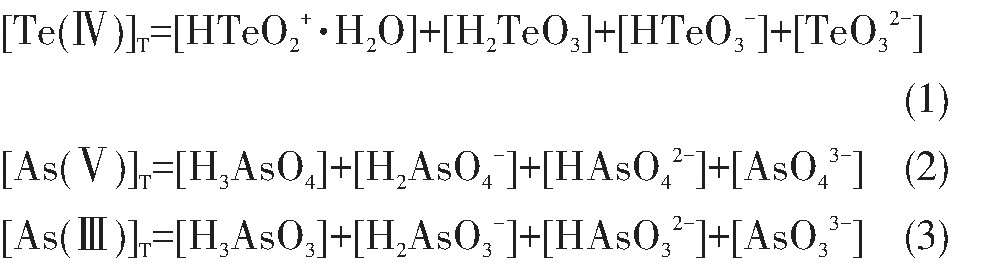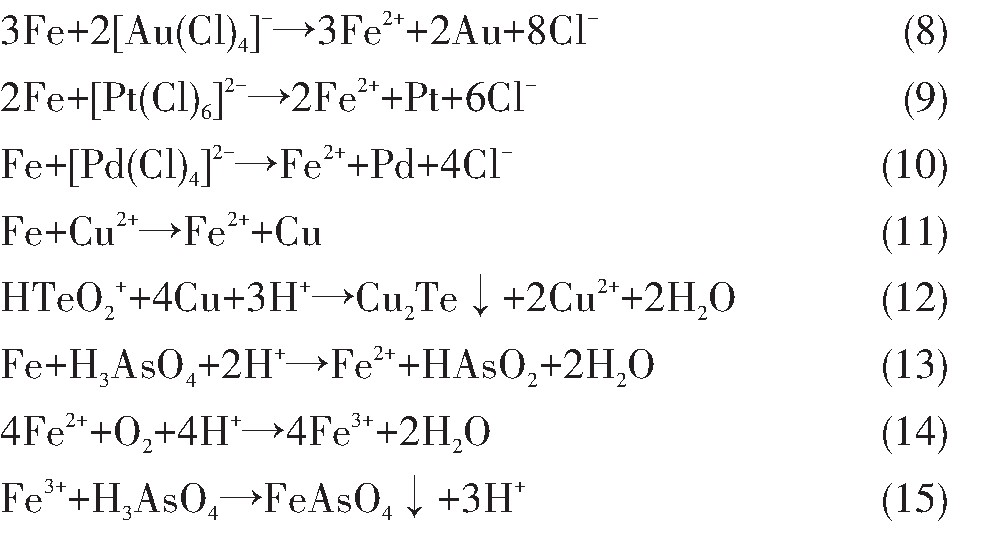网络首发时间: 2019-08-12 15:49
铜阳极泥分铜液高效分离稀散金属碲
安徽工业大学冶金工程学院
摘 要:
以铜阳极泥硫酸化焙烧-酸浸脱铜所得分铜液为研究对象,采用原位还原制得高活性、细微粒海绵铜作为还原剂,在线高效沉淀分离稀散元素碲、协同去除部分有害元素砷,考察了反应温度、反应时间、还原剂过量系数、搅拌速度和氧化剂加入量等因素对碲、砷沉淀率的影响。结果表明,在反应温度95℃、反应时间2 h、还原剂过量系数3、搅拌速度250 r·min-1和未添加氧化剂的优化条件下,碲的沉淀率达85.02%,砷的沉淀率为19.18%。还原渣的主要成分为Cu,Te,As和Se,含量分别为45.93%,15.34%,4.61%和2.69%(质量分数),贵金属Au,Ag,Pt和Pd的总含量为211.0 g·t-1,X射线衍射(XRD)分析表明产物的主要物相组成为Cu2-xTe和FeAsO4,此外还出现了Te和Cu的衍射峰。该工艺过程简单、环境友好、成本低廉,对分铜液的综合利用具有一定理论价值和实际意义。
关键词:
中图分类号: TF811;TQ125.3
作者简介:张金池(1996-),男,河北邯郸人,硕士研究生,研究方向:稀贵金属综合回收,E-mail:zjczhangjinchi@163.com;*张福元,教授,电话:13939816876,E-mail:sanzhfy@163.com;
收稿日期:2019-06-30
基金:国家自然科学基金联合基金项目(U1703130);国家自然科学基金青年基金项目(51704011)资助;
Efficient Separation of Rare Metal Tellurium in Copper-Rich Solution from Copper Anode Slime
Zhang Jinchi Zhang Fuyuan Zhao Zhuo Xu Liang
School of Metallurgical Engineering,Anhui University of Technology
Abstract:
Copper anode slime was vital secondary source that contains many metals with high value,such as gold,silver,platinum,palladium,selenium and tellurium. It was usually used as an important raw material to extract rare and precious metals and also attract considerable attention by metallurgical industry. The treatment process of copper anode slime mainly included pyrometallurgy,hydrometallurgy and combination of pyro and hydrometallurgy. However,no matter which treatment process was adopted,the copper-rich solution containing valuable elements of tellurium,gold,platinum and palladium would be obtained in the metallurgical process. How to efficiently recover rare and precious metals from the copper-rich solution had become an urgent problem in metallurgical industry.Relationship diagrams between the forms of Te(Ⅳ),As(V),As(Ⅲ)and pH value were respectively drawn by calculation according to the thermodynamic data,and the speciation of Te(Ⅳ),As(V)and As(Ⅲ)in the copper-rich solution were analyzed. Based on the Nernst equation,the actual electrode potentials of important metal ions in the copper-rich solution were calculated. High-activity and fine-particle sponge copper was obtained by in-situ reduction method,which was used as a reducing reagent to effectively precipitate rare metal of tellurium and remove part of the harmful element of arsenic from the copper-rich solution. The reaction mechanism of insitu reduction was studied by depicting the potential-pH diagram of the Te-H2O system according to thermodynamic calculation. The effects of various factors on the precipitation efficiency of Te,As and precious metals of Au,Pt and Pd were investigated referring to the precipitation efficiency of Te. Amplification experiments were carried out under the optimal conditions,and the chemical composition and the crystal structures of the reduction residue were analyzed by X-ray fluorescence spectrometry(XRF)and X-ray diffraction(XRD),respectively. The results of thermodynamic analysis showed that Te(Ⅳ),As(Ⅴ)and As(Ⅲ)respectively existed as the forms of HTeO2+·H2O,H3AsO4 and H3AsO3 in the copper-rich solution,of which the fractions could be reached as high as about 100%.The results of electrochemical analysis illustrated that the reduction order of ions coexisting in the copper-rich solution are[Au(Cl)4]-,[Pt(Cl)6]2-,HTeO2+,[Pd(Cl)4]2-,H3AsO4,Cu2+and HAsO2. The potential-pH diagram of the Te-H2O system showed that Cu2+could be reduced to generate fine-particle sponge copper,which was used to reduce HTeO2+to Cu2Te. Fe2+produced by reduction reaction,through oxidation,could react with H3AsO4 to form FeAsO4. The precipitation efficiency of Te significantly increased from 10.77% to 83.72% with the rise of reaction temperature from 55 to 95 ℃. A high temperature could effectively promote the precipitation reaction of FeAsO4 toward positive reaction,so the precipitation efficiency of As gradually increased from 8.74% to 20.17%. As reaction time prolonging,the precipitation efficiency of Te and As gradually increased. The dynamic equilibrium of the precipitation of Te and As reached at 2 h,and the precipitation efficiency of Te and As were 83.72% and 20.17%,respectively. With the rise of reductant excess coefficient,ions with high actual electrode potential preferentially reduced,such as[Au(Cl)4]-,[Pt(Cl)6]2-,[Pd(Cl)4]2-and H3AsO4. As the reductant excess coefficient further increasing,fine-particle sponge copper was produced to reduce Te(Ⅳ)to Cu2Te,causing the precipitation efficiency of Te increasing gradually. Because As(Ⅴ)was preferentially reduced to As(Ⅲ)instead of As,the precipitation efficiency of As first decreased and then increased. As the stirring speed improving,Te precipitation efficiency gradually dropped from 78.84% to 69.08% and the precipitation efficiency of As gradually increased from 18.79% to 28.20%,indicating that the precipitation reaction of As might be controlled by diffusion. Under the condition of high acidity and oxidant added,Te could be oxidized back to the solution,which made Te precipitation efficiency first decrease and then increase. The precipitation efficiency of As significantly increased as a result of oxidation of Fe2+and HAsO2 in the solution to obtain FeAsO4. The optimal conditions were selected as follows:reaction temperature of 95 ℃,reaction time of 2 h,the reductant excess coefficient of 3,stirring speed of 250 r·min-1 and no oxidant addition. In the amplification experiments,85.02% Te and 19.18% As were precipitated. The main components of the reduction residue were Cu,Te,As and Se,with the corresponding contents of 45.93%,15.34%,4.61% and 2.69%,respectively,and the total contents of precious metals of Au,Pt and Pd were 211.0 g·t-1. XRD pattern showed that the main phases of the residue were Cu2-xTe and FeAsO4. In addition,the typical diffraction peaks of Te and Cu were also observed.
