Trans. Nonferrous Met. Soc. China 23(2013) 3561-3567
Kinetic nucleation of primary α(Al) dendrites in Al-7%Si-Mg cast alloys with Ce and Sr additions
Zhong-wei CHEN, Xiao-lei HAO, Jing ZHAO, Cui-ying MA
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 15 March 2012; accepted 10 October 2013
Abstract:
Nucleation of dendritic primary α(Al) phase with addition of element Ce and Sr in hypoeutectic Al-7%Si-Mg cast alloy was investigated by using differential scanning calorimetry (DSC) and scanning electron microscopy. DSC results were used to calculate the activation energy and nucleation work of primary α(Al) phase. The results show that the values of activation energy and nucleation work are decreased and the nucleation frequency is increased with the additions of Ce and Sr to the alloys. Moreover, the grain size of dendritic α(Al) phase is well refined, and the nucleation temperatures of primary α(Al) dendrites are decreased with the additions of Ce and Sr. The effects of elements Ce and Sr additions on kinetic nucleation of primary α(Al) phases were also discussed in hypoeutectic Al-7%Si-Mg cast alloy.
Key words:
aluminium alloy; primary α dendrite; nucleation; grain refinement; activation energy; nucleation work; Ce; Sr;
1 Introduction
Al-7Si-Mg alloys are the most important Al-based cast alloys, and possess wide applications in aircraft, automobile, marine and electrical industries because of their excellent casting characteristics, weldability and corrosion resistance. Microstructure of Al-7Si-Mg alloy consists of a large fraction of primary α(Al) dendrites and secondary eutectic phases distributed in the interdendritic area. The mechanical properties of most cast alloys strongly depend on their microstructure characterization. Therefore, additions of grain refiner and modified agent for Al-7Si-Mg cast alloys are a popular melt treated processing.
The most common grain refiner for hypoeutectic Al-7Si cast alloys is Al-5Ti-lB master alloy [1,2]. It was assumed that Al3Ti particles must be the heterogeneous nucleation sites as they are a pro-peritectic phase, which could nucleate the solid through a peritectic reaction and furthermore have very good lattice matching with Al. ZHANG et al [3] concluded that Al3Ti (I4/mmm, a=0.385 nm, c=0.429 nm) is a more powerful nucleating substrate for Al alloy than TiC, TiB2 and AlB2 due to its better edge-to-edge matching in both close packed directions and close packed planes of the primary α(Al) phase (m3m, a=0.404 nm). On the other hand, some other literatures [4,5] agreed on the concept that solute elements segregate and restrict the growth of the solid-liquid interface of new grains and therefore cause grain refinement.
The structural transformation of the eutectic silicon phase from a coarse plate-like or flake-like structure to a fine fibrous structure in hypoeutectic Al-7Si cast alloys occurs when elements such as strontium and sodium are added, which has been explained based on an impurity- induced twinning (IIT) mechanism [6]. The ideal ratio of the atomic radius of the impurity-element compared with silicon for an element to cause modification was calculated as 1.646. The elements causing modification have an atomic radius ratio close to 1.65 from the theory of IIT, and the fine fibrous eutectic modification of hypoeutectic Al-7Si alloys containing Sr, Na, Ca and Ba was all investigated [6-8]. The additions of rare earth element La, Sm and Ce were also reported to cause the eutectic modification [9-11].
However, the effect of the additions of the modifying elements on primary α(Al) phase in Al-7Si alloy has not been well investigated. LIAO et al [12] found that the addition of Sr in Al-11.6%Si alloys results in a considerable increase of the amount of primary α(Al) dendrites and improves the mechanical properties of the modified alloys. It was indicated that Sr addition for A357 alloy not only modifies the morphology of eutectic Si phase but also affects the structure of primary α(Al) dendrites [13]. Furthermore, it was reported that the multicomponent Ce-phases may act as nucleation sites for α(Al) crystals in hypoeutectic Al-Si alloys, and the liquidus temperature of primary α(Al) is slightly reduced by the addition of Ce [14]. The precipitation enthalpy in Al-Si-Mg alloy decreases with Ce addition, while precipitation takes place more rapidly and intensively, indicating increased reaction kinetics [15].
