J. Cent. South Univ. (2012) 19: 340-346
DOI: 10.1007/s11771-012-1010-9![]()
Extraction of nickel from molybdenum leaching residue of metalliferous black shale by segregation roasting and acid leaching
CHU Guang(楚广)1, ZHAO Si-jia(赵思佳)2, YANG Tian-zu(杨天足)1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Hunan Research Institute of Nonferrous Metals, Changsha 410015, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract:
The recovery of nickel from molybdenum leach residue by the process of segregation roasting-sulfuric acid leaching-solvent extraction was investigated. The residue was characterized by microscopic investigations, using X-ray fluorescence spectrometry (XRF) and X-ray diffractometry (XRD) techniques and the residue after segregation roasting was characterized by chemical phase analysis method. A series of experiments were conducted to examine the mass ratio of activated carbon (AC) to the residue, segregation roasting time and temperature, sulfuric acid concentration, liquid-to-solid ratio, leaching time, leaching temperature, addition amount of 30% H2O2, stirring speed (a constant) on the leaching efficiency of nickel. A maximum nickel leaching efficiency of 90.5% is achieved with the mass ratio of AC to the residue of 1:2.5, segregation roasting time of 2 h, segregation roasting temperature of 850 ℃, sulfuric acid concentration of 4.5 mol/L, liquid-to-solid ratio of 6:1, leaching time of 5 h, leaching temperature of 80 ℃, addition of 30% H2O2 of 0.6 mL for 1 g dry residue. Under these optimized conditions, the average leaching efficiency of nickel is 89.3%. The nickel extraction efficiency in the examined conditions is about 99.6%, and the nickel stripping efficiency in the examined conditions is about 99.2%.
Key words:
metalliferous black shale; segregation roasting; nickel leaching; PC-88A; solvent extraction;
1 Introduction
Metalliferous black shale is organic-rich rock presented in specific horizons which abundantly contains metallic elements. For instance, in the northwest of Hunan Province, China, the Lower Cambrian metalliferous black shale sequence of the Niutitang Formation deposited at several hundreds of meters below the ground [1-2]. Some black shale contains economically significant concentrations of metals such as Mo, Ni, platinum group elements (PGE), Cu, Zn, As, Se and Ba [3].
The main minerals of nickel in the metalliferous black shale in this area are vaesite and millerite. For molybdenum, the main mineral in the metalliferous black shale in this area is molybdenum sulfide [4-6]. The useful components of minerals are difficult to be treated by mineral processing and metallurgy technologies because such ores are low-grade multiple-metal complex minerals. So far, there is no very effective and mature technology available on this subject. In industry, after roasting, molybdenum was leached out and recovered from metalliferous black shale [7]. However, nickel was not recovered from the residue in this procedure.
A number of studies have been addressed to extract nickel from nickel oxide ore, manganese nodule and other residues. The Caron Process of “reducing roast- atmospheric leaching” was used to recover nickel and cobalt from metallic grains [8]. Segregation roasting by using CaCl2 alone [9-10] or with coke, char or chlorine was involved to recover nickel from nickel oxide ore [11-12]. Chloridizing segregation by MgCl2 and magnetic separation of low-grade nickel laterites was used to recover nickel and cobalt from low-grade laterites [13]. Segregation roasting by using NaCl alone or with CaF2, CaCO3 or Ca(OH)2 was involved to recover nickel from nickel oxide ore or deep sea nodules [14-15]. In addition, the mechanism and technology of segregation roasting of oxide nickel ores and pure nickel sulphate were studied [16-17]. Pre-roasting can change the mineral structure [18], and VALIX and CHEUNG [19] even proved that pre-roasting can lead to the dehydroxylation of the serpentine or magnesium hydrosilicate phase and form an amorphous magnesium silicate phase. And dehydroxylation of goethite due to heating causes a topotactic transformation to hematite which occurs via minor modification in the goethite structure [20]. Leaching laterite with various acids was studied by McDONALD and WHITTINGTON [21-22], and mainly due to economical reasons that sulfuric acid was preferred. Segregation roasting followed by oxidizing pressure leaching with sulfuric acid [16] was involved to recover nickel from nickel oxide ore. Pre-roasting followed by hydrochloride acid leaching was also applied to recovering nickel from laterite [23].
