
Preparation of titanium dioxide photocatalytic hollow spheres
PANG Xue-man(庞学满), XU Ming-xia(徐明霞), HOU Feng(侯 峰), LI Ming-li(李明利)
School of Materials Science and Engineering,
Key Laboratory for Advanced Ceramics and Machining Technology of Ministry of Education,Tianjin University, Tianjin 300072, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
With coaxial nozzle system, TiO2 hollow spheres were prepared and the optimum parameters of forming TiO2 hollow spheres were fix on as follows: acrylamide (AM) was used as monomer up to 30.3%, acetone was used as vesicant, the mass fraction of initiator was 0.4%, the forming temperature was in the range from 90 ℃ to 95 ℃. The photocatalistic performance of TiO2 hollow spheres was characterized by degradation of methyl orange. Compared with nano-TiO2 powders, hollow spheres can be recycled after cleanout and drying, taking on similar efficiency of photocatalistic.
Key words:
inorganic compounds; chemical synthesis; catalytic properties;
1 Introduction
The photocatalytic performance of titanium dioxide thin film photocatalysts have been investigated in many different types in order to achieve the efficient of photocatalytic activities as high as possible. Since the pioneering work by Honda and Fujishima, small particle semiconductor of photocatalyst by TiO2 have been extensively studied due to their relatively high photocatalytic activity and stability, and most of the work has been devoted to the study of reactions associated with the photodecomposition of H2O into H2 and O2[1-3]. A variety of techniques have been employed to improve the photocatalytic performance of titanium dioxide thin film photocatalyst, for which the connection between the fine particle of titanium dioxide and the carrier can not be fast enough and the carrier has a negative effect on photochemical catalyst[4, 5]. In order to solve these problems, a lot of research work have been made. A new method that produces hollow sphere catalyst, is presented in this paper which is ideal for cleaning water since the spheres work as thin film catalyst and have no problem mentioned above.
Among the methods which are capable of producing a broad rang of materials in spherical form, coaxial nozzle systems have been designed to facilitate the creation of hollow spheres with diameter in several millimeter. This thesis describes the design, fabrication and photocatalytic performance of TiO2 hollow spheres prepared by coaxial nozzle system.
2 Experimental2.1 Preparation of titanium dioxide hollow sphere catalyst
Titanium dioxide hollow spheres were prepared by coaxial nozzle system design in the lab. Fig.1 shows the scheme of the equipments of coaxial nozzle system. The most important fixture is the coaxial nozzle, which contains two coaxial tubes where the inner tube injects gas, so called inner jet, and a slurry flows in the annular opening between the inner jet and the outer tube.
The hollow sphere formation in this process usually follows the sequence described as follows: the slurry exists in the nozzle form a piece of film in the bottom because of surface tension of the liquid, and then the film changes into a bubble with the help of airflow in the inner jet. The bubble forms a neck and closes, and the sphere breaks free when the inner jet inflates the bubble until the gas pressure inside the bubble equals that of the outer. A model explaining the growth and expansion rate of a sphere based on the balance of surface tension of the liquid film, the viscous stress due to the liquid motion, and the gas pressure inside the bubble, has been proposed
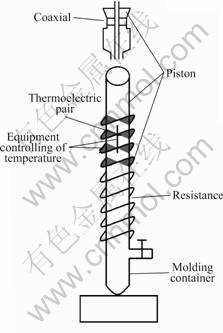
Fig.1 Scheme of equipments of coaxial nozzle system
by Chapman and Cochran[6, 7].
The hollow spheres fell through vertical tube tower with 1.0 m in height and 30 cm in diameter to complete molding process in hot medium oil under heating in the tube tower to form gel-spheres. Then, the gel-spheres have to be dried to gain enough green strength to handling. After calcining, they can be used as photocatalyst in cleaning polluted water.
2.2 Characterization
The absorption spectra were taken with a 7230G spectrophotometer. The morphologies of hollow spheres were observed by vidicon. The photocatalytic activity under ultraviolet light was evaluated by photocatalytic degradation of methyl orange as model reaction.
3 Results and discussion3.1 Investigation in technical process of producing TiO2 hollow spheres
The slurry was immitted in coaxial nozzle container, forming liquid film at the annular opening, then the film was shaped in sphere bubble under the action of the inner jet inflation and dropped in the hot oil to complete molding. The vesicant vaporized under heating, inflating in the vacuole, to form hollow spheres with thin wall, while monomer in slurry polymerized and intertwisted under high temperature to accomplish gelation and enwrap TiO2 particles to mold in the drop of the bubble. The monomer converted to polymer by a way of ionization of solicitation (NH4)2S2O8[8, 9]:
(NH4)2S2O8?2NH4++2S![]() (1)
(1)
SO4?+CH2CHOCNH2?SO4CH2CH?CONH2 (2)
SO4CH2CH?CONH2+nCH2CHCONH2?SO4CH2CHCONH2[CH2CHCONH2]nCH2CH?CONH2 (3)
It is shown that the amount of monomer, the temperature of reaction, the type of vesicant and the mass fraction of initiator are important process parameters controlling the time of forming and the structure of the hollow sphere.