Keyword:
copper anode slime; copper-rich solution; rare metals; tellurium; arsenic;
Received: 2019-06-30
碲是7种重要的稀散元素之一。碲及其化合物因具有优异的物理化学性能,在合金、探测、热电、太阳能、橡胶等领域应用广泛
目前,提取碲的原料
目前,从分铜液中回收碲的方法主要有中和法
1 实验
1.1 实验原料
分铜液来自国内某铜冶炼厂稀贵车间,p H值为0.58,该料液呈深蓝色、清澈透亮,其成分如表1所示。
由表1可知,分铜液主要成分Cu的含量为60.88 g·L-1,As的含量2.677 g·L-1,其中化学分析As(Ⅲ)占3.54%,As(Ⅴ)占96.46%,As主要以高价态形式存在;稀散元素Te的含量为1.764 g·L-1,贵金属Au,Ag,Pt,Pd的含量分别为0.37,0.17,1.24,2.12 mg·L-1,稀贵金属Te,Au,Ag,Pt和Pd具有较高的回收价值。
1.2 实验方法
取一定体积分铜液于烧杯中,将烧杯置于水浴锅中,设定水浴锅温度,待达到目标温度时,启动搅拌按实验所需缓慢向分铜液中加入还原剂,保温反应一段时间后,真空抽滤进行固液分离。采用化学滴定法分析溶液中Te和As的含量,并计算Te和As的沉淀率。
1.3 分析方法
采用X射线荧光光谱仪(XRF)对还原渣进行多元素分析,日本理学D/max-TTR III型X射线衍射仪(XRD)分析物相组成,Au,Ag,Pt和Pd含量参照GB/T7739.1-2007采用火试金-电感耦合等离子体发射光谱仪(ICP)分析,采用碘量法测定Cu含量,采用溴酸钾滴定法测定As含量,采用重铬酸钾返滴定法测定Te含量。
2 热力学分析
2.1 碲和砷的组态分析
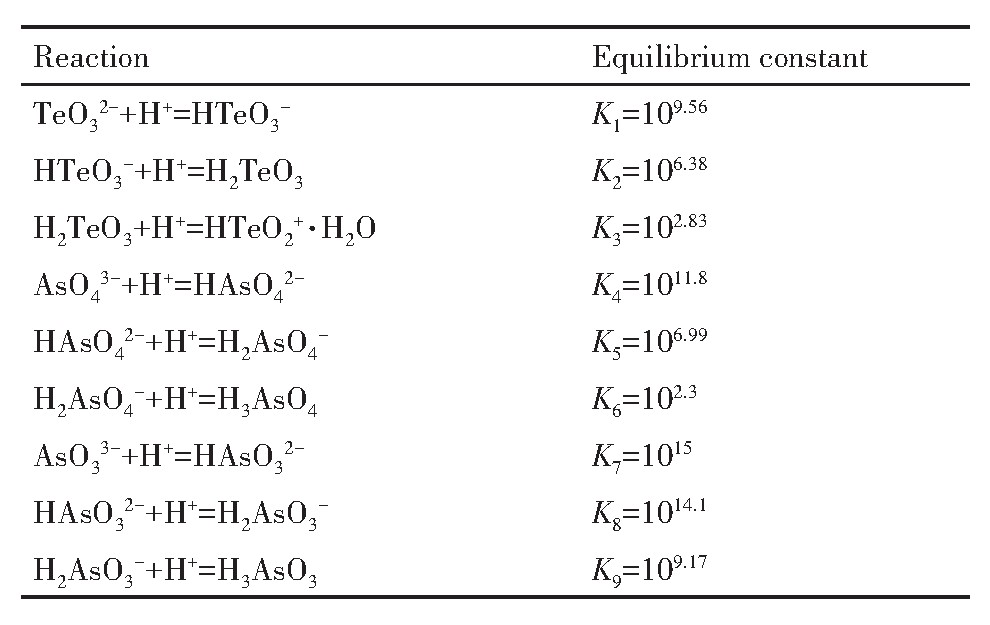
铜阳极泥分铜液的p H=0.5~1,As主要呈As(Ⅴ),Te主要为Te(Ⅳ),在铜阳极泥硫酸化焙烧过程中即使有少量Te过氧化生成Te(Ⅵ),也会在酸浸过程中与Cu2+结合成Cu3Te O6沉淀而进入分铜渣。在水溶液中Te O32-,As O43-和As O33-均为弱酸根离子,可与H+发生加质子反应,有关反应及平衡常数
由表2可知,水溶液中Te(Ⅳ)的存在形态有HTe O2+·H2O,H2Te O3,HTe O3-和Te O32-,As(Ⅴ)的存在形态分别为H3As O4,H2As O4-,HAs O42-,As O43-,As(Ⅲ)的形态分别为H3As O3,H2As O3-,HAs O32-,As O33-。溶液中Te(Ⅳ),As(Ⅴ)和As(Ⅲ)的总浓度如式(1)~(3)。
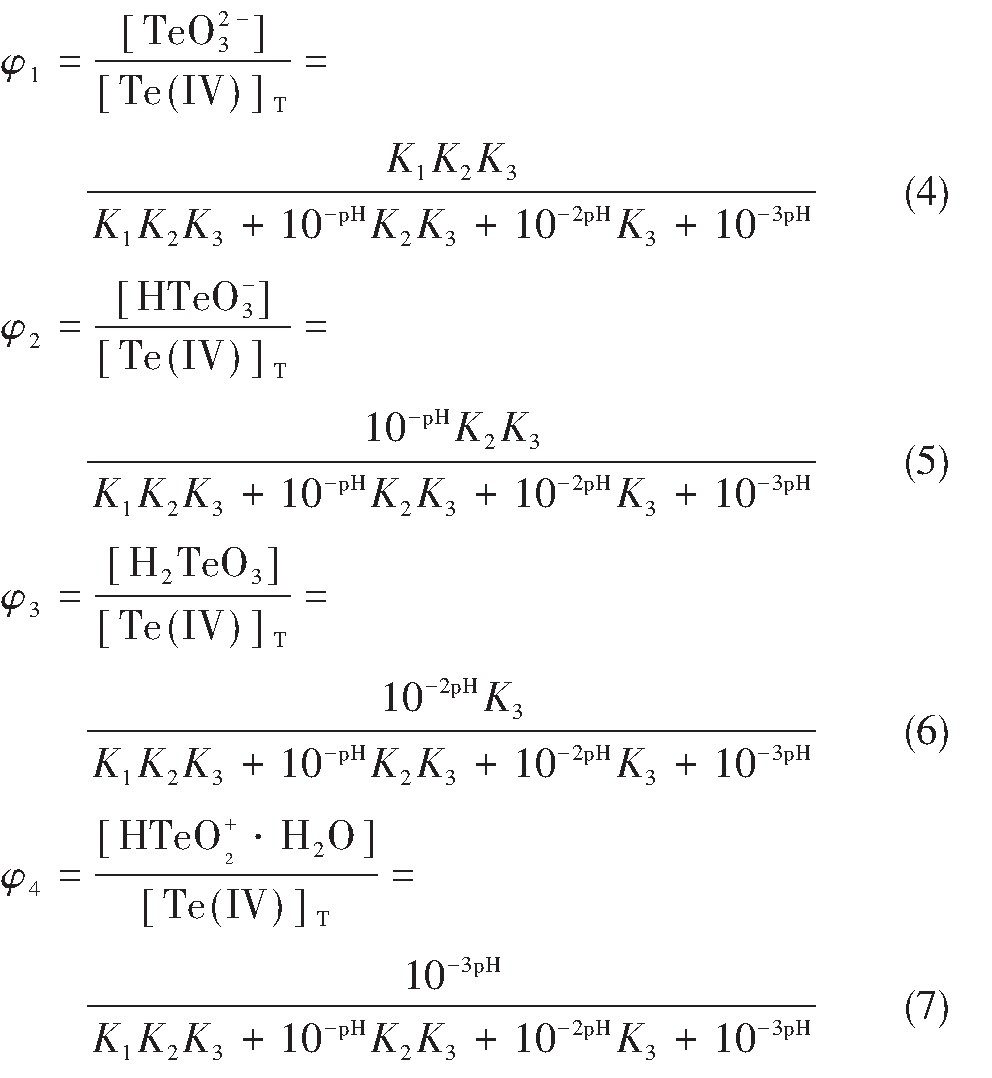
