
Effect of adding methods of metallic phase on microstructure and thermal shock resistance of Ni/(90NiFe2O4-10NiO) cermets
LAI Yan-qing(赖延清), ZHANG Yong(张 勇), TIAN Zhong-liang(田忠良),
SUN Xiao-gang(孙小刚), ZHANG Gang(张 刚), LI Jie(李 劼)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 4 December 2006; accepted 14 May 2007
Abstract:
Ball mixing and electroless plating were respectively used as the adding methods of metallic phase to prepare Ni/(90NiFe2O4-10NiO) cermets for the inert anode in aluminum electrolysis. The microstructure and thermal shock resistance of cermet samples were studied. The results show that, for the samples prepared by ball mixing method, aggregation of metallic phase is found in either the green blocks or sintered samples and the extent of aggregation increases with the increase of metal content. For 6.5Ni/(90NiFe2O4-10NiO) cermets prepared with electroless plating method, the homogeneous and fine metallic particles are found in either the green compacts or sintered samples, but the relative density and thermal shock residual strength decrease by 3% and 28%-58% respectively, compared with samples prepared with ball mixing method.
Key words:
Ni/(90NiFe2O4-10NiO) cermets; microstructure; thermal shock resistance; inert anode; aluminum electrolysis;
1 Introduction
The novel technics of aluminum electrolysis with inert anodes and wetted cathodes has been an important research item in aluminum electrolysis area for several decades, which will resolve the ultimate disadvantages, such as high energy, carbon consumption, environmental pollution[1-2], by eliminating carbon materials used in current aluminum electrolysis industry. In recent years, the research on inert anodes is mainly focused on alloys and cermets. The NiFe2O4-based cermets are deemed to be the most potential inert anode materials with combination of good electrical conductivity of metals and low corrosion of ceramics[3-4].
A lot of studies have been curried out on the corrosion behavior and electrical conductivity of cermet inert anodes, but the studies on their mechanical properties are not enough, and the essential problems were to be resolved, including the realization of anticipated efficacy (to improve mechanical properties and electrical conductivity of cermets), combination of advantages and offset of disadvantages of metallic phase and ceramic phase. Mechanical mixing was mostly adopted to mix metallic component and ceramic component in the preparation process of cermet inert anodes[5]. In order to get a continuous distribution of metallic phase in cermets, WEYAND et al[6] conceived to prepare NiFe2O4-based cermets with NiFe2O4-based ceramic powder covered with copper based alloy, but there was no research work reported afterward. YANG et al[7] prepared cermets with the NiFe2O4-NiO powder covered by copper by electroless plating, and found that the sintering property and electrical property of NiFe2O4- NiO-based cermets were improved obviously, compared with the cermets from the mechanically mixed powder of NiFe2O4-NiO ceramic and Cu. LI et al[8] mentioned that some cermets was prepared with oxides packed with metal by electroless plating, but the effect of electroless plating on material properties was not evaluated.
In order to get a perfect corrosion resistance and improve the mechanical properties, thermal shock resistance and electrical conductivity of cermet inert anodes simultaneously, a continuous distribution of metallic phase in the ceramic matrix should be got when the metallic phase content was limited at a low level, to improve the combining strength between metallic phase and ceramic phase, so effectively exerting its toughening effect. In this study, based on our previous study with ascertained 90NiFe2O4-10NiO as the best ceramic composition [9], the ball mixing and electroless plating were respectively used as the adding methods of metallic phase to prepare Ni/(90NiFe2O4-10NiO) cermets. The microstructure and thermal shock resistance of cermet samples were studied, to offer instructive guidance of the metallic phase adding methods for preparation of cermet inert anodes.
2 Experimental
2.1 Preparation of (90NiFe2O4-10NiO) ceramic powder
Reagent grade raw materials of NiO and Fe2O3 were mixed well by ball milling in certain proportion and calcined at 1 150 ℃ in the presence of air for 6 h to obtain the 90NiFe2O4-10NiO ceramic powder. It was proved that there were no impurities in the calcined powder with the X-ray diffraction analysis.
2.2 Electroless plating Ni on (90NiFe2O4-10NiO) ceramic powder
The ceramic powder 90NiFe2O4-10NiO was added into the solution made from 100 g/L SnCl2-6H2O, 150 g/L NaOH and 175 g/L KNaC4H6O6 and stirred synchronously for sensitizing. Powder was rinsed before being activated in 0.1 g/L PdCl2 solution and then rinsed again. The process of electroless plating Ni was completed in the solution made from 30 g/L NiCl2-6H2O, 50 g/L NH4Cl, 80 g/L NaC6H10O8 and 15 g/L NaH2PO2- H2O. After that, the powder was rinsed and dried in vacuum at 70-80 ℃. The content of metal Ni was ascertained by chemistry analysis methods and controlled by the content of raw material, concentration of solution and the times of electroless plating Ni. The content of metal Ni in cermet powder obtained by electroless plating is 6.5%.