The residue of extracted molybdenum from the metalliferous black shale used in this work was obtained from Hunan Province, China, which was dissolved very slowly in hydrochloric acid, sulfuric acid or nitric acid through dead roasting. Earlier studies suggested that selective leaching of nickel and iron from the residue was not successful. Segregation roasting was proved to be fairly effective to reduce the residue and it gave high yield of nickel leaching efficiency. To solve this problem, in this work, the process of “segregation roasting-H2SO4 leaching-solvent extraction” was proposed and used to recover nickel from the residue. This process was new and flexible. The recovery of valuable metals from the molybdenum leaching residue not only protected environment but also improved the utilization of resources.
2 Experimental
2.1 Residue samples
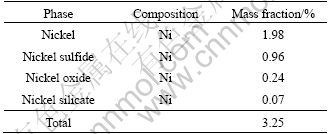
The residue used in the experiments was obtained from the factories in northwestern Hunan, China, which is specialized in extracting molybdenum from the metalliferous black shale. The sample included about 24% moisture, and the composition of the residue is listed in Table 1. Therefore, iron, sodium, calcium, aluminum, zinc and nickel are the primary components of the residue. Mineralogical analysis performed using X-ray diffraction indicates that NaAlSiO4, Fe3O4 and ZnS are the main mineralogical phases in the residue and the phases of nickel compounds are not revealed. The reason for this is that the contents of nickel compounds are less than 3% (mass fraction). Thus, NiFe2O4 was supposed to be the main phase of nickel compounds in the residue [24]. Before leaching, the residue was dried at 200 ℃ for 30 min and then was crushed and ground to powders with a particle size of <0.9 mm. The particle size distribution of residue after grinding is listed in Table 2. Chemical phase analysis performed on the segregation roasting residue showed that the main phases of nickel compounds were nickel, nickel sulfide, nickel oxide and nickel silicate. The result of chemical phase analysis of nickel compounds of the residue sample through segregation roasting is given in Table 3.
2.2 Reagents
The segregation roasting-sulfuric acid leaching tests were carried out with sulfuric acid. 30% H2O2 and AC were of analytical grade while graphite was of chemical grade. In addition, PC-88A and kerosene oil were both commercially pure. Pulverized coal contained 65.32% carbon. The particle size of AC was <112 μm (98.02%). The particle size of pulverized coal was <112 μm (88.24%). The particle size of graphite was <112 μm (93.13%).
Table 1 X-ray fluorescence spectrometry result of residue (mass fraction, %)
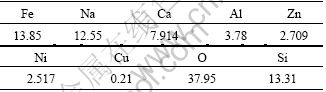
Table 2 Particle size distribution of residue after grinding
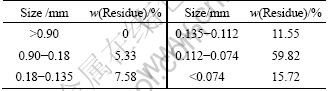
Table 3 Chemical phase analysis result of nickel compounds for residue sample through segregation roasting
2.3 Experimental procedure
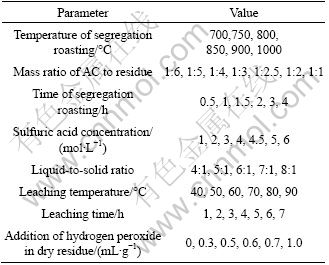
According to our previous experience in related works and preliminary tests, the following eight conditions were chosen as controlling parameters: 1) the temperature of segregation roasting, 2) the addition of AC, 3) the time of segregation roasting, 4) the concentration of sulfuric acid, 5) liquid-to-solid ratio, 6) the leaching temperature, 7) the leaching time, and 8) the addition of hydrogen peroxide. For each condition, the experiments were repeated twice, and the arithmetic averages of the results were used. The conditions for leaching tests are given in Table 4. Then, iron, copper, calcium, magnesium and zinc were removed from the leaching liquor and purified nickel sulfate was obtained. Finally, nickel was extracted by PC-88A and stripped by hydrochloric acid.