根据表2及式(1),计算可得Te O32-,HTe O3-,H2Te O3和HTe O2+·H2O分别占Te(Ⅳ)总浓度的分数,如式(4)~(7):
式中,φ1,φ2,φ3,φ4分别表示Te O32-,HTe O3-,H2Te O3和HTe O2+·H2O占Te(Ⅳ)总浓度的分数;p H代表H+浓度指数;K1,K2,K3为表2中所列数据。
表1 分铜液的主要成分分析结果 下载原图
Table 1 Main components of copper-rich solution(g·L-1)

*:represent mg·L-1
表2 Te O32-,As O43-和As O33-的加质子反应及平衡常数 下载原图
Table 2 Addition proton reactions and equilibrium con-stants
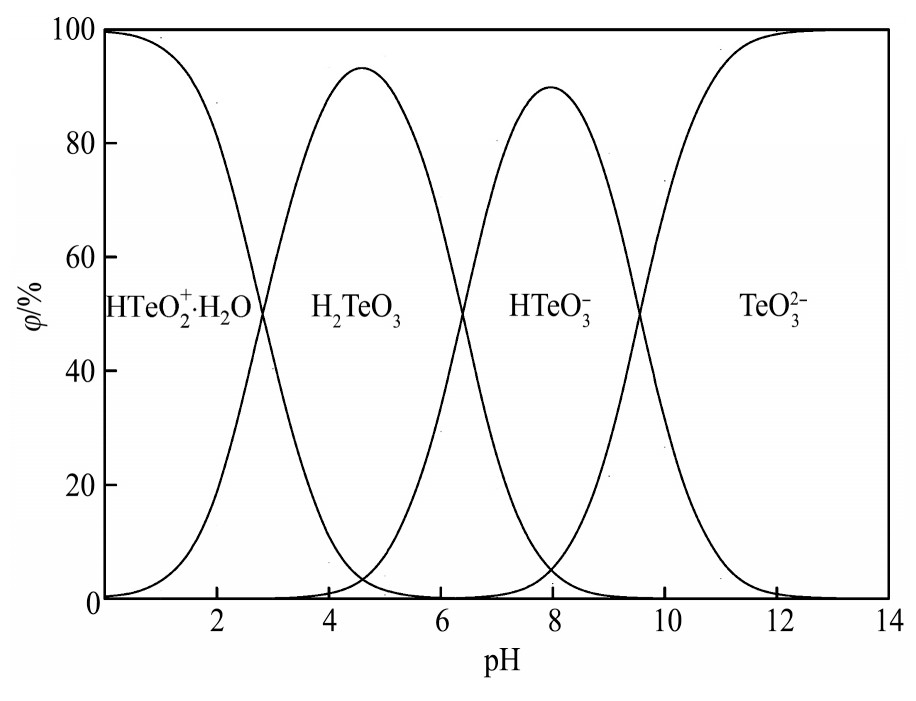
根据表2及上式可得Te(Ⅳ)各组态浓度分数与p H值的关系,如图1所示。
由图1可见,当溶液p H>12时,Te(Ⅳ)主要以Te O32-形态存在,随p H值的降低,Te O32-与H+发生加质子反应生成HTe O3-,Te O32-组分逐渐减少、HTe O3-组分逐渐增多,p H值为8左右时,Te(Ⅳ)主要以HTe O3-形态存在,占比为95%左右;随p H值的进一步降低,HTe O3-组分减少、H2Te O3组分增多,p H值达到4.6时,Te(Ⅳ)主要以H2Te O3形态存在,占比为98%左右;当溶液中p H<1时,Te(Ⅳ)主要以HTe O2+·H2O形态存在。分铜液的p H值为0.58,因此Te(Ⅳ)在分铜液中的存在形态主要为HTe O2+·H2O,约占99.2%。
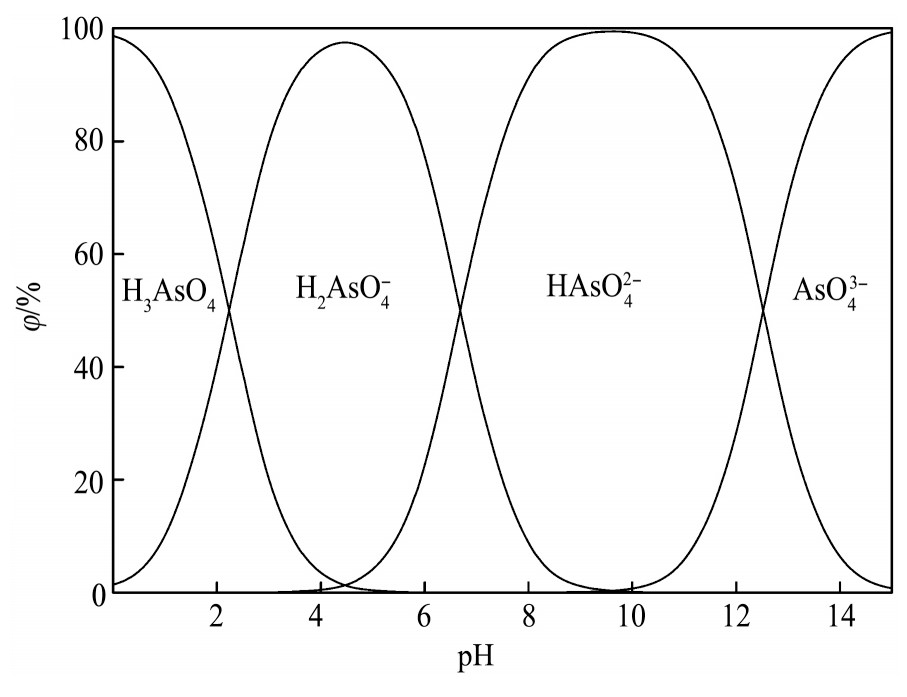
同理可得As(Ⅴ)和As(Ⅲ)各组态浓度分数与p H值的关系,分别如图2和图3所示。
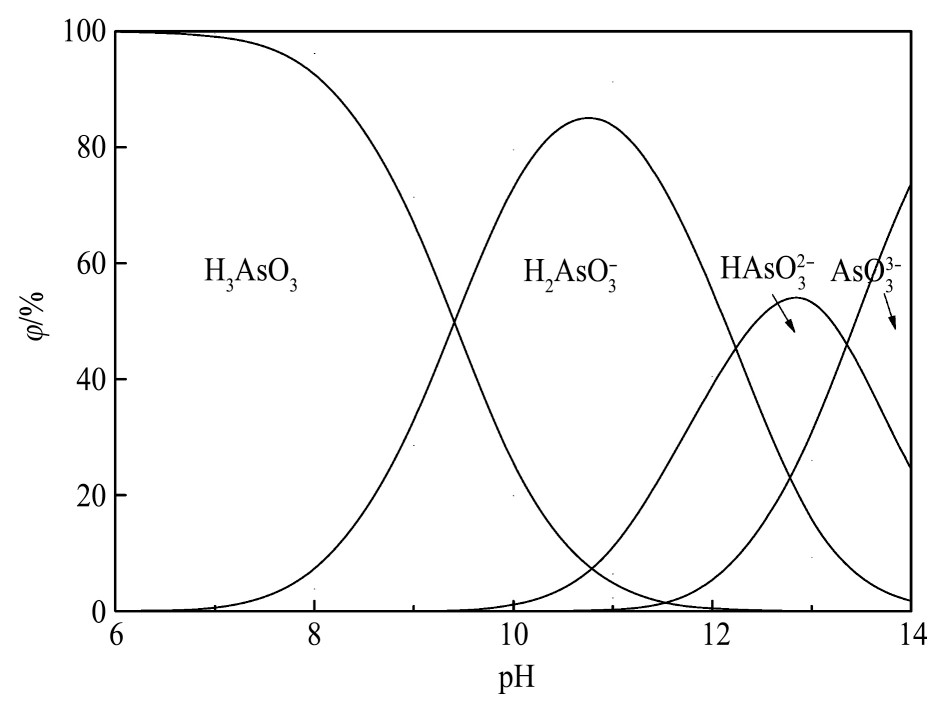
由图2可见,当溶液p H>14时As(Ⅴ)主要以As O43-形态存在,随溶液酸度的增加,As O43-与H+结合生成HAs O42-,As O43-组分逐渐减少、HAs O42-组分逐渐增多,当p H=12.5时,As O43-和HAs O42-组分各占50%;当p H值降至9.6时,As(Ⅴ)主要以HAs O42-形式存在,占比接近100%,当p H值为6.7时,HAs O42-和H2As O4-组分各占50%;当p H值达到4.5时,As(Ⅴ)主要以H2As O4-形式存在,随p H值的进一步降低,H2As O4-组分减少、H3As O4组分增多,当p H值达到2.2时,H2As O4-和H3As O4组分相同;当溶液中p H<1时,As(Ⅴ)主要以H3As O4的形式存在。所以,分铜液中占绝对优势的As(Ⅴ)主要以H3As O4形态存在,占比约为96.3%。