2.3 Preparation of cermet inert anodes
The ceramic powder and Ni were mixed and micronized by ball milling in the media containing dispersant and adhesive. According to the country criterion GB/T 16536─1996 [10], the samples with size of 4 mm×3 mm×38 mm were required for testing of bending strength. As the shrinkage of samples was considered during sintering, the dried powders were pressed into bar with size of 4.5 mm×3.5 mm×42 mm at the biaxial pressure of 200 MPa. The green samples were heated up to 600-800 ℃ slowly and the inert gas was used to remove the adhesive in samples, then sintered at 1 250-1 350 ℃ in vacuum furnace for 4-6 h to get the desired samples with regular shape and prospective phases [11]. For the powder obtained by electroless doping of Ni, the processes of pressing, degreasing and sintering were carried out according to the above technics.
2.4 Measurement methods
Relative density and porosity of the cermet samples were tested with the ASTM Archimedes Method C373-88 (1999). Microstructure analysis was carried out by scanning electron microscope (JSM-5600LV).
Thermal shock resistance of material was appraised by comparing the residual strength after thermal shock testing with intrinsic strength [10, 12]. The temperature difference (?T) controlled respectively were 100, 200 and 300 ℃. Three-point bending strength was tested by using electron omnipotence machine CSS-44100 with span of 30 mm and cross-head speed of 0.5 mm/min. Peak load was obtained when sample was fractured and the average value of five samples was received.
3 Results and discussion
3.1 Effect of metallic phase adding methods on micro- structure
In order to observe the distribution of Ni in sintering bodies, the fracture analysis of green compacts and sintered samples with different adding methods of metallic phase was completed. Fig.1 shows the fracture microstructures of 5Ni/(90NiFe2O4-10NiO) and 17Ni/ (90NiFe2O4-10NiO) prepared by ball mixing. There are some big particles covered with small bright white pellets in Fig.1(b) and many big bright white particles in Fig.1(d). The EDS analysis for the bright white phase shows that there are elements such as Ni, Fe and O and the content of O and Fe is very low. So, it can be considered that Fe is brought from ball mixing and the main component is Ni. This indicates that the metallic phase Ni aggregates for Ni/(90NiFe2O4-10NiO) cermets in either green compacts or sintered samples with increasing of Ni content. The reason for Ni aggregation in green compacts is that ceramic particles are brittle and broken up easily, however, Ni powder is pliable and distorted easily. Thus, the distorted Ni is likely to cold weld together to form big conglomeration, and the aggregation will be more serious with increasing of Ni content. The reason for Ni aggregation in sintering samples is that Ni has good ductibility and becomes soft at high temperature, and Ni powder aggregated in the process of ball mixing is extruded to big particles due to the shrinkage force in sintering process.
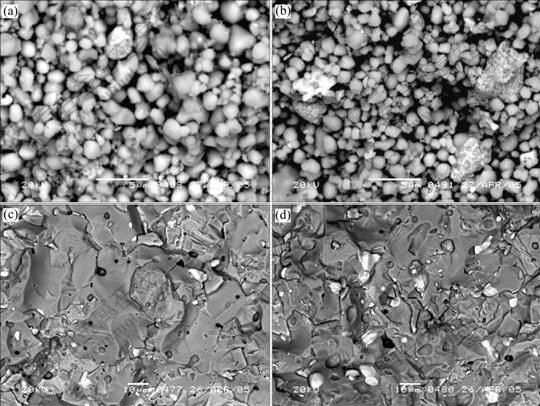
Fig.1 SEM photographs of cermet green compacts and sintered samples prepared by ball mixing: (a) 5Ni/(90NiFe2O4-10NiO), green compact; (b) 17Ni/(90NiFe2O4-10NiO), green compact; (c) 5Ni/(90NiFe2O4-10NiO), sintered sample; (d) 17Ni/(90NiFe2O4-10NiO), sintered sample
Fig.2 shows fracture microstructures of 6.5Ni/ (90NiFe2O4-10NiO) prepared by different adding methods of metallic phase. As shown in Figs.2(a) and (b), when the content of Ni is 6.5%, homogeneous distribu- tion of metallic particles is obtained in green compacts by electroless plating Ni, and dispersed distribution is achieved. However, for the green sample prepared by mechanical mixing, there are a few of ceramic particles covered with metal Ni. As shown in Figs.2(c) and (d), the metallic particles are fine in sintered sample prepared by electroless plating, but there are many coarse particles in sintered samples prepared by mechanical mixing. This indicates that metallic phase distributes more uniformly and dispersedly in sample obtained by the method of electroless plating Ni than mechanical mixing.