Each dry residue sample in this experiment was about 50 g. AC was mixed uniformly with dry residue with a certain mass ratio. Then, the residue was reductively roasted at 850 ℃ for 2 h in sealed corundum crucible in an electric furnace. Leaching tests at high temperature (40-90 ℃) were carried out in a heating magnetic whisk. 30% H2O2 was added into the solution of sulfuric acid and residue to improve nickel extraction. Leaching liquor and leaching residue were separated by air pump filtration. Subsequently, the residue was washed until pH value of the washing water was greater than 6.0, then the residue was dried in electric dry chamber. The solid and solution samples were collected and analyzed for nickel concentration by atomic absorption spectrometry. All the experimental data error was about ±5%.
Table 4 Condition of segregation roasting-sulfuric acid leaching for residue
3 Results and discussion
3.1 Effect of various factors on segregation roasting process
All residue samples were leached under leaching temperature of 80 ℃, leaching time of 5 h, sulfuric acid concentration of 4.5 mol/L, L/S of 6:1 and the stirring speed of 900 r/min. The mass ratio of carbon reducing agent to residue was 1:2.
3.1.1 Effect of species of carbon reducing agent and segregation roasting temperature
Compared with residue and residue after segregation roasting, the main phase of nickel changes from NiFe2O4 to metal nickel and nickel sulfide. The nickel leaching efficiency of residue after segregation roasting is much higher than that of the residue without segregation roasting by direct sulfuric acid leaching. Thus, segregation roasting of the residue is found to be necessary. Therefore, three kinds of carbon reductants are selected during segregation roasting.
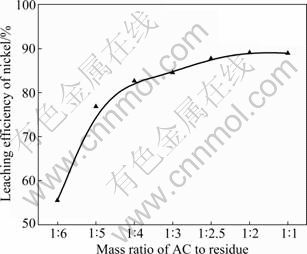
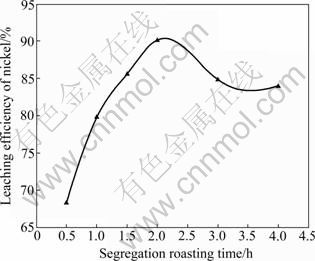
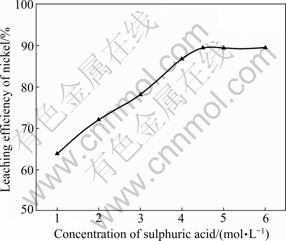
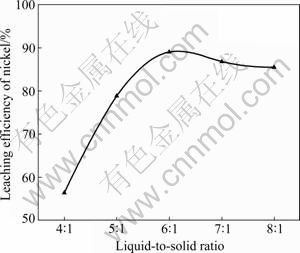
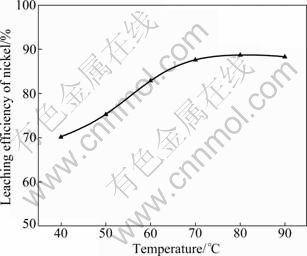
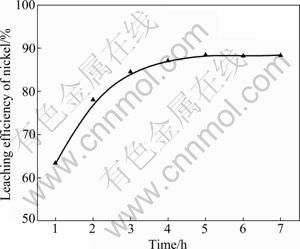
During sulfuric acid leaching, the ratio of 30% H2O2 to dry residue was 0.3:1 mL/g. The experimental results are shown in Fig. 1. It can be obviously seen in Fig. 1 that AC is the most efficient reductant among the three kinds of carbon reductants and nickel leaching efficiency of 84.0% is obtained. Segregation roasting by pulverized coal or graphite gives much lower nickel extraction, varying from 28.1% to 69.0%. Generally, the reducing capacity of carbon reductants is in the order of graphite< pulverized coal Fig. 1 Effect of carbon reductive agents on leaching efficiency of nickel (mass ratio of AC to dry residue 1:2, segregation roasting time 2 h, sulfuric acid concentration 4.5 mol/L, liquid- to-solid ratio 6:1, leaching temperature 80 ℃, leaching time 5 h, 900 r/min, addition of 0.3 mL 30% H2O2 for 1 g dry residue) It can also be observed that nickel leaching efficiency strongly depends on the segregation roasting temperature (Fig. 1). It increases rapidly before 850 ℃ and reaches a maximum of 84.0% at 850 ℃. However, the nickel leaching efficiency is decreased when segregation roasting temperature is even higher. This phenomenon might be due to soluble nickel compounds encapsulated by silicate with low melting point, or due to the mineral formed such as phosphate, which makes nickel compounds insoluble. The leaching efficiency of nickel is less than 84.0% when the temperature of segregation roasting is lower than 850 ℃. The reason is that the segregation reaction of the insoluble nickel compounds with AC is not complete. 