Trans. Nonferrous Met. Soc. China 24(2014) 1179-1186
Selective recovery of lead from zinc oxide dust with alkaline Na2EDTA solution
Qing LIU, Sheng-hai YANG, Yong-ming CHEN, Jing HE, Hao-tian XUE
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 13 January 2012; accepted 25 September 2012
Abstract:
The selective recovery of lead from the zinc oxide dust using an alkaline Na2EDTA solution was investigated. The effects of temperature, leaching time, Na2EDTA concentration and initial NaOH concentration on the leaching rates of lead and zinc were studied. The following optimized leaching conditions were obtained: liquid-to-solid ratio 5:1 mL/g, stirring speed 650 r/min, Na2EDTA concentration 0.12 mol/L, initial NaOH concentration 0.5 mol/L, leaching temperature 70 °C, leaching time 120 min. Under the optimized conditions, the average leaching rates of lead, zinc, fluoride and chloride are 89.92%, 0.94%, 62.84% and 90.02%, respectively. The filtrate was used to electrowin lead powders. The average current efficiency of electrowinning is about 93% and lead content is higher than 98% under the conditions of temperature of 60 °C, current density of 200 A/m2, H3PO4 concentration of 1.5 g/L, and lead ion concentration of above 5 g/L. The consumption of Na2EDTA and the direct current are about respectively 0.218 kg and 0.958 kW·h for per kilogram of lead powder.
Key words:
lead; zinc oxide dust; alkaline Na2EDTA solution; electrowinning; leaching;
1 Introduction
The sediment soot zinc oxide dust (ZOD) is produced in the process of fuming pyrometallurgical slag from zinc and lead smelting. The dust contains 30%-65% Zn, 9%-25% Pb, and high content impurities of fluorine, chlorine, arsenic and antimony. About 1×106 t ZOD accumulates annually due to lead and zinc plants operation in China [1]. Due to its high metal content and environmental impact, ZOD is regarded as a secondary source of zinc rather than as a waste. The ZOD cannot be used directly for zinc hydrometallurgy, and must be pretreated for corrosion problems using electrolysis [2]. In 1999, Big River Zinc Co., installed a zinc oxide washing and recovering plant to minimize the impact of halides on its electrolysis circuit [3]. However, this washing plant produced a large amount of wastewater.
Recently, extensive studies have been carried out on the treatment of ZOD by pyrometallurgical or hydrometallurgical processes. These studies have found pyrometallurgical processes to be less eco-friendly due to its environmental pollution and high energy consumption [4,5]. Therefore, the research has focused on different hydrometallurgical processes for the recovery of zinc and lead from the ZOD. Many researches have been done using hot sulfuric acid to extract zinc from ZOD [6-8]. Lead is enriched into a semi-soluble PbSO4 residue during a H2SO4 leaching stage. However, the residue is not suitable for lead smelting because it contains low lead content [9]. These stockpile residues cause serious environmental pollution due to the presence of toxic elements, such as lead and arsenic. In order to reduce the environmental pollution as well as create a new source, many new methods have been proposed, such as the flotation [10] and chemical conversion of PbSO4 into more easily treatable compounds like PbCl2 [11], PbCO3 [12], or PbS [5,9]. Besides the H2SO4 leaching techniques mentioned above, other leaching agents such as HCl, CH3COOH, and NaOH have also been reported [13,14]. A disadvantage of these methods is that both lead and zinc dissolve at the same time, which needs an additional step to separate them.