图1 Te(Ⅳ)各组态与p H值的关系
Fig.1 Relationship between forms of Te(Ⅳ)and p H value
图2 As(Ⅴ)各组态与p H值的关系
Fig.2 Relationship between forms of As(Ⅴ)and p H value
由图3可见,当溶液p H=14时As(Ⅲ)以As O33-,HAs O32-和H2As O3-的形态存在,占比分别为79.77%,19.94%和0.29%;随p H值的降低,As O33-与逐步H+结合生成HAs O32-和H2As O3-,As O33-组分减少、HAs O32-和H2As O3-组分增多,当p H值为12.8时,HAs O32-的组分达到最大值,占比为53.7%,As O33-和H2As O3-组分相同,占比为23.15%;随溶液酸度的增加,As O33-和HAs O32-的组分减少、H2As O3-和H3As O3的组分增多,当p H值达到10.8时,H2As O3-的组分达到最大值,占比为85.24%;在p H值小于7的广泛区域内,As(Ⅲ)主要以H3As O3的形式存在,占比接近100%。所以,分铜液中少量的As(Ⅲ)主要以H3As O3的形态存在,占比超过99.99%。
图3 As(Ⅲ)各组态与p H值的关系
Fig.3 Relationship between forms of As(Ⅲ)and p H value
综上可知,分铜液中Te(Ⅳ),As(Ⅴ)和As(Ⅲ)分别主要以HTe O2+·H2O,H3As O4和H3As O3的形态存在,占比均接近100%。
2.2 分铜液中相关反应电化学分析
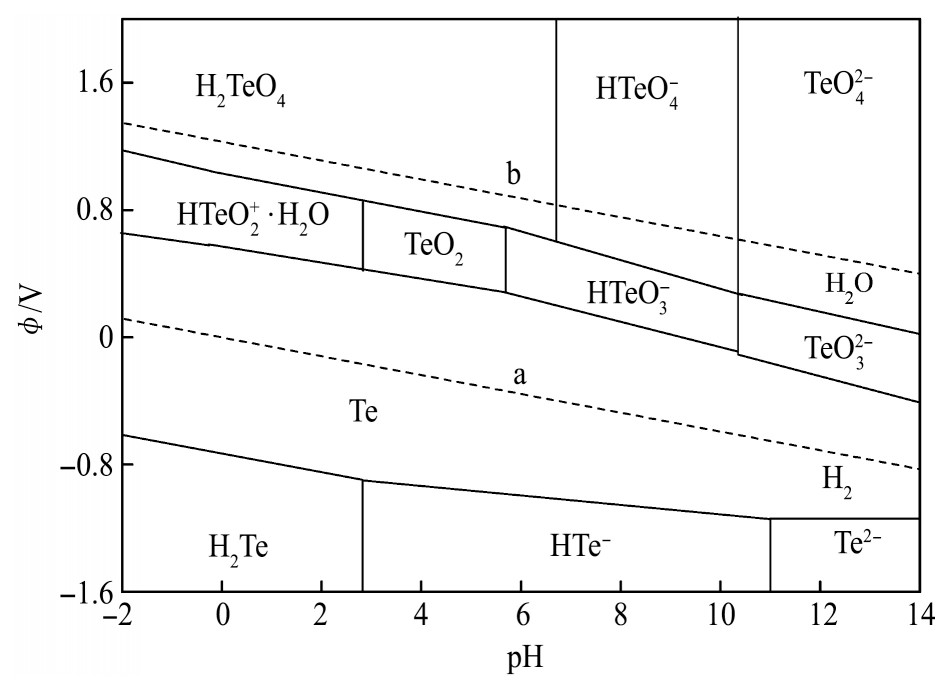
实验所涉及电极反应、标准电极电位及Nernst方程计算的实际电位如表3所列,Te-H2O系φ-p H图见图4。
对于As,无论是标准电位或是分铜液中实际电位,都存在?H3As O4/HAs O2>?H3As O4/As>?HAs O2/As,即在还原过程中As优先发生As(Ⅴ)还原为As(Ⅲ)的反应,其次发生As(Ⅲ)被还原为As单质的反应,热力学角度不发生As(Ⅴ)直接被还原为As单质的反应。铜的实际还原电位0.341 V较标准还原电位0.342 V基本不变,这得益于Cu2+的高浓度,同时?Cu2+/Cu=0.341 V>?HAs O2/As=0.157 V,在Cu2+未被还原至极低浓度前,As不会被还原为单质形式。因此,As和Cu的还原顺序为H3As O4,Cu2+,HAs O2。