3.1.2 Effect of amount of activated carbon Residue samples were reductively roasted with AC at 850 ℃ for 2 h. The addition amount of H2O2 was 0.6 mL vs 1 g dry residue in sulfuric acid leaching. The relationship between nickel leaching efficiency with mass ratio of AC to residue is displayed in Fig. 2. It can be seen from Fig. 2 that leaching efficiency of nickel increases with the raising of the mass ratio of AC to residue from 1:6 to 1:2 and reaches a maximum of 89.1% at 1:2. Any further rise is not attempted until the mass ratio of AC to residue reaches 1:1. The reason for this is that insoluble nickel compounds have almost been reductively roasted to soluble nickel compounds by AC. Fig. 2 Effect of activated carbon amount on leaching efficiency of nickel (segregation roasting time 2 h, segregation roasting temperature 850 ℃, sulfuric acid concentration 4.5 mol/L, liquid-to-solid ratio 6:1, leaching temperature 80 ℃, leaching time 5 h, 900 r/min, addition of 0.6 mL 30% H2O2 for 1 g dry residue) 3.1.3 Effect of segregation roasting time During the process of segregation roasting, AC was used as the carbon reducing agent for the segregation of the residue at 850 ℃. While for sulfuric acid leaching, the addition amount of 30% H2O2 was 0.6 mL vs 1 g dry residue. The influence of segregation roasting time on nickel leaching efficiency is shown in Fig. 3. It can be seen from Fig. 3 that the leaching efficiency of nickel increases with the raising of segregation roasting time from 0.5 to 2 h and decreases subsequently, and reaches a maximum of 90.0% at 2 h. The reason might be that too long segregation roasting time perhaps will lead to the production of insoluble nickel ferrite. Fig. 3 Effect of segregation roasting time on leaching efficiency of nickel (mass ratio of AC to dry residue 1:2, segregation roasting temperature 850 ℃, sulfuric acid concentration 4.5 mo/L, liquid-to-solid ratio 6:1, leaching temperature 80 ℃, leaching time 5 h, 900 r/min, addition of 0.6 mL 30% H2O2 for 1 g dry residue) 3.2 Effect of various factors on process of sulfuric acid leaching All residue samples were reductively roasted using AC at 850 ℃ for 2 h (the mass ratio of AC to residue is 1:2). The particle size of the residue was 74-112 μm (59.82%). The stirring speed was 900 r/min in sulfuric acid leaching. 3.2.1 Effect of sulfuric acid concentration The effect of sulfuric acid concentration from 1 to 6 mol/L on leaching efficiency of nickel was studied with the ratio of liquid to solid of 6:1, addition of 0.6 mL 30% H2O2 for 1 g dry residue and temperature 80 ℃. As seen in Fig. 4, the increase in sulfuric acid concentration enhances the leaching efficiency of nickel and the leaching efficiency of nickel reaches a maximum of 89.4% at 4.5 mol/L. Nevertheless, it is observed from Fig. 4 that further increase in sulfuric acid concentration has no significant effect on the increasing of leaching efficiency of nickel. This phenomenon may be due to the adsorption of sulfuric acid and other salts on the surface of the residue when the concentration of sulfuric acid further increases [25], which hinders the reaction of sulfuric acid and the residue. In addition, metallic nickel (Table 3) is difficult to be dissolved with further addition of sulfuric acid. Therefore, the optimum concentration of sulfuric acid is 4.5 mol/L and all further experiments are carried out at this concentration. Fig. 4 Effect of sulfuric acid concentration on leaching efficiency of nickel (mass ratio of AC to dry residue 1:2, segregation roasting temperature 850 ℃, segregation roasting time 2 h, liquid-to-solid ratio 6:1, leaching temperature 80 ℃, leaching time 5 h, 