The present study aims to investigate the effect of Ce and Sr additions on nucleation of primary α(Al) phase in hypoeutectic Al-7%Si-Mg cast alloys by differential scanning calorimetry (DSC) to calculate the activation energy, nucleation work and the nucleation frequency, and ascertain the kinetic nucleation of primary α(Al) phase.
2 Nucleation theory
According to the classical nucleation theory, the nucleation frequency I in melts and alloys is calculated from [16]
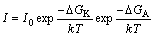 (1)
(1)
where I0 is a coefficient, △GK is the nucleation work and △GA is the activation energy, k is Boltzmann’s constant, and T is the nucleation temperature. The equation shows that the nucleation frequency is inversely proportional to the activation energy and nucleation work.
Then, the activation energy can be expressed using the Kissinger method [17]:
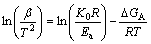 (2)
(2)
where K0 is a coefficient, β is the heating rate, R is the gas constant, and T is the temperature. Furthermore, equation (2) shows that a plot of ln(β/T2) against 1/T should give a straight line with the gradient -△GA/R.
According to Ref. [18], there is
 (3)
(3)
where TE is the formation temperature (890.3 K) of α(Al) phase during solidification in Al-7Si alloy, T is the peak temperature of α(Al) phase on the exothermic curves and △T is the degree of undercooling. A=16πσ3f(θ)/ 3k(△GV/△T)2, △GK=16πσ3f(θ)/3(△GV)2 [16], where △GV is the driving force for nucleation, σ is the solid-liquid interfacial energy, and θ is the contact angle at the solid-nucleus-liquid triple point. So, there is a following equation:
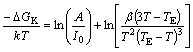 (4)
(4)
Equation (4) gives a plot of ln[β(3T-TE)/T2(TE-T)3] against -1/T and shows a straight line with gradient △GK/k. And the value of the nucleation work can be calculated using equation (4).
3 Experimental
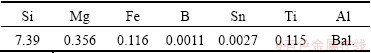
Table 1 shows the chemical composition of the commercial Al-7%Si-Mg ingots used in the experiment. The as-received commercial Al-7%Si-Mg ingots were melted in a SiC crucible by a 12 kW electrical resistance furnace. The SiC crucible was preheated to 400 °C before charging the commercial Al-7%Si-Mg ingots and then the alloy melt was heated up to 750 °C, carefully skimmed to remove dross and other impurities. Two of the alloy melts were respectively treated by Ce and Sr, the levels of 0.03% and 0.8% in the form of the Al-10Sr alloy and the pure cerium which was wrapped in aluminum foil were added to adjust the chemical compositions. Finally, the alloy melts were cast into the pre-heated cast-iron molds.
Table 1 Chemical composition of commercial Al-7%Si-Mg ingot (mass fraction, %)
The effect of additions of Ce and Sr on the microstructures was studied by a field emission scanning electron microscopy (FE-SEM, ZEISSSUPRA55). Electron backscattering diffraction (EBSD) tests for grain size were performed on a ZEISS SUPRA55 with HKL channel 5. DSC (NETZSCH STA 409 CD) was used to investigate the kinetics of α(Al) nucleation by varying the heating rate and cooling rate in the range of 5-25 K/min.
4 Results
4.1 Microstructure
Figure 1 gives the microstructures of Al-7%Si-Mg alloys with different treatments. The results show that the eutectic Si crystals were modified as fine fibrous with the addition of Ce and Sr, while the secondary dendritic arm spacing of primary α(Al) phases seems to be not changed with different treatments. Distribution of α(Al) grain size in Al-7%Si-Mg alloys with different treatments is shown in Fig. 2. With Ce and Sr addition, the dominated grain size of α(Al) phase decreases from 150 μm to 90 μm. For the grain size less than 300 μm, the distribution value of α(Al) grains was 0.685 in untreated alloy, 0.990 in Ce-treated alloy and 0.755 in Sr-treated alloy, respectively. It shows that the grain refinement effect of Ce addition is more marked than that of Sr addition.

