
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 865-872
Convenient synthesis of silver nanoplates with adjustable size through seed mediated growth approach
YI Zao1,2, ZHANG Jian-bo1, HE Hua2, XU Xi-bin1,2, LUO Bing-chi1, LI Xi-bo1, LI Kai1, NIU Gao1,
TAN Xiu-lan1, LUO Jiang-shan1, TANG Yong-jian1,3 , WU Wei-dong1, YI You-gen1, 2
1. Research Center of Laser Fusion, China Academy of Engineering Physics, Mianyang 621900, China;
2. School of Physical Science and Technology, Central South University, Changsha 410083, China;
3. Institute of Atomic and Molecular Physics, Sichuan University, Chengdu 610065, China
Received 29 April 2011; accepted 24 October 2011
Abstract:
Silver nanoplates, with average thickness about 5 nm and average tunable size from 40 to 500 nm, were synthesized via a simple room-temperature solution-phase chemical reduction method in the presence of appropriate concentration of trisodium citrate and silver seeds. The optical in-plane dipole plasmon resonance bands of these silver plates could be tuned from 520 to 1100 nm. Control experiments were explored for understanding of the growth mechanism. It is found that both the amount of citrate ions and the small silver seeds added to the growth solution are the key to controlling the silver nanoplates without changing their thickness and crystal structure. Small silver seeds are found to play an important role in the formation of large thin silver nanoplates when poly(vinylpyrrolidone) (PVP) are used as capping agent.
Key words:
silver nanoplate; trisodium citrate; poly (vinylpyrrolidone) (PVP); seed-mediated method; surface plasmon resonance (SPR);
1 Introduction
Metal nanostructures play important roles in many areas due to their fascinating properties. Their optical properties [1], together with their electrical [2] and catalytic [3] properties, etc, are most attractive in gold and silver nanostructures. The optical properties of the nanostructures are strongly dependent on their shape and size [4-6]. Therefore, there has been an explosion of interest in developing versatile methods for shape- controlled synthesis of these nanostructures for extending their optical properties according to human wishes.
A variety of methods have been reported to control the synthesis of silver nanoparticles with two- dimensional (2D) morphologies for desirable properties, such as the photo-induced method [7], electrochemical method [8], ultrasonic-assistant method [9], solvo- thermal method [10] and templating method (e.g., “soft” reverse micelles and “hard” polystyrene spheres) [11]. Seed-mediated process is extremely interesting and attractive in the synthesis of silver nanomaterials of two-dimensional (2D) morphologies. It has been applied to synthesizing silver nanoplates [12-15]. However, there is only limited success in the size-controlled synthesis of silver nanoplates by using easy approach. For example, although the size of silver nanoplates can be controlled through the reaction condition, a wide variety of other shapes such as rodlike shapes would be simultaneously produced [12,13]. Some approach needs vigorous reaction condition, such as thermal [14] and irradiated [15] process. Recently, KILIN et al [16] demonstrated in the ab initio study that the approximate 3-fold symmetry of citrate matches that of Ag(111) and results in four Ag—O bonds. MPOURMPAKIS and VLACHOS [17] supposed the citrate ions serve as charge regulators as well as reducing agents and stabilizers through density functional theory simulations.
Here, we employed seed-mediated growth strategy to prepare silver nanoplates in the presence of citrate at room-temperature. The shape of the silver particles and the optical in-plane dipole plasmon resonance bands of these nanoplates was controlled by varying the experimental parameters such as the amount of citrate ions and the small silver seeds.
2 Experimental
2.1 Chemicals
Silver nitrate (AgNO3, 99.9%), trisodium citrate (99%), sodium borohydride (NaBH4, 99%), PVP (Mr= 55000) were all purchased from Sigma-Aldrich. All of the solutions were freshly made for the synthesis of silver nanoparticles, especially the freshly made NaBH4 aqueous solution, which was ice bathed before use. All solutions were prepared using triply distilled deionized water.
2.2 Preparation of silver seed
A typical procedure for the synthesis of silver seed hydrosol was as follows: 0.5 mL 1.0×10-2 mol/L AgNO3 solution and 2 mL 2.0×10-3 mol/L PVP solution were added to 17.5 mL distilled water in a two-neck round bottom flask and the solution was kept under ice cold condition for 15 min. Then 0.3 mL of 10-2 mol/L ice cold aqueous NaBH4 solution was added all at once with vigorous stirring and the stirring was continued for 45 s. The solution became golden yellow, indicating the formation of silver hydrosol. Resulting silver hydrosol was heated to 75-80 °C for 10 min to decompose excess NaBH4 present in the solution. Silver hydrosol thus prepared was aged at room temperature for 2 h before using as seed.