900 r/min, addition of 0.6 mL 30% H2O2 for 1 g dry residue) 3.2.2 Effect of liquid-solid ratio Figure 5 gives the nickel leaching efficiency as a function of liquid-to-solid ratio (volume of liquid to mass of solid) under leaching temperature of 80 ℃, leaching time of 5 h, sulfuric acid concentration of 4.5 mol/L and addition of 0.6 mL 30% H2O2iquid-to-solidday to DSS of 8:2) md is vr is for 1 g dry residue. From Fig. 5, it can be observed that the leaching rate of nickel increases with the increase of the liquid-to-solid ratio and reaches a maximum of 89.0% at liquid-to-solid of 6:1. However, the leaching efficiency of nickel decreases sharply when the liquid-to-solid ratio further rises. So, the liquid-to-solid ratio of 6:1 is selected. Fig. 5 Effect of liquid-to-solid ratio on leaching efficiency of nickel (mass ratio of AC to dry residue 1:2, segregation roasting temperature 850 ℃, segregation roasting time 2 h, sulfuric acid concentration 4.5 mol/L, leaching temperature 80 ℃, leaching time 5 h, 900 r/min, addition of 0.6 mL 30% H2O2 for 1 g dry residue) 3.2.3 Effect of leaching temperature The leaching efficiency of nickel is also affected by leaching temperature. High leaching efficiency of nickel may not be achieved in a short time at low temperature; however, high temperature has disadvantages such as higher consumption of energy. So, in the present work, a range of medium temperature from 40 to 90 ℃ was selected and its effect on leaching efficiency of nickel was studied. Figure 6 shows the effect of temperature under the similar conditions as previous experiment. Leaching efficiency of nickel increases from 69.2% to 88.7% when leaching temperature increases from 40 to 80 ℃, but a further increase in temperature to 90 ℃ has no significant effect. Therefore, the optimum temperature is 80 ℃ and all further experiments are carried out at this temperature. 3.2.4 Effect of leaching time The effect of leaching time from 1 to 7 h on the dissolution of nickel was investigated using liquid to solid ratio of 6:1, initial sulfuric acid concentration of 4.5 mol/L, addition of 0.6 mL 30% H2O2 for 1 g dry residue and leaching temperature of 80 ℃. The results presented in Fig. 7 show that the leaching efficiency of nickel increases with the increase of leaching time and reaches 89.3% after 5 h. Beyond 5 h, there is no significant increase in the nickel leaching efficiency. So, further experiments are not carried out for 7 h. Fig. 6 Effect of temperature on leaching efficiency of nickel (mass ratio of AC to dry residue 1:2, segregation roasting temperature 850 ℃, segregation roasting time 2 h, sulfuric acid concentration 4.5 mol/L, liquid-to-solid ratio 6:1, leaching time 5 h, 900 r/min, addition of 0.6 mL 30% H2O2 for 1 g dry residue) Fig. 7 Effect of leaching time on leaching efficiency of nickel (mass ratio of AC to dry residue 1:2, segregation roasting temperature 850 ℃, segregation roasting time 2 h, sulfuric acid concentration 4.5 mol/L, liquid-to-solid ratio 6:1, leaching temperature 80 ℃, 900 r/min, addition of 0.6 mL 30% H2O2 for 1 g dry residue) Meanwhile, 30% hydrogen peroxide is added into the mixture solution of sulfuric acid and residue. The repeated experiment results show that when the addition of 30% H2O2 for 1 g dry residue is 0.6 mL, the leaching efficiency of nickel increases from 6.2% to 11.5% under the same small-scale laboratory conditions. 