Fig. 1 SEM images of Al-7%Si-Mg alloys with different treatments
4.2 Activation energy
Figure 3 shows the effect of heating rate on the endothermic peaks of Al-7%Si-Mg alloys. It can be seen that the fusion peak temperature of α(Al) dendrite increases with increasing the heating rate. Figure 4 shows the plots of ln(β/T2) against 1/T using endothermic peak temperatures extracted from Fig. 3. According to equation (2), the gradient -△GA/R can be extracted from the straight lines in Fig. 4 and the value of △GA can be calculated and listed in Table 2.
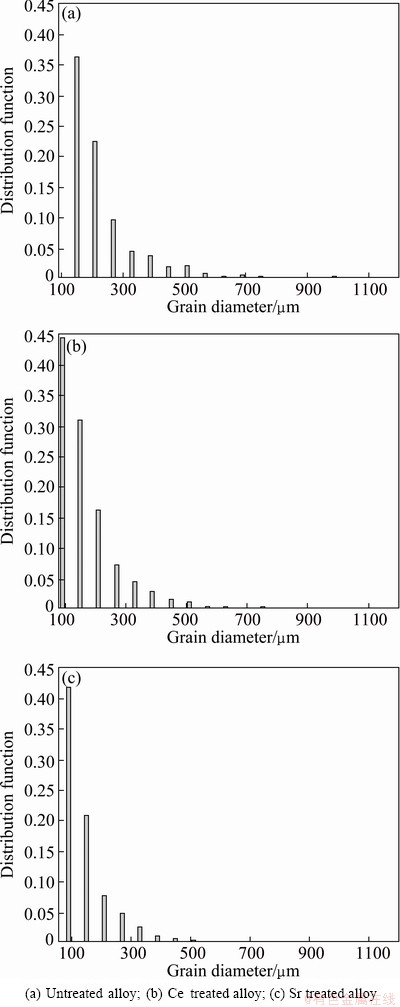
Fig. 2 Distribution of α(Al) grain size in Al-7%Si-Mg alloys with different treatments
4.3 Nucleation work
Figure 5 shows the effect of cooling rate on the exothermic peaks of Al-7%Si-Mg alloys. It can be seen that the solidification peak temperature of primary α(Al) dendrite decreases with increasing cooling rate. Figure 6 shows the plot of 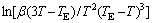 against 1/T using exothermic peak temperatures extracted from Fig. 5. According to equation (4), the gradient △GK/k can be extracted from the straight lines in Fig. 6 and the value of △GK can be calculated and listed in Table 2.
against 1/T using exothermic peak temperatures extracted from Fig. 5. According to equation (4), the gradient △GK/k can be extracted from the straight lines in Fig. 6 and the value of △GK can be calculated and listed in Table 2.
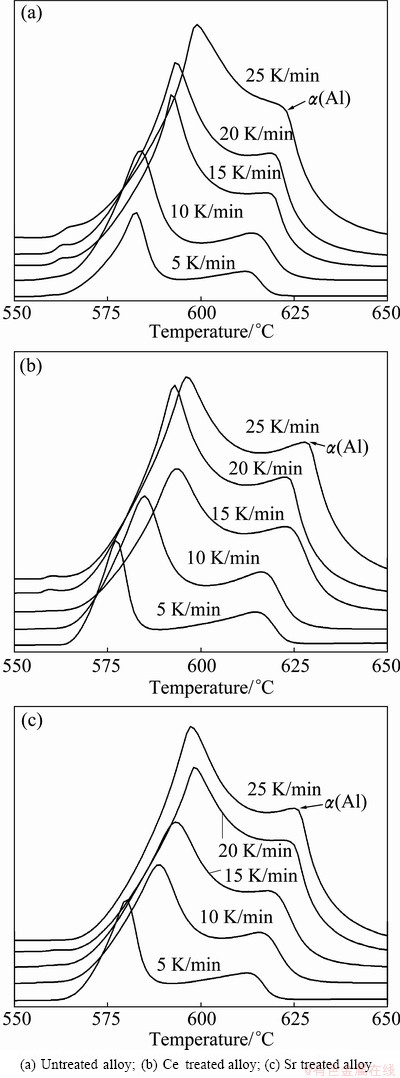
Fig. 3 DSC traces of endotherms of Al-7%Si-Mg alloys with different heating rates