由图4可知,Te(Ⅳ)在水溶液中有多种存在形态,随p H值的增加分别为HTe O2+·H2O,Te O2,HTe O3-和Te O32-,其热稳定区域在虚线b下方,在水溶液中均可稳定存在;随p H值的增加,HTe O2+·H2O,Te O2,HTe O3-和Te O32-的还原电位逐渐降低,还原电位最高的是HTe O2+·H2O,在p H<2.8的酸性溶液中,以HTe O2+·H2O形态存在的Te(Ⅳ)可被还原为单质Te,且单质Te存在的电位和p H均较为广泛,进一步降低还原电位可将Te(Ⅳ)还原为-2价。分铜液p H值为0.58左右,Te(Ⅳ)以HTe O2+·H2O形态存在,其实际电极电位?HTe O+2/Te=0.498 V,加入还原剂可将Te(Ⅳ)还原为Te和H2Te,并在相应区域稳定存在。
当溶液中的Cu2+被还原后生成新生态海绵铜,具有比表面积大、活性高的特性,能够将HTe O2+还原形成Cu2Te
表3 分铜液中部分元素标准电极电位(?θ)及其实际电极电位(?) 下载原图
Table 3 Standard electrode potential(?θ)and actual electrode potential(?)of some elements in copper-rich solution
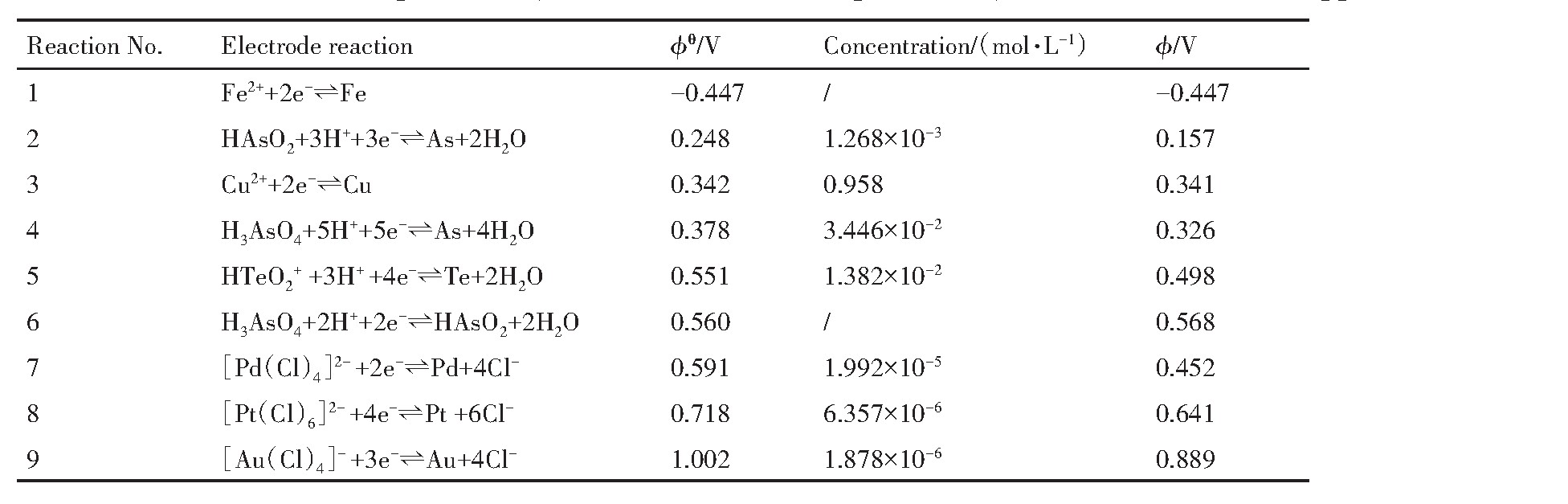
图4 Te-H2O系电位-p H图
Fig.4 Potential-p H diagram of Te-H2O system
通过上述分析,若控制合适的工艺参数,可以通过原位还原的方式在线沉淀分铜液中Te,Au,Pt和Pd等稀贵元素,协同去除部分有害元素As。在反应过程中,随着还原剂加入量的增加,溶液中的Au,Pt,Pd优先全部还原
3 结果与讨论
3.1 反应温度对碲、砷沉淀率的影响
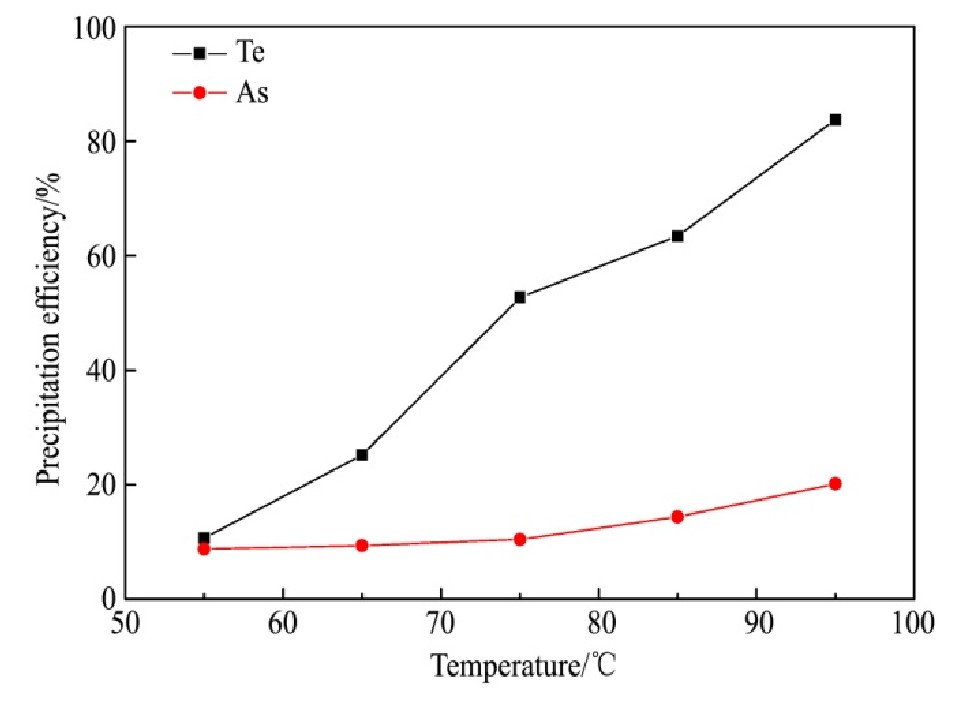
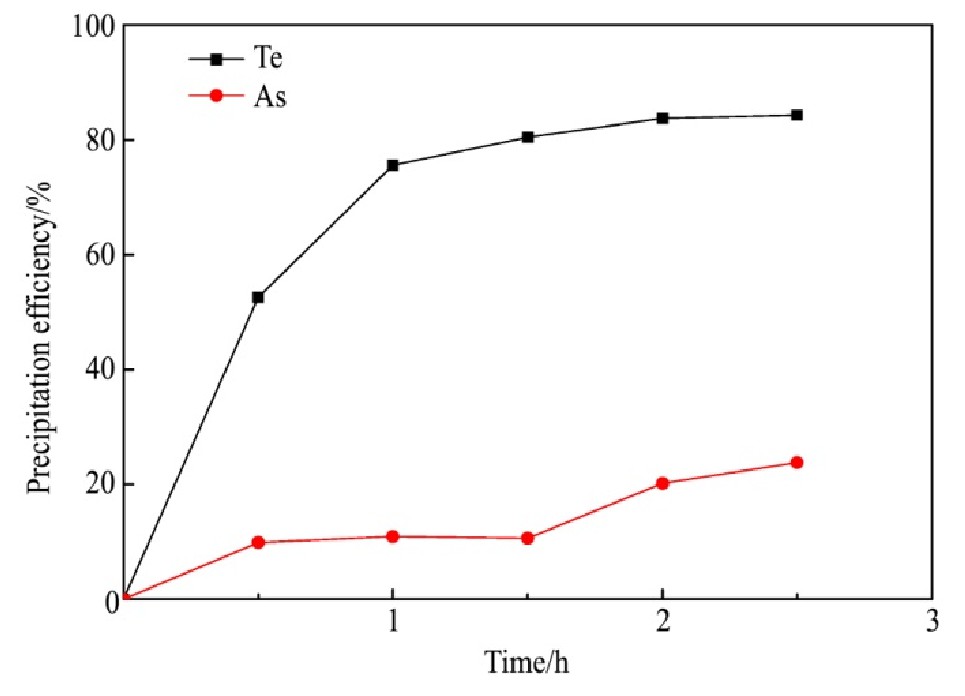
量取分铜液200 ml,在250 r·min-1的搅拌速度和还原剂过量系数为9的条件下反应2 h,反应温度对碲、砷沉淀率的影响结果如图5所示。
从图5可知,Te的沉淀率随温度的升高而显著增加,温度由55℃上升至95℃,Te的沉淀率由10.77%迅速增加至83.72%,As的沉淀率随温度的升高而缓慢增加,温度由55℃变化至75℃,As的沉淀率仅由8.74%变化至10.46%,温度升至95℃时,As的沉淀率增加至20.17%。反应温度升高时,分子运动速率增大,活化分子占比增加,从而增加了活化分子的有效碰撞概率,有利于还原反应的进行。同时,高温有利于Fe2+的氧化反应,形成了更多Fe As O4沉淀,从而使As的沉淀率增加。因此,95℃为适宜反应温度。
图5 反应温度对碲、砷沉淀率的影响
Fig.5 Effect of reaction temperature on precipitation efficien-cy of tellurium and arsenic