3.3 Purifying leach liquor containing nickel sulfate The content of the leach liquor of the residue is listed in Table 5. Table 5 Elemental contents of leach liquor (g/L) As seen in Table 5, there are higher concentrations of iron, zinc, magnesium, calcium and copper in the leach liquor. Thus, the removing process is arranged as follows: 1) Iron removal Adjusting the pH of the leaching liquor arranging from 3.0 to 3.5, then Fe3+ in the solution was hydrolyzed at 90 ℃ for 2 h, with moderate stirring. Leaching liquor and residue were separated by air pump filtration. After filtration, the solution was obtained with significantly lower concentration of iron. The chemical reaction is described by the following equation: Fe3++3H2O=Fe(OH) 3↓+3H+ (1) 2) Copper removal Adjusting the pH of the solution attained in Eq. (1) to 4.0, and then Na2S was added into the solution to deposit Cu2+ in the solution at 80 ℃ for 3 h. The addition of Na2S was four times as much as calculation amount from Eq. (2). After filtration, the solution was obtained with lower contents of ions of copper and zinc. The chemical reaction is described by the following equation: Cu2++S2-=CuS↓ (2) 3) Magnesium and calcium removal After adjusting the pH of the solution attained in Eq. (2) to 4.5, then NH4F was added into the solution to get rid of Ca2+ and Mg2+ in the solution at 85 ℃ for 1.5 h. The addition of NH4F was six times as much as calculation amount in Eqs. (3) and (4). After filtration, the solution was obtained with lower ion concentrations of magnesium and calcium. The chemical reaction is described by the following equation: Ca2++2F-=CaF2 ↓ (3) Mg2++2F-=MgF2 ↓ (4) 4) Zinc removal After adjusting the pH of the solution attained in Eq. (3) to 3.5, PC-88A was used to extract zinc from the solution. When VO/VA=1:3 and contacting time is 2 min, more than 99% of zinc is rejected from the solution. Then purged liquor is obtained. The content of purged liquor is listed in Table 6. Table 6 Elemental contents of purged liquor (mg/L) 3.4 Results of extraction of nickel and stripping of nickel Nickel concentration in feed solution is 2-3 g/L. The mixture of 30% PC-88A and diluted kerosene oil was used as an effective nickel extractant and solution of hydrochloric acid was used as stripping agent of nickel. Two-step cross-current extraction was carried out to recover nickel from feed solution, and one step stripping was carried out to recover nickel from organic phase. Under these optimized conditions, the nickel extraction efficiency under the examined conditions is about 99.6% and the nickel stripping efficiency under the examined conditions is about 99.2%. 3.5 Preparation of nickel oxalate and nickel monoxide Nickel concentration in strip liquor was about 0.5 mol/L. After the stripping, nickel oxalate was crystallized and precipitated at 70 ℃ and pH 8.0-8.5. The main chemical reaction can be described by the following equation: Ni2++H2C2O4→NiC2O4↓+2H+ (5) Then, the precipitants were filtered, washed with purified water repeatedly, air dried at 60 ℃ for 5 h and the purity of nickel oxalate was acquired, the quality of which can meet the standard industrial specification. After calcination of nickel oxalate at 700 ℃ for 4 h, celadon nickel monoxide was attained at last. The chemical reaction is described by the following equation: NiC2O4 4 Conclusions 1) The residue of extracted molybdenum from the metalliferous black shale in northwest Hunan of China through dead roasting dissolves very slowly in hydrochloric acid, sulfuric acid or nitric acid. Segregation roasting is proved to be fairly effective to reduce the molybdenum leach residue and it gives high yield of nickel extraction. Generally, the reducing capacity of carbon reductants is in the order of graphite< pulverized coal 2) A maximum nickel leaching efficiency of 90.5% is achieved with the mass ratio of AC to the residue of 1:2.5, segregation roasting time of 2 h, segregation roasting temperature of 850 ℃, sulfuric acid concentration of 4.5 mol/L, liquid-to-solid ratio of 6:1, leaching time of 5 h, leaching temperature of 80 ℃, addition of 30% H2O2 of 0.6 mL for 1 g dry residue. 