2.3 Preparation of silver nanoprisms
Growth solution containing aqueous solution of 0.5 mL 1.0×10-2 mol/L AgNO3, 10 mL 0.1mol/L PVP and 0.2 mL different concentrations of trisodium citrate solution was prepared in a 25 mL conical flask. Next, different volumes of seed solution were added to this growth solution under stirring for 10 min for well mixing. Then these solutions are stored in the darkroom at room temperature for about 24 h. The mixture in the bottles was muddy, and on the bottom of bottles some sediments appeared.
2.4 Instrumentation and measurements
A JEM-2010 transmission electron microscope (TEM) was used to observe the nanoparticles. All TEM samples were prepared by dropping the as-prepared colloids (centrifuged at 4000 r/min for 30 min and then redispersed in 2 mL of de-ionized water) on copper grids and letting dry in air. X-ray diffraction patterns (XRD) were recorded on an X’ Pert PRO X-ray diffractometer using Cu Kα (40 kV, 40 mA) radiation. All XRD samples were prepared by dropping some centrifuged colloid on clean glass slides and letting dry. Fourier transform infrared spectroscopy (FT-IR) was obtained with a Perkin-Elmer spectrophotometer, model 1600, using the KBr pellet technique, which involves mixing thoroughly the material to be tested with KBr before forming a pellet at high pressure. All UV-VIS-NIR spectra were recorded within a 1 cm optical length quartz cell on a Perkin-Elmer Lambda 12 spectrophotometer.
3 Results and discussion
3.1 TEM
TEM images of the initial silver nanoparticles and the products by seed-mediated growth approach in aqueous solvent are shown in Fig. 1. Silver nanoparticle seeds with an average diameter of 5 nm are obtained after reduction of AgNO3 with NaBH4 in the presence of PVP as a surface ligand for the nanoparticles (Fig. 1(a)). Figures 1 (b-f) show the TEM images of products deoxidized by trisodium citrate in aqueous solvent with different volumes of seed solution at 24 h. It can be seen that the size of nanoplates becomes larger as the amount of silver seeds decreases. When 0.6 mL seed solution is introduced into, the size of silver nanoplates in the range of 60-120 nm is obtained (Fig. 1(b)). When the amount of the seeds is decreased to 0.4 mL (Fig. 1(c)), the size of the nanoplates increases to 140-200 nm. Figure 1(d) shows the magnification of the region in Fig. 1(c). As shown in the image, the silver nanoplates are composed of triangle and hexagon. Further, the seed solution is decreased to 0.2 mL (Fig. 1(e)), resulting in the formation of larger nanoplates with the size between 230 and 300 nm. When the amount of the seeds is decreased to 0.1 mL (Fig. 1(f)), the size of the nanoplates increases to 380-500 nm. The yield of silver nanoplates relative to the total number of nanoparticles formed is more than 85%. As shown in Figs. 1(b)-(f), the thickness of the nanoplates with different edge lengths is almost the same (~5 nm). When silver ions in the growth solution are kept at a constant, more nucleation centers will evidently lead to fewer silver ions for a single nanoplate. So, it is reasonable that the size of nanoplates will decrease with increasing the amount of seed.
In order to comprehend the growth mechanism of the silver nanoplates, TEM was used to monitor the morphological evolution of the nanostructures from silver nuclei or nanoparticles to nanoplates at various time during the growth reaction. Figure 2 shows the TEM images of the as-synthesized silver nanoplates with different growth time. The volume of silver seed solution is 0.2 mL and concentration of trisodium citrate is 0.20 mmol/L. The silver nanoplate lengths increased up to 500 nm, whereas the thicknesses remained almost constant (~5 nm) throughout all stages of the growth reaction. During the initial stages of growth (after 1 h, Fig. 2(a)), the size of silver nanoparticles does not obvious change, indicating that the growth reaction is very slow. With increase of the growth time (Fig. 2(e), the type silver nanoparticle seeds with an average diameter of 5 nm changed from spherical to triangular shape with the edge length between 230 and 300 nm. After 30 h, most of the triangular silver nanoplates are completely converted into hexagonal silver nanoplates with edge length 400-500 nm. According to our experimental results, the suitable growth reaction time is 24 h to synthesize triangular silver nanoplates.