1) Factors affecting time of gelation
The amount of monomer has important effect on gelation time of accomplishing concretion in the course of bubbles falling down the heated tube. If the amount of monomer is not enough, the spheres will adhere to each other. Table 1 lists the effect of amount of monomer on sphere modality.
Table 1 Effect of amount of monomer on sphere modality

It is necessary for the formation of hollow spheres to increase the amount of monomer, which is attributed to the mechanism of gelation. Monomer free radical is engendered by the action of initiator and polymerized with other monomers, lengthening the polymer, as the equation below shows [10]:
RCH2CH?X+CH2CHX?RCH2CHXCH2CH?X×××? RCH2CHX[CH2CHX]nCH2CH?X (4)
It is considered that with the increasing of monomer concentration, polymer can be easily to lengthen and intertwist, which make the gelation apertures minished and gelation strength increased. Hollow spheres do not coherent to each other or huddle together for distortion at the bottom of the container with the enough amount of momomer, presenting considerable strength, which reveals that the gelation optimizing is carried out.
The initiator inspires monomer to polymerize under heating conditions to form gel-spheres with considerable strength. The content of initiator plays an important role in polymerization, controlling the time of gelation. To attain excellent hollow spheres, it is important to achieve gel-spheres in 105 s spent in falling down the heated oil in the tube. The optimal initiator content was determined to be 0.2%-0.4%, according to experiments.
It is well known that polymerization consists of three radical reaction, viz. initiation, lengthen and termination. It has also been reported that gelating velocity is described below in the case of [I] and [M] represent the concentration of initiator and monomer, K denotes velocity constant [11]:
![]() (5)
(5)
Therefore, in order to be quick enough to complete gelation, it is necessary to increase the concentration of initiator, according to the copolymerization kinetics that the initiator plays the role of touching off the reaction by ionizating and engendering monomer free radical.
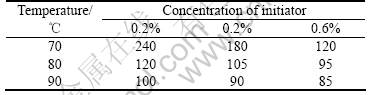
It is suggested that conglutination occurs in low oil temperature, but when the oil is heated excessively, vesicant will vaporize seriously to destroy the round shape of hollow spheres. The favorite oil temperature is between 75-95 ℃. Gelation time is shortened with rise of temperature, as shown in Table 2.
Table 2 Change of gelation time with temperature and concentration of initiator diversification110
The step in initiator degradation is proposed to involve the generation of initial free radical leading to the formation of monomer free radical to polymerize with other monomers [10]:
I?R? (6)
R?+CH2CX?RCH2CH?X (7)
It has been reported that the degradation of initiator is endothermic reaction, activating energy come up to 105-150 kJ/mol, which determines the efficiency of the whole polymerization. Therefore, it is feasible to shorten gelating time by increasing the temperature of reaction.
2) Factors affecting modality of hollow spheres
Factors affecting formation of hollow spheres includes solid load, vesicant species and dosage, the amount of initiator, oil temperature and so on, which engender hollow spheres with honeycomb or single hole with thin wall, as shown in Fig.2 and Fig.3.

Fig.2 Spheres with honeycomb
It is interesting to note that the formation progress of the hollow spheres can be described as follows: evaporation of acetone, function as vesicant, brings

Fig.3 Spheres with thin wall
considerable inflation in liquid bubbles which drops in heated oil to develop the sphere wall thinner and thinner in a short time, then preserves a big hole in gelation spheres. The intensity of pressure in the bubble keeps the same everywhere in order to make a consistent wall. With gelation accomplished at the same time, excellent hollow sphere with thin wall is obtained in the end. The effects of initiator concentration and reacting temperature on the formation of hollow spheres are investigated by employing different concentrations of initiator from 0.3% to 0.5% and temperature from 80 ℃ to 95 ℃ to determine the best point. It has been shown that hollow sphere owns its consummate modality to the interaction of initiator and temperature, which makes glelation complete as soon as forming a single hole with thin wall (Fig.4). The optimal concentration of initiator and reacting temperature have been agreed for 0.4% to 0.3% and 80 ℃ to 90 ℃ by the experiment.