3) The nickel extraction efficiency in the examined conditions is about 99.6%, and the nickel stripping efficiency in the examined conditions is about 99.2%. References [1] FAN De-lian, YANG Xiu-zhen, WANG Lian-fang, CHEN Nan-sheng. Petrological and geochemical characteristics of a nickel- molybdenum-multi-element-bearing Lower Cambrian black shale from a certain district in South China [J]. Geochimica, 1973(3): 143- 164. (in Chinese) [2] LI You-yu. Geochemistry of Ni-Mo polymetallic exhalation sediment ore deposit in Northwestern Hunan [J]. Geochimica, 1997, 26(3): 89-96. (in Chinese) [3] KRIBEK B, SYKOROVA I, PASAVA J, MACHOVIC V. Organic geochemistry and petrology of barren and Mo-Ni-PGE mineralized marine black shales of the Lower Cambrian Niutitang Formation (Southern China) [J]. International Journal of Coal Geology, 2007, 72(3/4): 240-256. [4] STEINER M, WALLIS E, ERDTMANN B D, ZHAO Yuan-long, YANG Rui-dong. Submarine-hydrothermal exhalative ore layer sin black shales from South China and associated fossils-insights into a Lower Cambrianfacies and bio-evolution [J]. PALA Eos, 2001, 169(3/4): 165-191. [5] MAO Jing-wen, LEHMANN B, DU An-dao, ZHANG Guang-di, MA Dong-sheng, WANG Yi-tian, ZENG Ming-guo, KERRICH R. Re-Os dating of polymetallic Ni-Mo-PGE-Au mineralization in Lower Cambrian black shales of South China and its geological significance [J]. Economic Geology, 2002, 97(5): 1051-1061. [6] ORBERGER B, VYMAZALOV? A, WAGNER C, FIALIN M, GALLIEN J P, WIRTH R, PA?AVA J, MONTAGNAC G. Biogenic origin of intergrown Mo-sulphide- and carbonaceous matter in Lower Cambrian black shales (Zunyi Formation, Southern China) [J]. Chem Geol, 2007, 238(3/4): 213-231. [7] PI Guan-hua, XU Hui, CHEN Bai-zhen, SHI Xi-chang, LI Jun-li. Study on recovering molybdenum from rocky-select Ni-Mo ores [J]. Hunan Nonferrous Metals, 2007, 23(1): 9-12. (in Chinese) [8] CARON M H. Ammonia leaching of nickel and cobalt ores [J]. Journal of Metals-Transaction AIME, 1950, 88: 67-99. [9] HOOVER M, HAN K N, FUERSTENAU D W. Reduction roasting of nickel, copper and cobalt from deep-sea manganese nodules [J]. International Journal of Mineral Processing, 1975, 2(2): 173-185. [10] TITOVA Z P, MAIOROV A D, REZNIK I D, POLOSINA E E, OSIPOVA A S. Extraction of nickel from iron-containing oxidized nickel ores by the segregation method [J]. Tsvetnye Metally, 1975, 48(1): 8-11. (in Russian) [11] MEHROTRA S P, SRINIVASAN V. Extraction of nickel from an Indian laterite by reduction roasting [J]. Mineral Processing and Extractive Metallurgy, 1994, 103: C97-C104. [12] ILIC I, KRSTEV B, CEROVIC K, STOPIC S. Study of chlorination of nickel oxide by chlorine and calcium chloride in the presence of active additives [J]. Scandinavian Journal of Metallurgy, 1997, 26(1): 14-19. [13] LIU Wan-rong, LI Xin-hai, HU Qi-yang, WANG Zhi-xin, GU Ke-zhuan, LI Jin-hui, ZHANG Lian-xin. Pretreatment study on chloridizing segregation and magnetic separation of low-grade nickel laterites [J]. Trans Nonferrous Met Soc China, 2010, 20(s1): s82- s86. [14] SUDZUKI R. Extraction of nickel from oxidized nickel ores by segregation roasting and magnetic separation [J]. Tsvetnye Metally, 1977(7): 9-12. (in Russian) [15] PAREKH B K, JEPSEN T L B, GOLDBERGER W M. Segregation roasting and beneficiation of deep