3.2 反应时间对碲、砷沉淀率的影响
量取分铜液200 ml,在反应温度为95℃、搅拌速度为250 r·min-1、还原剂过量系数为9的条件下,反应时间对碲、砷沉淀率的影响结果如图6所示。
从图6可知,当反应时间为0.5 h时,Te,As的沉淀率分别为52.59%,9.86%;继续延长反应时间至1.5 h,Te的沉淀率由52.59%快速增加至80.47%,而As的沉淀率增长缓慢,仅由9.86%增加至10.65%;随反应时间延长至2.5 h,Te的沉淀率缓慢增加,由80.47%增加至83.72%,As的沉淀率显著增加,由10.65%快速提升至23.80%,反应至2 h时碲、砷的沉淀反应基本达到动态平衡。综合考虑,选择2 h为适宜反应时间。
图6 反应时间对碲、砷沉淀率的影响
Fig.6 Effect of reaction time on precipitation efficiency of tel-lurium and arsenic
3.3 还原剂过量系数对碲、砷沉淀率的影响
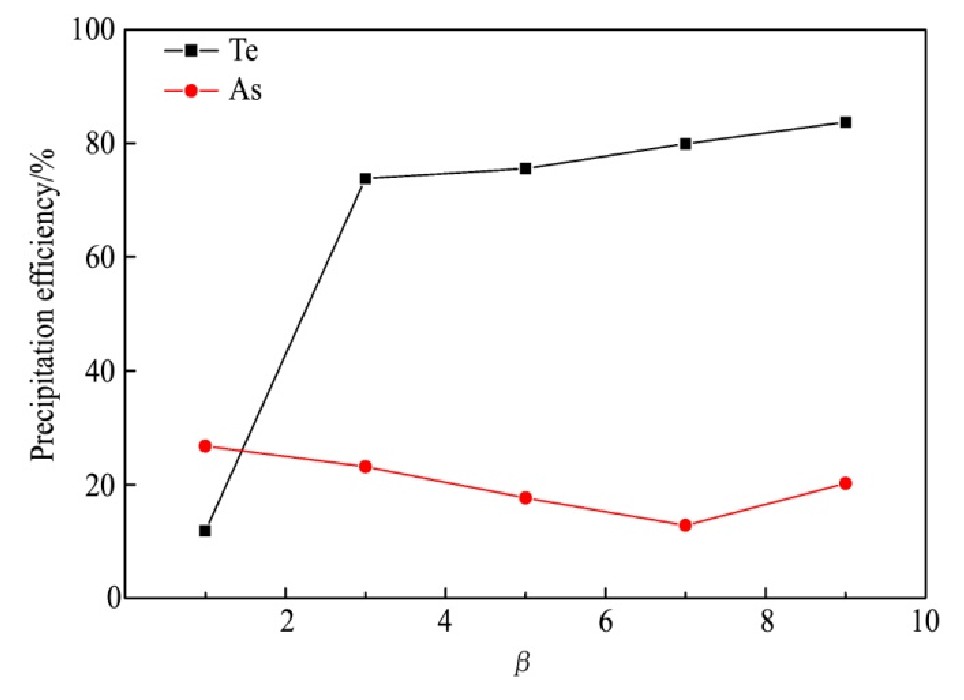
量取分铜液200 ml,在反应温度为95℃和搅拌速度为250 r·min-1的条件下反应2 h,还原剂过量系数(β)(β为1时理论上恰好能够形成Fe As O4沉淀)对碲、砷沉淀率的影响结果如图7所示。
从图7可知,Te沉淀率随还原剂过量系数的增大而增加,当β小于3时,Te的沉淀率从11.85%快速增加至73.83%,这是由于还原剂优先还原Au,Pt,As和Pd等电位高的离子,当还原剂过量时可得到新生海绵铜将Te(Ⅳ)还原为Cu2Te,使得Te沉淀率大大提升;当β由3增大至9时,Te沉淀率仅由73.83%逐渐增加至83.72%;As沉淀率随着β的增大先降低后增加,在β为7时As沉淀率仅有12.81%、达到最低值。β由1增大至7的过程中,As的沉淀率持续降低,这可能是因为还原剂将溶液中的As(Ⅴ)还原为As(Ⅲ),不利于Fe As O4的生成。随β增加至9,As沉淀率反而增加,可能由于随着大量还原剂的加入,溶液中As(Ⅴ)几乎全部被还原为As(Ⅲ)且有大量Cu2+被置换,As(Ⅲ)的浓度增加、Cu2+浓度降低,从而出现?HAs O2/As>?Cu2+/Cu的情况,使As(Ⅲ)直接还原为单质As,导致As沉淀率增加。在确保较高的Te,As沉淀率和避免分铜液中引入过多杂质的前提下,还原剂过量系数选择3较适合。
图7 还原剂过量系数对碲、砷沉淀率的影响
Fig.7 Effect of excess coefficient of reductant on precipitation efficiency of tellurium and arsenic
3.4 搅拌速度对碲、砷沉淀率的影响
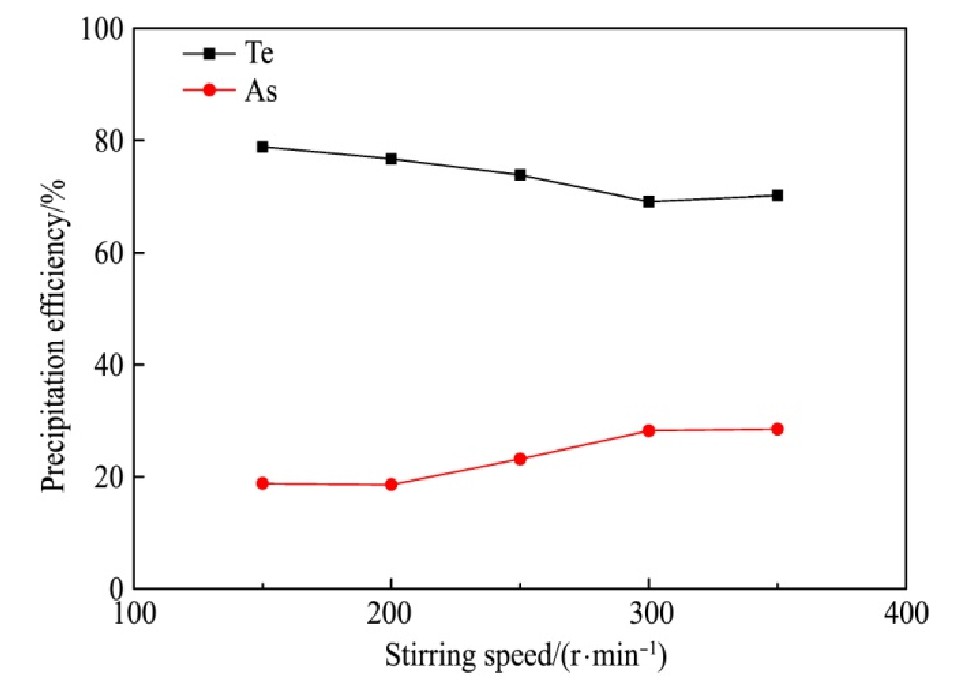