Fig. 1 TEM images of silver seeds (a), and products deoxidized by trisodium citrate solution ( 0.20 mmol/L ) with 0.6 mL (b), 0.4 mL (c, d), 0.2 mL (e) and 0.1 mL (f) of seed solution at 24 h
3.2 XRD and SAED pattern analysis
Figure 3(a) shows XRD pattern of the products. The three peaks are assigned to the diffraction of {111}, {200} and {220} planes of FCC silver. The cell parameter is calculated from this pattern to be 4.087 ? in agreement with the standard card (PCPDF No. 04—0783). It is noticed that the ratio between the intensities of {111} and {200} diffraction peaks is much higher than the conventional value (13 versus 4) [18]. This indicates that the nanoparticles are {111} oriented, and tend to lay with these planes parallel to the supporting substrate. Thus, diffraction intensity of the {111} plane should be greatly enhanced compared with that of other planes. Further evidence is offered by the selected-area electron diffraction analysis. Figure 3(b) shows the selected-area electron diffraction (SAED) pattern of one flat-lying nanoplate with its top surface perpendicular to the electron beam, in which the spots correspond to the {220}, {422} and 1/3{422} Bragg reflections of face-centered cubic (fcc) silver respectively. The existence of {220} reflection indicates that the nanoplates are single crystals with {111} planes as the basal planes, and the appearance of normally forbidden 1/3{422} reflection implies the presence of numbers of {111} stacking faults lying parallel to the {111} surface and extending across the entire nanoplate [18]. Thus, it is reasonable that the top and bottom surfaces of the nanoplates are bounded by atomically flat {111} planes [19]. From the structure analyses on the XRD and SAED patterns, it is clear that the products are nanoplates with {111} facets being the basal planes.
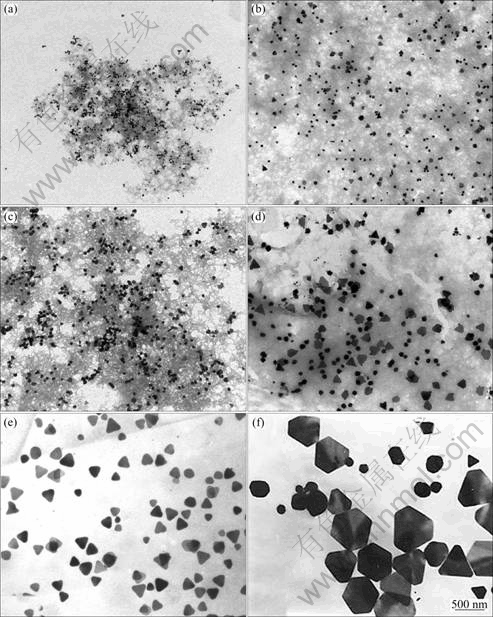
Fig. 2 TEM images of products with different growth time: (a) 1 h; (b) 6 h; (c) 12 h; (d) 18 h; (e) 24 h; (f) 30 h (Volume of seed solution is 0.2 mL and concentration of trisodium citrate is 0.20 mmol/L)
3.3 UV–VIS spectroscopic study
The UV-VIS absorption spectra of silver nanoplates in aqueous solvent are shown in Fig. 4. Absorption spectra of these products normally exhibit some peaks. A small peak at around 332 nm is attributed to the out-of-plane quadrupole resonance of silver nanoplates. A shoulder peak at 400-450 nm can be ascribed to the out-of-plane dipole resonance of silver nanoplates. The third peak at the longest wavelength side, which is very sensitive to the size and aspect ratio of the particles, is due to the in-plane dipole plasmon resonance of silver nanoplates. The in-plane dipolar band is always paid more attention as it holds the strongest peak and is the most sensitive to the changes of the anisotropy and component of the nanoplates [20]. Citrate ions, usually generated from citric acid or sodium citrate, have been extensively used in the synthesis of silver or gold nanoparticles [6,20,21]. In order to investigate the role in the synthesis of silver nanoplates, the trisodium citrate concentrations are adjusted from 0.05 mmol/L to 0.35 mmol/L and other parameters are kept constants (The amounts of silver seeds are 0.20 mL and grown time is 24 h.). Figure 4 (a) displays the absorption spectra of the products obtained with different trisodium citrate concentrations. In the absence