the defect and crack were obviously observed. EDS analysis showed that the mass proportion and atom number proportion of element Cu, Si, O were significantly decreased after bioleaching. The Cu on the surface was dissolved to an ionic state with the leaching action of bacteria. The silicon–oxygen bond was disrupted in the high alkalization condition, leading to the release of Si and O from chrysocolla or quartz.
3.5 Comparison of bioleaching and ammonia leaching
To investigate the bioleaching advantages compared to chemical leaching, ammonia leaching experiments were carried out under the same condition with bioleaching. The initial ammonia concentration in chemical leaching was 0.67 mol/L, which was equal to the value when the urea was completely hydrolyzed by the strain in urea medium. The results are shown in Fig. 11. After 152 h, the copper extraction rate of ammonia leaching was 30.59%, which was 21.1% lower than bioleaching. Results proved that bioleaching using JAT is more effective than ammonia leaching under the same experimental condition. Metabolites other than ammonia produced by strain must be involved in bioleaching, which will interact with the mineral and promote the dissolution of metals [18].
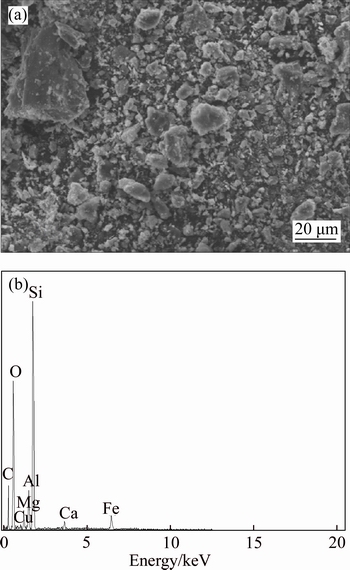
Fig. 9 SEM image (a) and EDS analysis (b) of copper ore
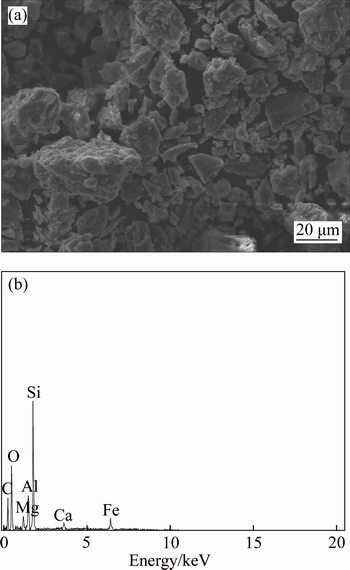
Fig. 10 SEM image (a) and EDS analysis (b) of leaching residue
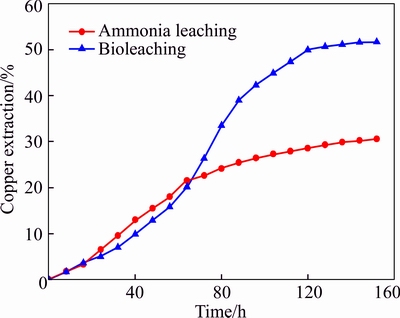
Fig. 11 Comparison of bioleaching and ammonia leaching
4 Conclusions
1) The heterotrophic strain, designated as Providencia JAT-1, can grow in the presence of urea and produce ammonia. The optimal growth conditions of bacteria were temperature of 30 °C, initial pH 8.0 and shaking rate of 180 r/min.
2) The activity of bacteria was improved after ultraviolet mutagenesis. The best irradiation time was 120 s. After irradiating for 120 s, cells density at the stationary phase increased by about 26% and ammonia production increased by about 12% compared to the original bacteria.
3) The bioleaching performance of bacteria was improved after mutation. The copper extraction rate with mutant bacteria was 10.6% higher compared with that of original bacteria. The dissolution of copper ore was related to the activity of bacteria and higher activity of bacteria leads to a higher copper extraction rate.
4) Free copper oxide and copper silicates could be bioleached out easily by using JAT. A small part of copper sulfide was leached out. SEM analysis indicated that the surface of copper ore was corroded and the fine particles were absent after bioleaching. The mass proportion and atom number proportion of Cu, Si and O decreased in the alkaline bioleaching condition.
5) Bioleaching using JAT is more effective than ammonia leaching. The copper extraction rate with mutant bacteria was 21.1% higher than ammonia leaching under the same experimental condition. Metabolites other than ammonia produced by strain were also involved in bioleaching.
References
[1] BRIERLEY C L. Biohydrometallurgical prospects [J]. Hydrometallurgy, 2010, 104(3): 324–328.
[2] PRADHAN N, NATHSARMA K C, RAO K S, SUKLA L B, MISHRA B K. Heap bioleaching of chalcopyrite: A review [J]. Minerals Engineering, 2008, 21(5): 355–365.
[3] BRIERLEY J A, BRIERLEY C L. Present and future commercial applications of biohydrometallurgy [J]. Hydrometallurgy, 2001, 59(2): 233–239.
[4] FALCO L, POGLIANI C, CURUTCHET G, DONATI E. A comparison of bioleaching of covellite using pure cultures of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans or a mixed culture of Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans [J]. Hydrometallurgy, 2003, 71(1): 31–36.
[5] LIU Jian-she, XIE Xue-hui, XIAO Sheng-mu, WANG Xiu-mei, ZHAO Wen-jie, TIAN Zhuo-li. Isolation of Leptospirillum ferriphilum by single-layered solid medium [J]. Journal of Central South University of Technology, 2007, 14(4): 467–473.