量取分铜液200 ml,在反应温度为95℃和还原剂过量系数为3的条件下反应2 h,搅拌速度对碲、砷沉淀率的影响结果如图8所示。
从图8可知,Te沉淀率随着搅拌速度的增加而缓慢降低,当搅拌速度小于300 r·min-1时,Te沉淀率由78.84%降低至69.08%;当搅拌速度大于300r·min-1时,Te沉淀率稳定在70%左右。As沉淀率随着搅拌速度的增加而缓慢增加,当搅拌速度小于200 r·min-1时,As沉淀率稳定在19%左右;当搅拌速度由200 r·min-1增加至300 r·min-1时,As沉淀率增加至28.20%,这可能是由于As的沉淀过程受扩散控制,搅拌速度的增加可有效消除扩散影响,有利于As的沉淀;当搅拌速度大于300 r·min-1时,As沉淀率稳定在28%左右。综合考虑,搅拌速度选择250 r·min-1较合适。
3.5 氧化剂加入量对碲、砷沉淀率的影响
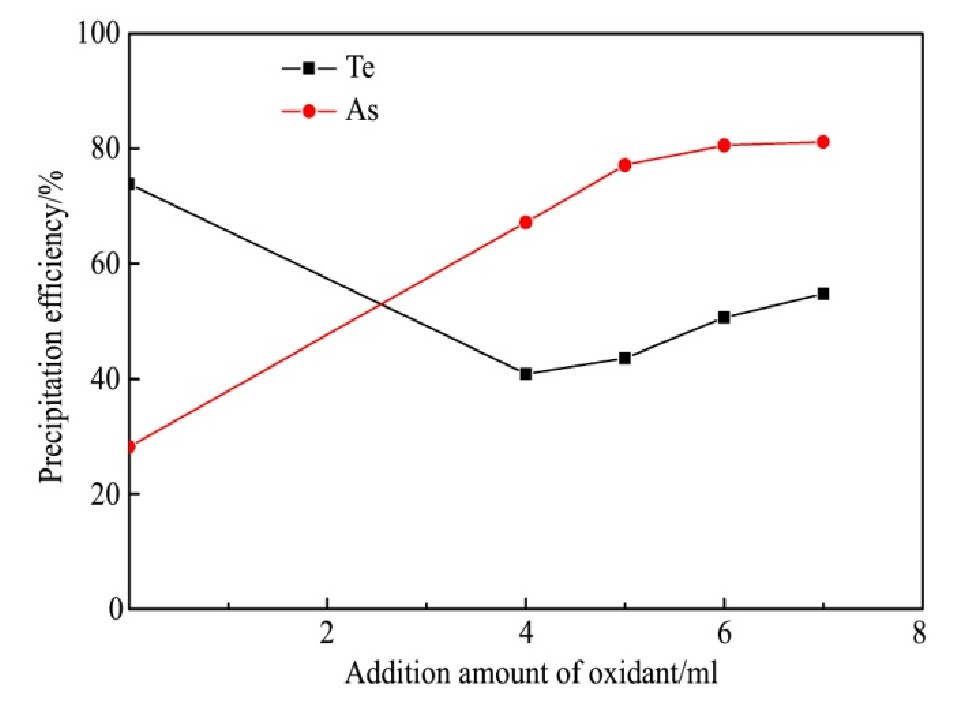
量取分铜液200 ml,在反应温度为95℃、搅拌速度为250 r·min-1和还原剂过量系数为3的条件下反应2 h后,向分铜液底部加入一定量双氧水,在上述的条件下继续反应1.5 h,氧化剂加入量对碲、砷沉淀率的影响结果如图9所示。
从图9可知,Te沉淀率随H2O2的加入由73.83%迅速降低至40.87%,这是由于被还原沉淀的单质Te在酸度较高的条件下部分被H2O2氧化重新进入溶液,导致Te的沉淀率大幅降低;Te沉淀率随H2O2加入量增加由40.87%增加至54.76%,这可能是由于新生细微Fe As O4颗粒的比表面积大,可吸附Te,Au,Pt和Pd等元素共沉淀,此驱动力大于Te被H2O2氧化进入溶液的氧化反应驱动力,从而使Te沉淀率随H2O2加入量的增加反而增加。As沉淀率随H2O2的加入由28.20%迅速增加至67.13%,溶液中的Fe2+和HAs O2分别被氧化为Fe3+和H3As O4,结合形成Fe As O4从而使As沉淀率迅速增加;As沉淀率随H2O2加入量的增加而增加,由4ml增加至7 ml,As沉淀率由67.13%增加至81.18%。综合考虑Te,As的沉淀效果,实验过程应不添加氧化剂。
图8 搅拌速度对碲、砷沉淀率的影响
Fig.8 Effect of stirring speed on precipitation efficiency of tel-lurium and arsenic
图9 氧化剂加入量对碲、砷沉淀率的影响
Fig.9 Effect of addition amount of oxidant on precipitation ef-ficiency of tellurium and arsenic
综上可得分铜液沉淀Te,As的优化工艺条件:温度为95℃、搅拌速度为250 r·min-1、还原剂过量系数为3、还原时间为2 h。