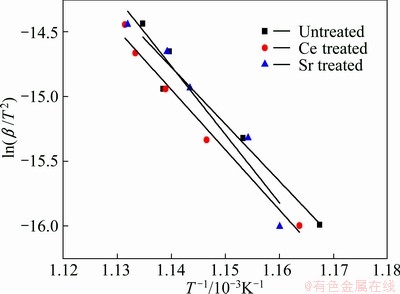
Fig. 4 Plot of ln(β/T2) vs 1/T using endothermic peak temperatures of primary α(Al)
Table 2 Results of gradient for activation energy and nucleation work extracted from Fig. 4 and Fig. 6 (R=8.314 J/(mol·K-1), k= 1.38×10-23 J/K)
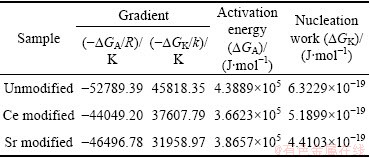
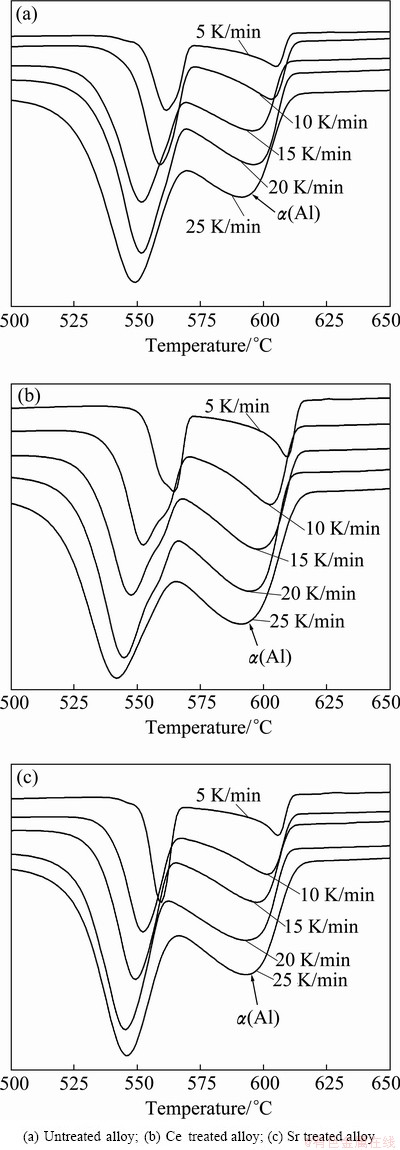
Fig. 5 DSC traces of solidification exotherms of Al-7%Si-Mg alloys with different cooling rates
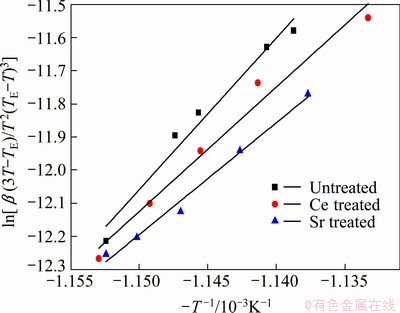
Fig. 6 Plot of ln[β(3T-TE)/T2(TE-T)3] vs 1/T using exothermic peak temperatures of primary α(Al)
4.4 Nucleation frequency
For the nucleation frequency given as equation (1), the ratio IM/IUn of the nucleation frequency for the modified alloy to the unmodified alloy is as follows:
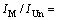
 (5)
(5)
where the subscripts “Un” and “M” present the unmodification and modification, respectively.
According to the DSC in Fig. 5, when the cooling rate is 5 K/min, the nucleation temperatures of primary α(Al) dendrite are 610.07, 614.15, and 611.39 °C for unmodified and modified of Al-7%Si-Mg alloys with addition of Ce and Sr, respectively. So, from equation (5), the ratio of nucleation frequency, ICe/IUn is exp(6.0996×1024) and ISr/IUn is exp(4.3399×1024), respectively. From the calculation above, the nucleation frequency of primary α(Al) dendrite in the modified Al-7%Si-Mg alloys with addition of Ce or Sr is much larger than that of the unmodified one, and the α(Al) nucleation frequency in the alloy modified with Ce is much larger than that of the modified one with Sr.