[6] EKMEKYAPAR A,  A, DEMIRKIRAN N. Investigation of leaching kinetics of copper from malachite ore in ammonium nitrate solutions [J]. Metallurgical and Materials Transactions B, 2012, 43(4): 764–772.
A, DEMIRKIRAN N. Investigation of leaching kinetics of copper from malachite ore in ammonium nitrate solutions [J]. Metallurgical and Materials Transactions B, 2012, 43(4): 764–772.
[7] PEACEY J, GUO Xian-jian, ROBLES E. Copper hydrometallurgy- current status, preliminary economics, future direction and positioning versus smelting [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(3): 560–568.
[8] LIU Zhi-xiong, YIN Zhou-lan, XIONG Shao-feng, CHEN Yi-guan, CHEN Qi-yuan. Leaching and kinetic modeling of calcareous bornite in ammonia ammonium sulfate solution with sodium persulfate [J]. Hydrometallurgy, 2014, 144: 86–90.
[9]  S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching [J]. Hydrometallurgy, 2005, 76(1): 55–62.
S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching [J]. Hydrometallurgy, 2005, 76(1): 55–62.
[10] WANG Hong-jiang, XIONG You-wei, WU Ai-xiang, WANG Heng, HUANG Ming-qing. Alkaline copper oxide ore bioleaching by ammonia-producing bacteria [J]. Journal of University of Science and Technology Beijing, 2013, 35(9): 1126–1130.
[11] SCHAAPER R M, DUNN R L, GLICKMAN B W. Mechanisms of ultraviolet-induced mutation: Mutational spectra in the Escherichiacoli lacI gene for a wild-type and an excision-repair- deficient strain [J]. Journal of Molecular Biology, 1987, 198(2): 187–202.
[12] DONG Y, LIN H, WANG H, MO X, FU K, WEN H. Effects of ultraviolet irradiation on bacteria mutation and bioleaching of low-grade copper tailings [J]. Minerals Engineering, 2011, 24(8): 870–875.
[13] YANG Yu, ZHANG Shuai, XU Ai-ling, ZOU Li-hong, LI Li, QIU Guan-zhou. UV-induced mutagenesis and bioleaching of Acidiphilium cryptum and Acidithiobacillus ferrooxidans [J]. Journal of Central South University: Science and Technology, 2010, 41(2): 393–399. (in Chinese)
[14] LIU Wei, TANG Mo-tang, TANG Chao-bo, HE Jing, YANG Sheng-hai, YANG Jian-guang. Dissolution kinetics of low grade complex copper ore in ammonia-ammonium chloride solution [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 910–917.
[15] ZHANG Jian-bin, ZHANG Tong, MA Kai, CHEN Guo-hua, ZHANG Dong-yan, WEI Xiong-hui. Isolation and identification of the thermophilic alkaline desulphuricant strain [J]. Science in China Series B: Chemistry, 2008, 51(2): 158–165.
[16] XU Ai-ling, XIA Jin-lan, ZHANG Shuai, YANG Yu, NIE Zhen-yuan, QIU Guan-zhou. Bioleaching of chalcopyrite by UV-induced mutagenized Acidiphilium cryptum and Acidithiobacillus ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(2): 315–321.
[17] BEGGS C B. A quantitative method for evaluating the photoreactivation of ultraviolet damaged microorganisms [J]. Photochemical & Photobiological Sciences, 2002, 1(6): 431–437.
[18] JAIN N, SHARMA D K. Biohydrometallurgy for nonsulfidic minerals-a review [J]. Geomicrobiology Journal, 2004, 21(3): 135–144.
[19] LIU Zhi-xiong, YIN Zhou-lan, HU Hui-ping, CHEN Qi-yuan. Leaching kinetics of low-grade copper ore containing calcium- magnesium carbonate in ammonia-ammonium sulfate solution with persulfate [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(11): 2822–2830.
(Edited by YANG Hua)
Cite this article as:
WU Ai-xiang, HU Kai-jian, WANG Hong-jiang, ZHANG Ai-qing, YANG Ying. Effect of ultraviolet mutagenesis on heterotrophic strain mutation and bioleaching of low grade copper ore [J]. Journal of Central South University, 2017, 24(10): 2245–2252.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-017-3634-2Foundation item: Project(2012BAB08B02) supported by the National Key Technologies R&D Program for the 12th Five-year Plan, China; Projects(51304011, 51374035) supported by the National Natural Science Foundation of China
Received date: 2015-11-26; Accepted date: 2016-03-25
Corresponding author: HU Kai-jian, PhD; Tel: +86–18810826909; E-mail: hukaijian1988@qq.com
Abstract: The effect of ultraviolet mutagenesis on a heterotrophic strain (Providencia JAT-1) mutation was studied and bioleaching of low grade copper ore with mutant bacteria was investigated. The results show that the activity of bacteria was improved after ultraviolet mutagenesis; the best irradiation time was 120 s. Compared to the original bacteria, the cells density of mutant bacteria at stationary phase increased by 26% and ammonia produced by mutant bacteria increased by 12%. Higher activity of bacteria leads to a higher copper extraction rate. The bioleaching performance of Providencia JAT-1 was improved after UV mutagenesis. The copper extraction rate with mutant bacteria increased by 10.6% compared to the original bacteria. The ore surface was corroded and the fine particles were absent after bioleaching. Free copper oxide and copper silicates could be leached out easily by using JAT-1; a small part of the copper sulfide can also be leached out. Bioleaching using JAT-1 is more effective than ammonia leaching and copper extraction rate with mutant bacteria was 21.1% higher than that by ammonia leaching under the same condition.