Recent decades have witnessed a rapid development of various leaching technologies for the remediation of heavy metals from contaminated soils. Ethylenediamine- tetraacetic acid (H2EDTA), discovered by BLAYLOCK et al [15] and WU et al [16], shows exceptional promise due to its strong complex ability and relatively low cost compared with other chelants. Compared with other chelating agents, EDTA2- used in soil washing achieves better heavy-metal removal [17]. PETER and SHEM [18] found that EDTA2- can substantially increase the release of heavy metals from contaminated soils, and overall removal efficiency of 97.2% was achieved after a six-stage washing with 0.05 mol/L fresh EDTA2- solution. However, the high cost and the generation of large volumes of wastewater containing metal-EDTA complexes hampered its widespread use in the remediation of metal-contaminated soils. Therefore, the main concern is to find a method of recovering metals, in which the complexing agent can be reused. Several EDTA recycling methods such as cementing Pb with magnesium metal or bimetallic mixtures of magnesium [19], electrochemical separation of Pb2+ and EDTA2- using an Al anode [20], were studied on a laboratory scale. The electrochemical method is a simple and efficient method for the treatment of wastewater, but forms negatively charged Al hydroxides at the anode. Above all, EDTA2- extracts zinc and lead removes into solution simultaneously under weak acid conditions. Furthermore, there is no practical and commercially available method to recycle EDTA2-.
In this work, a new process, involving selective leaching and elelctrowinning, to separate lead from ZOD was proposed. Alkaline Na2EDTA solution was introduced as a complexing agent to selectively extract Pb from ZOD, and lead powders were selectively electrodeposited from the filtrate. The spent electrolyte adding Na2EDTA and solid NaOH was recycled to leach fresh ZOD. The removal rates of fluoride and chloride from the ZOD were also investigated.
2 Experimental
2.1 Raw materials
The ZOD used came from Chenzhou Yuteng Chemical Industry Co., Ltd., Hunan Province, China. It was produced by processing lead slag from a blast furnace in a fuming furnace. The chemical composition of the ZOD is shown in Table 1.
Table 1 Chemical composition of zinc oxide dusts (mass fraction, %)
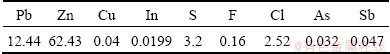
From Table 1, it can be seen that the contents of chloride and fluoride are high. It cannot be used directly in a hydrometallurgical zinc plant because the relatively high contents of chloride and fluoride pose malignant corrosion problems during electrolysis. Leaching agents were prepared using analytical reagent of Na2EDTA, NaOH and distilled water.
2.2 Experimental procedure
Leaching experiments were carried out in a 2 L plat-bottomed flask, with three necked tops for sample extraction. A mechanical stirrer and a mercury thermometer were placed in the solution. The temperature in the flask was adjusted by thermostatically controlling to ±0.5 °C. All the tests were conducted at temperatures between 30 °C and 90 °C. Agitation was provided by a mechanical stirrer, and a stirring speed was fixed at 650 r/min. For each test, 1.5 L solution containing desired concentrations of Na2EDTA and NaOH was added to the leaching reactor. After the temperature attained a desired value, a 300 g ZOD sample was added to the solution. 5 mL of slurry was extracted at appropriate time intervals. After being quickly filtered, 1.0 mL of filtrate was then diluted into 1 L for lead and zinc analysis. The hot slurry was filtered with Whatman41 filter paper in a Bticker funnel after each leaching experiment. The residue was weighed after being dried at 110 °C for 24 h and then subjected to analysis. The mixed filtrate containing 21.32 g/L Pb, 2.0 g/L Zn, and 0.12 mol/L Na2EDTA was used as the electrolyte. The electrolytic cell used a Pb-Ag alloy (1.0%Ag) as an anode, and a stainless steel plate as the cathode, respectively. One side of an anode and a cathode was used; the other side was coated with AB glue. The overall cathode surface was 12 cm×6 cm and the surface area ratio between the cathode and the anode was set at 1.2:1. The electrowinning experiments were carried out in a 2 L glass reaction vessel equipped with a mechanical stirrer fixed at 10 r/min. A thermostat was used for maintaining the desired electrolyte temperature. 1.0 mL of electrolytic solution sample was withdrawn from the electrolytic cell at selected time intervals and diluted to 1 L with distilled water. After electrowinning, the collected lead powders were analyzed and the spent electrolyte recycled back to the leaching stage.