of trisodium citrate, the UV-VIS absorption spectra of silver nanoplates keep immovability, indicating that trisodium citrate is necessary to silver nanoplates growth. As the concentration of trisodium citrate minished from 3.5 to 0.05 mmol/L, the in-plane dipolar SPR absorption band of these products red shifted to 851 nm from 520 nm. Among the curves b, c, d, e and h, the curve e has the maximum red shifted absorption intensity. As the surfactant, citrate ligands can effectively block the overgrowth of Ag onto the {111} facets and ensure anisotropic growth along the lateral direction. Ag ions were pretreated with citrate ions to form Ag-citrate complexes, which later can also release the citrate anion to help maintaining anisotropic growth without introducing any new ligand [22]. From the curves e, f, g and h, we know that when the concentration of silver citrate is 0.2 mmol/L, the in-plane dipolar SPR absorption band of products reaches the maximum red shifted absorption intensity, that is, the higher the concentration of citrate ions, the slower the reaction rate under the same conditions. This is probably caused by the formation of silver citrate complexes if more citrate ions exist in the reaction solution, which can decrease the reducing rate and hence the reaction time to reach the maximum absorption intensity [21]. When the concentration of silver citrate is 0.2 mmol/L, the in-plane dipolar SPR absorption band of products reaches the maximum red shifted absorption intensity. The change of the in-plane dipolar absorption band indicates that trisodium citrate is necessary to silver nanoplates formation.
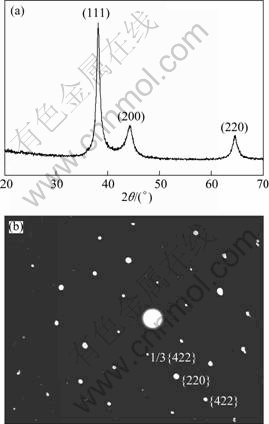
Fig. 3 XRD pattern of products deoxidized with 0.2 volume of seed solution at 24 h (a) and selected-area electron diffraction (SAED) pattern obtained by focusing electron beam onto one single nanoplate (b)
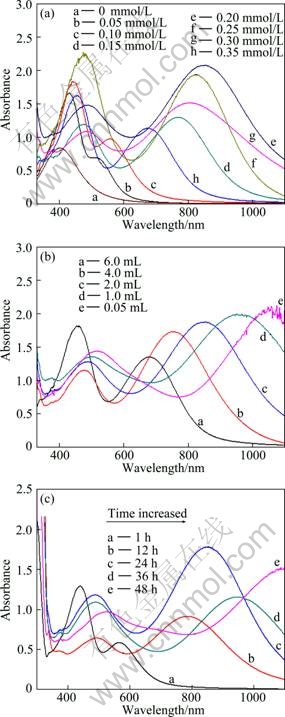
Fig. 4 UV-VIS absorption spectra of triangular silver nanoplates: (a) Products obtained from different concentrations of trisodium citrate; (b) Products obtained from different amounts of seeds; (c) Products obtained with different growth time
Using seed-mediated growth approach, the size of the silver nanoplates can be controlled by adding an amount of small silver seeds. Larger nanoplates can be obtained using a smaller amount of silver seeds. The in-plane dipolar SPR absorption band of these products red shifts because the size of plates increases. According to Fig. 4(b), the concentration of trisodium citrate is 0.20 mmol/L and growth time is 24 h. When the amount of seed is 6 mL, the other two peaks at 442 nm and 678 nm are assigned to the out-of-plane dipole resonance and in-plane dipolar SPR band. The shoulder peak at 415-453 nm is normally attributed to the out-of-plane dipole resonance of nanoplates. However, its relative intensity is much stronger than theoretically expected one. Since spherical silver particles may also have absorption band in this range, it may imply the existence of spherical particles in the aqueous solvent. When the seed amount is decreased to 2 mL, the two peaks red shift to 450 and 851 nm respectively, which runs parallel to the size increase of the triangular nanoplates [23]. The in-plane dipolar band red shifts with the increased plate size, and it can even reach 1060 nm in the near-IR region when the amount of seed is 0.05 mL.