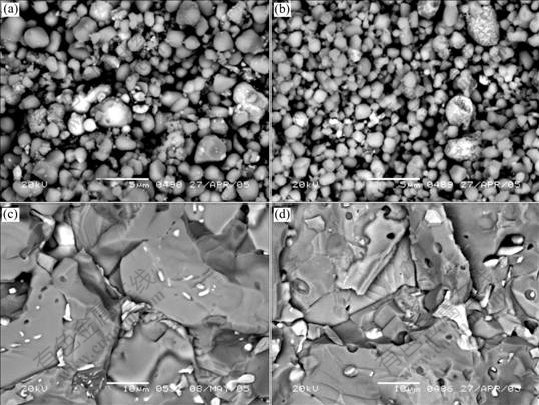
Fig.2 SEM photographs of 6.5Ni/(90NiFe2O4-10NiO) prepared by different metal adding methods: (a) Green compact prepared by electroless plating Ni; (b) Green compact prepared by mechanical mixing; (c) Sintered sample prepared by electroless doping Ni; (d) Sintered sample prepared by mechanical mixing
3.2 Effect of metallic phase adding methods on density and thermal shock resistance
As shown in Table 1, compared with the sample prepared by ball mixing, the relative density of samples prepared by electroless doping Ni reduces by 3%. There are several potential reasons. Firstly, Ni-P plating layer is adopted and phosphorus exists in sintered samples, which can be confirmed by phosphorus element surface scan for 6.5Ni/(90NiFe2O4-10NiO) cermet samples (Fig.3). A majority of phosphorus volatilize during sintering process, which increases holes and reduces density of cermet sample. Secondly, in order to eliminate the effect of grain size, ceramic powder is ball mixed for 150 min before electroless plating Ni, but electroless plating Ni may eliminate a part of crystal lattice flaw generated in the process of ball mixing, and the sintering driving force of cermet powder prepared by electroless plating Ni is smaller than that of cermet powder prepared by ball mixing. Otherwise, as shown in Fig.2(b), Ni particles are fine, homogeneous and dispersedly distributed in green compacts prepared by electroless plating Ni. In the last state of sintering, the densification process of samples is actual the process of grain recrystallizing and growing up [13-14], dispersed metallic particles located at the grain boundary cause steric hindrance and retard the densification of cermets, and this effect becomes more intensively with better dispersed distribution of second-phase metallic particles in ceramic body during sintering process.
Table 1 Effect of metallic phase adding methods on density and thermal shock resistance
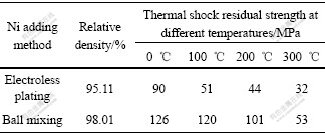
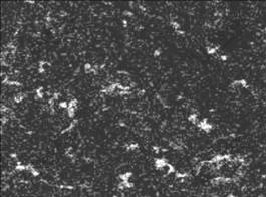
Fig.3 Surface scanning image of phosphorus element in fracture sample of 6.5Ni/(90NiFe2O4-10NiO)
As shown in Table 1 and Fig.4, though Ni distributes more homogeneously in the samples prepared by electroless plating Ni than those by ball mixing, the thermal shock resistance is not improved. On the contrary, thermal shock resistance reduces by 28%-58%. There are several reasons. Firstly, metallic phases in samples prepared by electroless plating Ni are fine and distribute homogeneously compared with samples prepared by ball mixing, and this generates dispersion strengthening that is better than particles strengthening of ball mixing [12,15]. Secondly, extensive application foreground, mature technics and facile raw material have been considered when selecting technics and recipe of electroless plating Ni. Ni-P electroless plating layer is used on ceramic powder and this causes some bad effect. On one side, the reduction of relative density affects thermal shock resistance of material; on the other side, phosphorus element has not volatilized and remains in material and causes bad effect on strength and toughness of material. This bad effect counteracts the effect generated by dispersion toughness, and induces bad thermal shock resistance of cermets prepared by electroless plating Ni. Of course, the specific reason should be further studied.