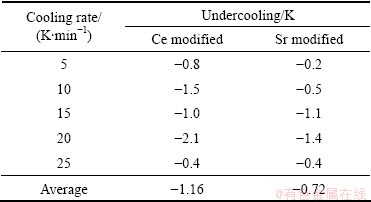
Table 3 shows the primary α(Al) peak depression of two modified alloys from Fig. 5. It shows that the nucleation temperatures of primary α(Al) dendrites are decreased with the addition of Ce or Sr. The average nucleation undercooling of primary α(Al) phase in modified alloy with Ce and Sr is -1.16 K and -0.72 K respectively, compared with unmodified alloy.
Table 3 Primary α(Al) undercooling of modified Al-7%Si-Mg alloys
5 Discussion
In the metal casting industry, it is common practice to introduce nucleating agents to a liquid metal, referred to be inoculation, for the formation of fine, uniform and equiaxed grains. Al-7%Si-Mg alloys based on inoculation with the Al-Ti-B-type master alloys are typical examples in this regard. Ti content in the experimental alloys is 0.115% as shown in Table 1, and is enough for inoculation. A model has been proposed for heterogeneous nucleation and grain formation on potent spherical particles during solidification on the basis of an adsorption and surface diffusion mechanism. For a spherical-cap nucleus to become a “transformation nucleus”, the linear dimension (d) of the flat substrate must exceed the critical nucleus size (2r*). This Turnbull criterion (d≥2r*) defines a minimum undercooling for grain formation, and effective inoculation on flat nucleating substrates [19]. The linear dimension (d ) is expressed as follows [19,20]:
 (6)
(6)
where rSL is the interfacial energy, Tm is the melting point, LV is the latent heat of fusion per unit volume of the crystal and △T is the undercooling. Equation (6) defines a minimum undercooling (△Tmin) for the formation of a transformation nucleus or a grain on a flat substrate.
In this study, the nucleation frequency of primary α(Al) phases in the well modified Al-7% Si-Mg alloy is about exp(1024) times more than the unmodified one by decreasing the activation energy and nucleation work. In other words, the activation energy and nucleation work of primary α(Al) phases in the modified alloy are lower than those of the unmodified alloy, and the energy of the effective nuclei is steady and the quantities of the effective nuclei are enough for nucleation. Then, the effective nucleation site is easier to obtain under a certain undercooling. This allows more particles to be active in nucleation and, consequently, increases the number density of active particles, giving rise to a finer grain size. In the modified alloys, the lower activation energy and nucleation work could be attributed to the decreasing of a minimum undercooling (△Tmin) for nucleation by the adsorption or wetting mechanism on potent/wettable substrates [21]. The further discussion is beyond the scope of this work.
On the other hand, it is well accepted that for effective grain refinement some solute is required in the melt to restrict the growth of the solid even if potent nucleating particles with a favourable physical nature are present [22,23]. It was also shown that for four contributions to the total solidification undercooling (kinetic, curvature, thermal, and solutal) the solutal or constitutional undercooling (△Tcs) is the controlling term under typical aluminum alloy casting conditions [24]. The most recent analytical model for constitutional undercooling driving grain formation and grain size prediction was given in Refs. [5,25, 26].
In this study, the average undercoolings of primary α(Al) phase with Ce or Sr addition are -1.16 K and -0.72 K, respectively. It revealed that the solute elements in the liquid ahead of the growing crystals reduce the growth velocity of the nucleated crystals and increase the maximum undercooling achievable before recalescence, and then result in a finer grain size. Therefore, based on the kinetics of primary α(Al) phase nucleation, the grain refinement of primary α(Al) phase in modified alloy with Ce or Sr addition is attributed to the promoted nucleation and restricted growth. Furthermore, since the influence of Ce addition on the promoted nucleation and restricted growth is much stronger than that of Sr addition, the effect of grain refinement of Ce addition is more marked compared with Sr addition.
6 Conclusions
1) The dominated grain size of α(Al) phase in Al-7%Si-Mg alloys modified by Ce or Sr elements is decreased from 150 μm to 90 μm, but the effect of grain refinement with Ce addition is more marked than that with Sr addition.
2) Ce or Sr addition in Al-7%Si-Mg alloys can decrease the activation energy and nucleation work for α(Al) nucleation and increase the nucleation frequency of the primary α(Al).