As the surface plasmon resonance (SPR) absorption of metal nanoparticles like gold and silver is very sensitive to the changes of the size and shape. This sensitivity can be used as a tool to monitor the shape of the particles through the optical extinction spectrum. The growth of products is monitored by UV-VIS spectroscopy. Figure 4(c) exhibits surface plasmon resonance (SPR) absorption band with different grown time. The amount of seeds is 0.20 mL and the concentration of trisodium citrate is 0.20 mmol/L. Metal nanoparticles SPR absorption band is produced by the particles of collective oscillation that the surface particles of conduction band electrons are driven by photoelectric field. The peak absorption is affected by the morphology and scale of particle, the dielectric constant of the medium, the character of particle surface-coupled molecules, the degree of aggregation of particles and other factors [24]. Curve a in Fig. 4(c) shows that when the reaction begins about 1 h, the in-plane dipolar SPR absorption band peak is around 567 nm. This weak peak suggests that the nanoplates were growing up. When the reaction proceeds for 12 h, the in-plane dipolar SPR absorption band peak red shifts to 786 nm. In the period from 18 h to 48 h, the in-plane dipolar SPR absorption band peak red shifts to 1100 nm, indicating that the size of silver nanoplates is bigger and bigger.
3.4 Role of PVP in growth solution
The infrared spectra (IR) of PVP and silver nanoplates are shown in Fig. 5(a). It is found that all peaks are identical in both silver nanoplates and pure PVP, which confirms the PVP adheres to the nanocomposites, the stretching vibration peak of C=O at 1670 cm-1. The stretching vibration absorption peak of carbonyl (1642 cm-1) of the silver nanoplates is shifted 28 cm-1 to the right in comparison with the stretching vibration absorption peak of carboxyl (1670 cm-1) of pure PVP, indicating that the action of silver atoms with PVP can affect the part IR character of PVP [25]. The reason is that PVP adheres to the nanoparticles through a charge-transfer interaction between the pyrrolidone rings and surface metal atoms so as to form strong —C=O—Ag bonds. These C=O vibrational peaks will gradually shift to low frequency [26]. The lone pair of electrons from the nitrogen and oxygen atoms in the polar groups of the PVP repeated unit may be donated into sp hybrid orbitals of Ag+ ions to construct complex compounds; an sp hybrid orbital usually forms a linear coordinative bond [27]. Thus, the two possible bonding styles, PVP molecule intra- and interchain interactions, may occur when Ag+ ions interact with PVP molecules. The two kinds of coordinative types can effectively decrease chemical potential and further enable the PVP-bound Ag+ ions to be reduced more easily. The chain length of protecting polymer PVP plays a significant role in controlling the growth rate of crystals planes [28]. Figure 5(b) shows the TEM image of the verge of one silver nanoplate. There is an internal difference color in contrast between its verges, suggesting that the cross-linking PVP can form polymeric shell around the silver nanoplate. As shown in Fig. 5, there is a certain chemical action between silver nanoparticles and the carboxyl of the PVP in silver nanoplate.

Fig. 5 Infrared transmittance spectra of PVP powders and silver nanoplates (a) and TEM image of single silver nanoplate (b)
4 Conclusions
1) Silver nanoplates with different sizes were prepared by seed-mediated method in the presence of trisodium citrate and poly (vinyl pyrrolidone) at room temperature. The experimental conditions, such as the trisodium citrate concentration, the amount of silver seeds and the growth time were optimized to prepare silver nanoplates. It is found that both the amount of citrate ions and the small silver seeds added to the growth solution are the key to control the silver nanoplates without changing their thickness and crystal structure.
2) The optical in-plane dipole plasmon resonance bands of these plates can be tuned from 520 to 1100 nm. The changing of the in-plane dipolar absorption band indicating trisodium citrate and seeds is necessary for silver nanoplates growth. Based on our experimental results, PVP was proposed to play a vital role in synthesis of silver nanoplates as it has restricted silver nanoparticles to diffuse and aggregate interaction pool.
References
[1] HIDEYUKI N, KYLE J M B, BARTLOMIEJ K. Photoconductance and inverse photoconductance in films of functionalized metal nanoparticles [J]. Nature, 2009, 460(16): 371-375.
[2] GOLDEN M S, BJONNES A C, GEORGIADIS R M. Distance- and wavelength-dependent dielectric function of Au nanoparticles by angle-resolved surface plasmon resonance imaging [J]. J Phys Chem C, 2010, 114: 8837-8843.