3.6 优化工艺条件放大试验
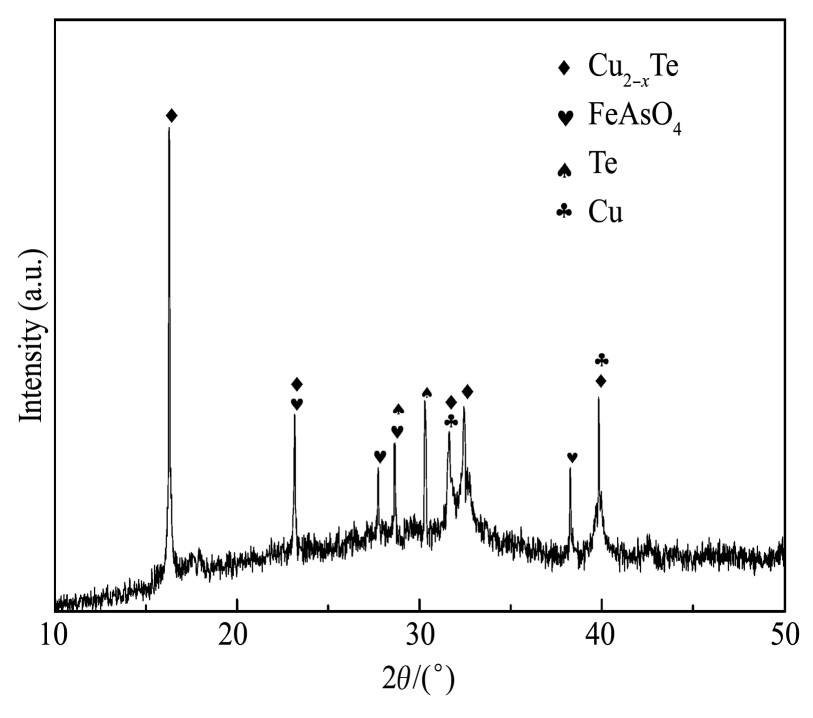
在上述优化工艺条件下,对1.5 L分铜液进行了3次试验,结果如表4所示,还原渣的化学成分如表5所列、XRD谱如图10所示。
由表4可知,在优化实验条件下,分铜液中Te由1.764 g·L-1降低至0.2643 g·L-1,沉淀率为85.02%;As由反应前的2.677 g·L-1降低至2.164 g·L-1,沉淀率为19.18%,As的沉淀率较低,可能是该反应发生在还原气氛中,Fe2+不易被氧化,同时H3As O4优先被还原为H3As O3,难以产生Fe As O4沉淀,最终导致As的沉淀率较低。
表4 优化实验结果 下载原图
Table 4 Experimental results of optimum conditions
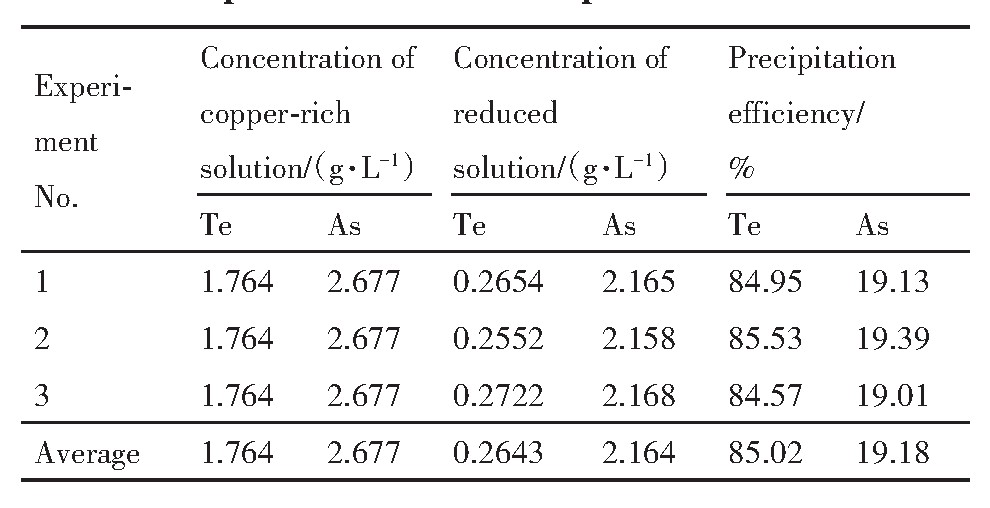
表5 还原渣的主要成分 下载原图
Table 5 Main components of reduction residue(%,mass fraction)
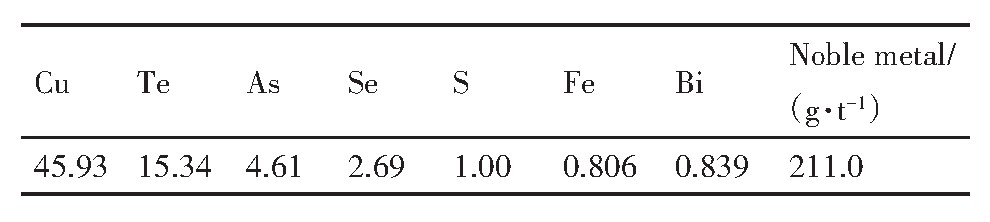
由表5可知,还原渣中的主要元素为Cu,Te,Se和有害元素As,含量分别为45.93%,15.34%,2.69%和4.61%,贵金属Au,Pt,Pd的总含量为211.0 g·t-1,该产物为高品位贵金属精矿。
由图10中可以看出,还原渣主要出现Cu2-xTe和Fe As O4物相;Cu2-xTe由原位还原制得的海绵铜在线还原HTe O2+所得,高温时Fe2+被氧化为Fe3+,再与H3As O4结合得到Fe As O4。除此之外,渣中还出现了单质Cu和Te的衍射峰,Cu衍射峰的出现可能是由于还原剂过量导致新生海绵铜未完全消耗,还原剂直接将HTe O2+还原为单质Te可能是Te衍射峰出现的原因。
图1 0 还原渣的XRD图谱
Fig.10 XRD pattern of reduction residue
4 结论
1.本文采用原位还原工艺,高效分离富集铜阳极泥分铜液中有价元素Te,Au,Pt,Pd,协同去除部分有害元素砷,在温度95℃、搅拌速度250r·min-1、还原剂过量系数3和反应时间2 h的优化放大条件下,Te的沉淀率达85.02%,As的沉淀率为19.18%,实现了分铜液中Te的高效分离和部分有害元素As的去除。
2.还原渣主要成分为Cu,Te,As,Se等,含量分别为45.93%,15.34%,4.61%和2.69%等,贵金属Au,Pt,Pd的总含量为211.0 g·t-1,渣中的主要物相为Cu2-xTe和Fe As O4,同时出现了单质Cu和单质Te的衍射峰,为高品位贵金属精矿。
参考文献