3) The average nucleation undercoolings of primary α(Al) phase in modified alloys with Ce and Sr addition are respectively -1.16 K and -0.72 K, compared with the unmodified alloy.
4) The grain refinement of primary α(Al) phase in modified alloy with Ce or Sr addition is attributed to the promoted nucleation and restricted growth.
References
[1] GREER A L, BUNN A M, TRONCHE A, EVANS P V, BRISTOW D J. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al-Ti-B [J]. Acta Materialia, 2000, 48(11): 2823-2835.
[2] KORI S A, MURTY B S, CHAKRABORTY M. Development of an efficient grain refiner for Al-7Si alloy and its modification with strontium [J]. Materials Science and Engineering A, 2000, 283(1): 94-104.
[3] ZHANG M X, KELLY P M, EASTON M A, TAYLOR J A. Crystallographic study of grain refinement in aluminum alloys using the edge-to-edge matching model [J]. Acta Materialia, 2005, 53: 1427-1438.
[4] SCHMID-FETZER R, KOZLOV A. Thermodynamic aspects of grain growth restriction in multicomponent alloy solidification [J]. Acta Materialia, 2011, 59: 6133-6144.
[5] MEN H, FAN Z. Effects of solute content on grain refinement in an isothermal melt [J]. Acta Materialia, 2011, 59: 2704-2712.
[6] LU S Z, HELLAWELL A. The mechanism of silicon modification in aluminum-silicon alloys: Impurity induced twinning [J]. Metallurgical and Materials Transactions A, 1987, 18: 1721-1733.
[7] NOGITA K, KNUUTINEN A, MCDONALD S D, DAHLE A K. Mechanisms of eutectic solidification in Al-Si alloys modified with Ba, Ca, Y and Yb [J]. J Light Metals, 2001, 1: 219-228.
[8] McDONALD S D, NOGITA K, DAHLE A K. Eutectic nucleation in Al-Si alloys [J]. Acta Materialia, 2004, 52: 4273-4280.
[9] TSAI Y C, CHOU C Y, LEE S L, LIN C K, LIN J C, LIM S W. Effect of trace La addition on the microstructures and mechanical properties of A356 (Al-7Si-0.35Mg) aluminum alloys [J]. Journal of Alloys and Compounds, 2009, 487: 157-162.
[10] YE B J, LOOPER C R, LU D Y, KANG C S. An assessment of the role of rare earth in the eutectic modification of cast aluminum-silicon alloys [J]. Transaction of America Foundry Society, 1984, 92: 533-544.
[11] CHEN Z W, MA C Y, CHEN P. Modifying agent selection for Al-7Si alloy by Miedema model [J]. International Journal of Minerals, Metallurgy and Materials, 2012, 19(2): 131-135.
[12] LIAO H C, SUN Y, SUN G X. Correlation between mechanical properties and amount of dendritic a-Al phase in as-cast near-eutectic Al-11.6%Si alloys modified with strontium [J]. Materials Science and Engineering A, 2002, 335: 62-66.
[13] CHEN Z W, ZHANG R J. Effect of strontium on primary dendritic and eutectic temperature of A357 aluminium alloy [J]. China Foundry, 2010, 7(2): 149-152.
[14]  , SCHMID-FETZER R. Thermodynamic aspects of the constitution, grain refining, and solidification enthalpies of Al-Ce-Si alloys [J]. Metallurgical and Materials Transactions A, 2004, 35: 3349-3362.
, SCHMID-FETZER R. Thermodynamic aspects of the constitution, grain refining, and solidification enthalpies of Al-Ce-Si alloys [J]. Metallurgical and Materials Transactions A, 2004, 35: 3349-3362.
[15]  M, KORES S, MRVAR P, MEDVED J. Effect of Ce on solidification and mechanical properties of A360 alloy [J]. Journal of Alloys and Compounds, 2011, 509: 7349-7355.
M, KORES S, MRVAR P, MEDVED J. Effect of Ce on solidification and mechanical properties of A360 alloy [J]. Journal of Alloys and Compounds, 2011, 509: 7349-7355.