[3] PEREZ J J, SANTOS I P, MARZAN L M. Gold nanorods: Synthesis, characterization and applications [J]. Coordin Chem Rev, 2005, 249: 1870-1901.
[4] JIANG D L, XIE J M, CHEN M, LI D, ZHU J J, QIN H R. Facile route to silver submicron-sized particles and their catalytic activity towards 4-nitrophenol reduction [J]. Journal of Alloys and Compounds, 2011, 5: 1975-1979.
[5] YI Z, XU X B, LI X B, LUO J S, WU W D, TANG Y J, YI Y G. Facile preparation of Au/Ag bimetallic hollow nanospheres and its application in surface-enhanced Raman scattering [J]. Applied Surface Science, 2011, 258: 212-217.
[6] XUE C, MIRKIN C A. pH-switchable silver nanoprism growth pathways [J]. Angew Chem, 2007, 10: 2082-2084.
[7] JIN R, CAO Y, MIRKIN C A. Photoinduced conversion of silver nanospheres to nanoprisms [J]. Science, 2001, 294: 1901-1903.
[8] ROCHA T C R, ZANCHE D. Structural defects and their role in the growth of Ag triangular nanoplates [J]. J Phys Chem C, 2007, 111: 6989-6993.
[9] LIU G Q, CAI W P, KONG L. Vertically cross-linking silver nanoplate arrays with controllable density based on seed-assisted electrochemical growth and their structurally enhanced SERS activity [J]. J Mater Chem, 2010, 20: 767-772.
[10] WASHIO Y, XIONG Y, YIN Y, XIA Y N. Reduction by the end groups of poly (vinyl pyrrolidone): A new and versatile route to the kinetically controlled synthesis of Ag triangular nanoplates [J]. Adv Mater, 2006, 18: 1745-1749.
[11] LU Q, LEE K J, LEE K B. Investigation of shape controlled silver nanoplates by a solvothermal process [J]. Journal of Colloid and Interface Science, 2010, 1: 8-17.
[12] LIANG H P, WAN L J, BAI C L, JIANG L. Gold hollow nanospheres: Tunable surface plasmon resonance controlled by interior-cavity sizes [J]. J Phys Chem B, 2005, 109: 7795-7800.
[13] HAO E, KELLY K L, HUPP J T, SCHATZ G C. Synthesis of silver nanodisks using polystyrene mesospheres as templates [J]. J Am Chem Soc, 2002, 124(51): 15182-15183.
[14] SUN Y G, MAYERS B, XIA Y N. Transformation of silver nanospheres into nanobelts and triangular nanoplates through a thermal process [J]. Nano Lett, 2003, 3(5): 675-679.
[15] GUEVEL X L, WANG F Y, STRANIK O, ROBERT N, VLADIMIR G, COLETTE M. Synthesis, stabilization, and functionalization of silver nanoplates for biosensor applications [J]. J Phys Chem C, 2009, 113(37): 16380-16386.
[16] KILIN D S, PREZHDO O V, XIA Y. Shape-controlled synthesis of silver nanoparticles: Ab initio study of preferential surface coordination with citric acid [J]. Chem Phys Lett, 2008, 2: 113-116.
[17] MPOURMPAKIS G, VLACHOS D G. Insights into the early stages of metal nanoparticle formation via first-principle calculations: The roles of citrate and water [J]. Langmuir, 2008, 24: 7465-7473.
[18] YI Zao, ZHANG Jian-bo, CHEN Yan, CHEN Shan-jun, LUO Jian-shan, TANG Yong-jian, WU Wei-dong, YI You-gen. Triangular Au-Ag framework nanostructures prepared by multi-stage replacement and their spectral properties [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 2049-2055.
[19] WU X M, Peter L R, LIU H T, CHEN Y Y, MICHAEL S, LOUIS B. Photovoltage mechanism for room light conversion of citrate stabilized silver nanocrystal seeds to large nanoprisms [J]. J Am Chem Soc, 2008, 130: 9500-9506.
[20] CHEN S H, CARROLL D L. Silver nanoplates: Size control in two dimensions and formation mechanisms [J]. J Phys Chem B, 2004, 18: 5500-5506.
[21] JIANG X C, CHEN C Y, CHEN W M. Role of citric acid in the formation of silver nanoplates through a synergistic reduction approach [J]. Langmuir, 2010, 6: 4400-4408.