Fig.4 Spheres of round and smooth
3.2 Study on photocatalistic performance of TiO2 hollow spheres comparing with nano-TiO2 powders
Fig.5 shows the rate of methyl orange degradated by TiO2 powders and TiO2 hollow spheres made of the same powder. It can reach a conclusion that powder and spheres both present a certain degree of photocatalisis. Powder shows higher property in the first 15 min mainly
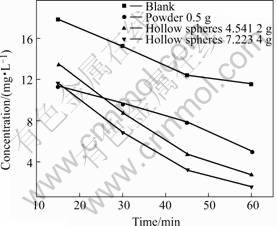
Fig.5 Curves of methyl orange concentration during degradation with TiO2 powders and TiO2 hollow spheres made by same powder
for its sorption easier than spheres towards methyl orange. It can be seen that degradation is observed during the first 30 min of reaction, which indicates that under these conditions photonic efficiency is very high. After a certain time the surface achieves equilibrium adsorption and process slows down. It must be taken into account that improving the sorption of methyl orange depending on increasing mass of hollow spheres plays an important role in photonic efficiency improvement. It is detected that degradation is not increased when one sphere is divided into two pieces with the purpose of renewing surface area which indicates that the surface area of hollow spheres unsintered mainly contributes to adsorption and photocatalistic.
Hollow spheres can be recycled after cleanout and drying, taking on similar efficiency of photocatalistic, as shown in Fig.6. So the hollow spheres can be callback
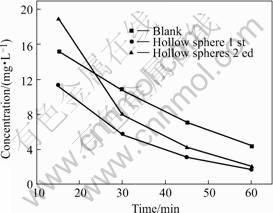
Fig.6 Curves of hollow spheres recycled after cleanout and drying, taking on similar efficiency of photocatalistic
and reused for many times. Hence, purifying equipment for water polluted made of TiO2 hollow spheres have several advantages of powders or film. The equipment for purifying polluted water based on TiO2 hollow spheres has been under research and design.
4 Conclusions
Titanium dioxide hollow spheres were produced by coaxial nozzle system using titanium dioxide powders prepared in the lab. The optimum parameters of forming TiO2 hollow spheres were as follows: AM used as monomer took up 30.3%, acetone was used as vesicant, the mass fraction of initiator was 0.4%, the forming temperature was in the range from 90 ℃ to 95 ℃. The studies on photocatalistic performance of TiO2 hollow spheres and a comparison with nano-TiO2 powders show that degradation is observed during the first 30 min of reaction, after the surface achieves equilibrium adsorption and process slows down. Hollow spheres can be recycled after cleanout and drying, taking on similar efficiency of photocatalistic.
References[1] HOFFMANN M R, MARTIN S T, BAHNEMANN D W, et al. Environmental Applications of Semiconductor Photocatalysis [J]. J Chem Rev, 1995, 95(1): 69-96.
[2] MUNEER M, PHILIP R, DAS S. Photocatalytic degradation of wastewater pollutants titanium dioxide-mediated oxidation of a textile acid blue 40#[J]. J Res Chem Intermed, 1997, 23(3): 233-246.
[3] HONDA K, FUJISHIMA A. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238: 37-38.
[4] MILLS A, LE HUNTE A. An overview of semiconductor photocatalysis[J]. J Photochem Photobiol A, 1997, 108: 1-35.
[5] RAJA P, BANDARA J, GIORDANO P, KIWI J. Innovative supported composite photocatalyst for the oxidation of phenolic waters in reactor processes[J]. Ind Eng Chem Res, 2005, 44: 8959-8967.
[6] ALKOY S. Piezoelectric Hollow Sphere Transducers: The “BBs”[D]. Pennsylvania: The Pennsylvania State University, 1999. 11.
[7] CHAPMAN A T, COCHRAN J K, BRITT J M Jr, HWANG T J. Thin-wall Hollow Spheres[R]. Slurries Annual Reports, ORNL Subcontent 86X22043C. Washington D C: Department of Energy, 1987-1989.
[8] JANNEY M A., OMATERE O O, STREHOLW R A. A new ceramic forming process[J]. Ceramic Bulletin, 1991, 70(10): 1641-1640.
[9] JANNEY M A, OMATERE O O. Method for Molding Ceramic Powder Using a Water-Based Gelcasting Process[P]. US 4145908, 1992.
[10] PAN Z R. Macromolecule Chemistry[M]. Beijing: Chemical Industry Press, 1997. 18-69.(in Chinese)
[11] XUE Y D. The Study on Gelcasting Forming Process of Si3N4 Ceramic Material[D]. Tianjin: Tianjin University, 2003. 26-28.
Corresponding author: XU Ming-xia; Tel.: +86-22-27890489, Fax: +86-22-27404724. Email: xumingxia@tju.edu.cn