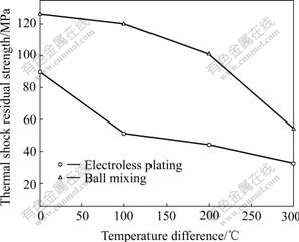
Fig.4 Thermal shock residual strength of 6.5 Ni/(90NiFe2O4- 10NiO) prepared by different Ni adding methods
4 Conclusions
1) Metallic phase aggregates in green compacts and sintered samples of Ni/(90NiFe2O4-10NiO) cermets when ball mixing is used to add Ni, and aggregation becomes serious with increasing of metallic phase content. Ni powder is distorted easily and welded together due to the mechanical force of ball mixing, then forms conglomeration in green compacts; Ni powder is extruded to big particles due to the shrinkage force of ceramic body in sintering process.
2) Metal Ni particles distribute finely and homogeneously in green compacts and sintered samples of 6.5Ni/(90NiFe2O4-10NiO) cermets prepared by electroless plating Ni. However, because the side effect of phosphorus introduced by electroless plating Ni and the reduction of sintering driving force, relative density and thermal shock residual strength of 6.5Ni/ (90NiFe2O4-10NiO) samples prepared by electroless plating Ni reduce by 3% and 28%-58% respectively, compared with those of the samples prepared by ball mixing.
References
[1] LI Jie, LAI Yan-qing, ZHOU Ke-chao, LI Zhi-you, LIU Ye-xiang. Preparation and preliminary testing of cermet inert anode for aluminum electrolysis [J]. Trans Nonferrous Met Soc China, 2003, 13(3): 663-670.
[2] KENIRY J. The economics of inert anodes and wettable cathodes for aluminum reduction cells [J]. JOM, 2001, 53(5): 43-47.
[3] SADOWAY D R. Inert anodes for the Hall-Héroult cell: The ultimate materials challenge [J]. JOM, 2001, 53(5): 34-35.
[4] PAWLEK R P. Inert anodes: An update [C]// Wolfgang Schneider. Light Metals 2002, TMS. USA: Warreudale PA, 2002. 449-456.
[5] SUN Xiao-gang. Studies on densification and mechanical property of Ni-NiFe2O4-NiO cermet inert anodes [D]. Changsha: Central South University, 2005. (in Chinese)
[6] WEYAND J D, DEYOUNG D H, RAY S P, TARCY G P, BAKER F W. Inert anodes for aluminum smelting (final report) [R]. Alcoa Center, 1986.
[7] YANG Jian-hong, WANG Hua-zhang, LIU Ye-xiang, XIE Xin-jun. Studies on preparation and property of NiO-NiFe2O4 based cermets for aluminum electrolysis [J]. Journal of Central South Institute of Mining and Metallurgy, 1993, 24(3): 326-331. (in Chinese)
[8] LI Guo-xun, WANG Chuan-fu, QU Shu-ling HUANG Ai-qin, LI Guo-bin. Studies on preparation and corrosion resistance of inert anodes for aluminum electrolysis [J]. Nonferrous Metals, 1993, 45(2): 53-57. (in Chinese)
[9] LI Jie, DUAN Hua-nan, LAI Yan-qing, TIAN Zhong-liang, LIU Ye-xiang. Effect of NiO content on the corrosion behaviour of Ni-xNiO-NiFe2O4 cermets in Na3AlF6-Al2O3 melts [J]. Trans Nonferrous Met Soc China, 2004, 14(6): 1180-1186.
[10] State Bureau of Technical Supervision of China. Thermal shock resistance experimental method of engineering ceramics [S]. GB/T 16536─1996. 1997-04-01. (in Chinese)
[11] LAI Yan-qing, SUN Xiao-gang, LI Jie, DUAN Hua-nan, LI Xing-zheng, ZHANG Gang, TIAN Zhong-liang. Densification of Ni-NiFe2O4-NiO cermets for aluminum electrolysis [J]. Trans Nonferrous Met Soc China, 2005, 15(3): 666-670.
[12] ZHANG Qing-chun. Mechanical properties of ceramics [M]. Beijing: Science Press, 1987. (in Chinese)
[13] HUANG Pei-yun. Powder metallurgical principle (edited) [M]. Beijing: Metallurgical Industrial Press, 1997. (in Chinese)
[14] SHI Jian-lin. Solid state sintering (Ⅰ): Pore microstructural model and thermodynamic stability, densification equations [J]. Journal of the Chinese Ceramic Society, 1997, 25(5): 499-513. (in Chinese)
[15] MU Bai-chun. Tenacious of ceramics [M]. Beijing: Metallurgical Industrial Press, 2002. (in Chinese)
Foundation item: Project(50474051) supported by the National Natural Science Foundation of China; Project(2005CB623703) supported by the National Basic Research Program of China; Project(03JJY3080) supported by the Natural Science Foundation of Hunan Province, China
Corresponding author: LAI Yan-qing; Tel: +86-731-8876454; E-mail: 13975808172@126.com