[22] ZHANG Q, HU Y X, GUO S R, GOEBL J, YINi Y D. Seeded growth of uniform Ag nanoplates with high aspect ratio and widely tunable surface plasmon bands [J]. Nano Lett, 2010, 10(12): 5037-5042.
[23] SAMANTA S, PYNE S, SARKAR P, SARKARA P, GOBINDA P, HAREKRISHNA B. Synthesis of silver nanostructures of varying morphologies through seed mediated growth approach [J]. Journal of Molecular Liquids, 2010, 2: 170-173.
[24] AN J, TANG B, ZHENG X L, ZHOU J, DONG F X, XU S P, WANG Y, ZHAO B, XU W Q. Sculpturing effect of chloride ions in shape transformation from triangular to discal silver nanoplates [J]. J Phys Chem C, 2008, 112: 15176-15182.
[25] ZHAO T, SUN R, YU S H, ZHANG Z J, ZHOU L M, HUANG H T, DU R X. Size-controlled preparation of silver nanoparticles by a modified polyol method [J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2010, 366: 197-202.
[26] LI C C, CAI W P, CAO B Q, SUN F Q, LI Y, KAN C X. Mass synthesis of large, singlecrystal Au nanosheets based on a polyol process [J]. Adv Funct Mater, 2006, 16(1): 83-90.
[27] JIANG P, LI S Y, XIE S S, GAO Y, SONG L. Machinable long PVP-stabilized silver nanowires [J]. Chem Eur J, 2004, 10(19): 4817-4821.
[28] BORODKO Y, HABAS S E, KOEBEL M, YANG P, FREI H, SOMORJAI G A. Probing the interaction of poly (vinylpyrrolidone) with platinum nanocrystals by UV-Raman and FTIR [J]. J Phys Chem B, 2006, 110(46): 23052-23059.
简易种子调停法制备大小可调的三角形银纳米盘
易 早1,2, 张建波1, 何 花2, 徐习斌1,2, 罗炳池1, 李喜波1, 李 恺1, 牛 高1,
谭秀兰1, 罗江山1, 唐永建1,3, 吴卫东1, 易有根1, 2
1. 中国工程物理研究院 激光聚变研究中心,绵阳 621900;
2. 中南大学 物理科学与技术学院,长沙 410083;
3. 四川大学 原子与分子物理研究所,成都 610065
摘 要:通过柠檬酸钠还原银离子,以银纳米粒子为种子,在室温下制备出平均厚度约为5 nm,尺寸40到500 nm可调的三角形银纳米片。通过种子调停法可实现银纳米盘的面内偶极表面等离子体共振峰(SPR)从最初的520 nm红移至1100 nm。通过控制实验参数能够很好地理解其生长机制。柠檬酸根离子和增加到生长液里面的银纳米种子是2个重要的参数,可以控制银纳米盘的大小却不改变银纳米盘的厚度以及晶面结构。采用PVP作为包覆剂,其作用机理还不是很清楚,需要进一步的研究。
关键词:银钠米盘;柠檬酸钠;聚乙烯吡咯烷酮;种子调停法;表面等离子体共振
(Edited by LI Xiang-qun)
Foundation item: Project (10804101) supported by the National Nature Science Foundation of China; Project (2007CB815102) supported by the National Basic Research Program of China; Project (2007B08007) supported by the Science and Technology Development Foundation of Chinese Academy of Engineering Physics
Corresponding author: YI You-gen; Tel: +86-816-2484233; E-mail: myyz1984@yahoo.cn
DOI: 10.1016/S1003-6326(11)61258-2
Abstract: Silver nanoplates, with average thickness about 5 nm and average tunable size from 40 to 500 nm, were synthesized via a simple room-temperature solution-phase chemical reduction method in the presence of appropriate concentration of trisodium citrate and silver seeds. The optical in-plane dipole plasmon resonance bands of these silver plates could be tuned from 520 to 1100 nm. Control experiments were explored for understanding of the growth mechanism. It is found that both the amount of citrate ions and the small silver seeds added to the growth solution are the key to controlling the silver nanoplates without changing their thickness and crystal structure. Small silver seeds are found to play an important role in the formation of large thin silver nanoplates when poly(vinylpyrrolidone) (PVP) are used as capping agent.