[16] KURZ W, FISHER D J. Fundamentals of Solidification [M]. 4th ed. Switzerland, Germany, UK, USA, 1998: 29.
[17] KISSINGER H E. Reaction kinetics in differential thermal analysis [J]. Analytical Chemistry, 1957, 29: 1702-1706.
[18] HO C R, CANTOR B. Heterogeneous nucleation of solidification of Si in Al-Si and Al-Si-P alloys [J]. Acta Metallurgica et Materialia, 1995, 43: 3231-3246.
[19] TURNBULL D. Theory of catalysis of nucleation by surface patches [J]. Acta Metallurgica, 1953, 1: 8-14.
[20] CAHN J W. Emergence of modern nucleation theory [C]//Phase Transitions in Condensed Systems—Experiments and Theory. Pittsburgh (PA): Materials Research Society, 1987: 41-55.
[21] QIAN M. Heterogeneous nucleation on potent spherical substrates during solidification [J]. Acta Materialia, 2007, 55: 943-953.
[22] HUNT J D. Steady state columnar and equiaxed growth of dendrites and eutectic [J]. Materials Science and Engineering, 1984, 65: 75-83.
[23]  M, TRIVEDI R, KURZ W. Nucleation ahead of the advancing interface in directional solidification [J]. Materials Science and Engineering A, 1997, 226-228: 763-769.
M, TRIVEDI R, KURZ W. Nucleation ahead of the advancing interface in directional solidification [J]. Materials Science and Engineering A, 1997, 226-228: 763-769.
[24] QUESTED T E, DINSDALE A T, GREER A L. Thermodynamic modelling of growth-restriction effects in aluminium alloys [J]. Acta Materialia, 2005, 53: 1323-1334.
[25] QIAN M, CAO P, EASTON M A, MCDONALD S D, STJOHN D H. An analytical model for constitutional supercooling-driven grain formation and grain size prediction [J]. Acta Materialia, 2010, 58: 3262-3270.
[26] CHEN Z W, HE Z, JIE W Q. Growth restriction effects during solidification of aluminium alloys [J]. Transactions of Nonferrous Metals Society of China,2009, 19: 410-413.
Al–7%Si–Mg铸造铝合金添加Ce与Sr元素后初生α(Al)枝晶的动力学形核
陈忠伟,郝小雷,赵 静,马翠英
西北工业大学 凝固技术国家重点实验室,西安 710072
摘 要:利用差示量热法(DSC)和扫描电镜研究Al-7%Si-Mg亚共晶铸造铝合金添加Ce与Sr元素后初生α(Al)枝晶的动力学形核。根据DSC结果计算初生α(Al)相的激活能和形核功。结果表明,合金中添加Ce与Sr元素后,激活能和形核功降低,形核率增加;同时,初生α(Al)晶粒细化,其形核温度降低。讨论添加Ce与Sr元素对Al-7% Si-Mg亚共晶铸造铝合金初生α(Al)枝晶动力学形核的影响。
关键词:铝合金;初生α枝晶;形核;晶粒细化;激活能;形核功;Ce;Sr
(Edited by Hua YANG)
Foundation item: Project (42-QP-009) support by Research Fund of the State Key Laboratory of Solidification Processing, China; Project (B08040) supported by the Program of Introducing Talents of Discipline to Universities (“111” Project), China
Corresponding author: Zhong-wei CHEN; Tel: +86-29-88493450-8001; E-mail: chzw@nwpu.edu.cn
DOI: 10.1016/S1003-6326(13)62901-5
Abstract: Nucleation of dendritic primary α(Al) phase with addition of element Ce and Sr in hypoeutectic Al-7%Si-Mg cast alloy was investigated by using differential scanning calorimetry (DSC) and scanning electron microscopy. DSC results were used to calculate the activation energy and nucleation work of primary α(Al) phase. The results show that the values of activation energy and nucleation work are decreased and the nucleation frequency is increased with the additions of Ce and Sr to the alloys. Moreover, the grain size of dendritic α(Al) phase is well refined, and the nucleation temperatures of primary α(Al) dendrites are decreased with the additions of Ce and Sr. The effects of elements Ce and Sr additions on kinetic nucleation of primary α(Al) phases were also discussed in hypoeutectic Al-7%Si-Mg cast alloy.