sea nodules [J]. Marine Mining, 1988, 7(4): 417-429. [16] REZNIK I D, TARASOV A V, MAYOROV A D. Mechanism and technology of reduction roasting of oxide nickel ores with subsequent calcine flotation and concentrate leaching [C]// SCHLESINGER M E. EPD Congress 2004. Charlotte City, America: The Minerals, Metals and Materials Society, 2002: 725-737. [17] KAR B B, DATTA P M, ISRA V N. Thermal study of segregation roasting of pure nickel sulphate [J]. Mineral Processing and Extractive Metallurgy, 2004, 113(2): 118-120. [18] RUAN H D, FROST R L, KLOPROGGE J T, DUONG L. Infrared spectroscopy of goethite dehydroxylation: II. Effect of aluminium substitution on the behaviour of hydroxyl units [J]. Spectrochim Acta: Part A, 2002, 58(3): 479-491. [19] VALIX M, CHEUNG W H. Study of phase transformation of laterite ores at high temperature [J]. Miner Eng, 2002, 15(8): 607-612. [20] LANDERS M, GILKES R J. Dehydroxylation and dissolution of nickeliferous goethite in New Caledonian lateritic Ni ore [J]. Appl Clay Sci, 2007, 35(3/4): 162-172. [21] McDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review: Part I. Sulphuric acid technologies [J]. Hydrometallurgy, 2008, 91(1/2/3/4): 35-55. [22] McDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review: Part II. Chloride and bio-technologies [J]. Hydrometallurgy, 2008, 91(1/2/3/4): 56-69. [23] LI Jin-hui, LI Xin-hai, HU Qi-yang, WANG Zhi-xing, ZHOU You-yuan, ZHENG Jun-chao, LIU Wang-rong, LI Ling-jun. Effect of pre-roasting on leaching of laterite [J]. Hydrometally, 2009, 99: 84-88. [24] ZHAO Si-jia. Recovery and preparation of nickel compounds from residue of metalliferous black shale [D]. Changsha: School of Metallurgical Science and Engineering, Central South University, 2010: 18-30. (in Chinese). [25] ZHONG Zhu-qian, MEI Guang-gui. Hydrometallurgical processes [M]. Changsha: Central South University of Technology Press, 1988: 83. (in Chinese) (Edited by YANG Bing) Foundation item: Project(2007CB613604) supported by the National Basic Research Program of China Received date: 2011-03-12; Accepted date: 2011-11-08 Corresponding author: CHU Guang, Professor, PhD; Tel: +86-731-88836791; E-mail: chuguang2006@163.com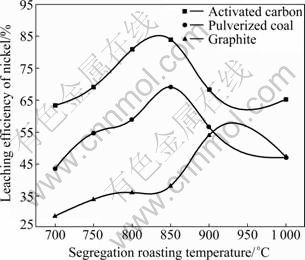
![]()
![]()
![]() NiO+CO↑+CO2↑ (6)
NiO+CO↑+CO2↑ (6)
Abstract: The recovery of nickel from molybdenum leach residue by the process of segregation roasting-sulfuric acid leaching-solvent extraction was investigated. The residue was characterized by microscopic investigations, using X-ray fluorescence spectrometry (XRF) and X-ray diffractometry (XRD) techniques and the residue after segregation roasting was characterized by chemical phase analysis method. A series of experiments were conducted to examine the mass ratio of activated carbon (AC) to the residue, segregation roasting time and temperature, sulfuric acid concentration, liquid-to-solid ratio, leaching time, leaching temperature, addition amount of 30% H2O2, stirring speed (a constant) on the leaching efficiency of nickel. A maximum nickel leaching efficiency of 90.5% is achieved with the mass ratio of AC to the residue of 1:2.5, segregation roasting time of 2 h, segregation roasting temperature of 850 ℃, sulfuric acid concentration of 4.5 mol/L, liquid-to-solid ratio of 6:1, leaching time of 5 h, leaching temperature of 80 ℃, addition of 30% H2O2 of 0.6 mL for 1 g dry residue. Under these optimized conditions, the average leaching efficiency of nickel is 89.3%. The nickel extraction efficiency in the examined conditions is about 99.6%, and the nickel stripping efficiency in the examined conditions is about 99.2%.