2.3 Analysis methods
The elemental contents of lead and zinc in the ZOD and the residues were analyzed by EDTA titration. Other metal elements such as copper, indium, and antimony were analyzed using an atomic absorption spectrophotometer (AAS-TAS990, Beijing Purkinje General Instrument Co., Ltd., China). Chlorine and fluoride were measured with a spectrophotometer (JK-VS-722G visible spectrophotometer, Shanghai Jingke Scientific Instrument Co., Ltd., China). Sulfur was measured with a sulfur and carbon analyzer (LECO SC-444, Leco Co., Ltd., US).
The zinc and lead in the solution as well as the lead in the leached residues were analyzed by an AAS. The current efficiency (CE) was calculated from the decrease of zinc and lead concentrations in electrolytic solution. The concentrations of EDTA in filtrates and electrolytic solutions were determined spectrophotometrically according to the procedure outlined by HAMANO et al [21]. The pH measurements were carried out with a pHS-3C digital pH meter (LEICI pHS-3C, Shanghai Leici Device Works, China).
3 Results and discussion
3.1 Effect of parameters on leaching rates of lead and zinc
3.1.1 Effect of leaching time
The leaching intervals were ranged from 15 to 180 min. The effects of leaching time on the leaching rates of lead and zinc are shown in Fig. 1.
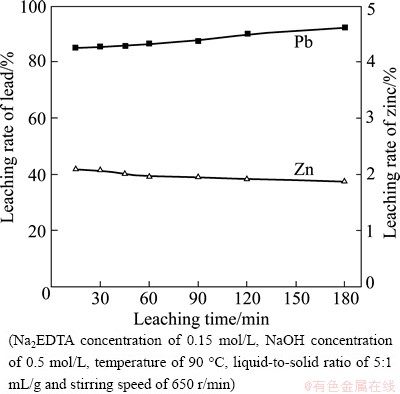
Fig. 1 Effect of leaching time on leaching rates of lead and zinc
Figure 1 shows that the leaching rate of lead increases slightly, but with a slower growth rate as time goes on, whereas the leaching rate of zinc decreases slowly. The leaching rates of lead and zinc only reach 92.10% and 1.88% at the end of 180 min, starting from 85.02% and 2.10% at the beginning of 15 min, respectively. This demonstrates that prolonging the leaching time has little influence on the leaching rates of lead and zinc. So, the suggested leaching time is set at 120 min for lower energy consumption and higher productivity.
3.1.2 Effect of initial Na2EDTA concentration
EDTA can form coordinate chemical bonds with most divalent metals (complexes) in a 1:1 molar ratio and facilitate their solubilization from the solid into the leaching solution. The effects of Na2EDTA concentration on the leaching rates of lead and zinc were investigated at Na 2EDTA concentrations ranging from 0.1 to 0.25 mol/L. The results are shown in Fig. 2. It can be seen from Fig. 2 that the leaching rate of lead increases gradually as Na2EDTA concentration increases. On the other hand, the leaching rate of zinc increases significantly. This may be due to that the lead concentration is about 0.12 mol/L if the lead oxide in the ZOD dissolves completely. The EDTA2- ions used as ligands bind with lead ions to form stable complexes in a 1:1 molar ratio in an alkaline Na2EDTA solution. EDTA2- will not bind with zinc oxide if lead oxide is present in the ZOD due to the lower EDTA2- affinity for Zn2+ than Pb2+, as seen in Eq. (1).
Zn-EDTA+PbO+H2O=Pb-EDTA+Zn(OH)2 (1)
However, once the concentration of Na2EDTA surpasses a certain point, excessive EDTA2- will bind with zinc, which leads to substantial increase in zinc concentration. With this information, the appropriate concentration of Na2EDTA is chosen to be 0.12 mol/L.

Fig. 2 Effect of Na2EDTA concentration on leaching rates of lead and zinc
3.1.3 Effect of NaOH concentration
NaOH concentration is the most complicated factor in lead and zinc extraction. When NaOH concentration varies from 0 to 1.2 mol/L, the effects of NaOH concentration on the leaching rates of lead and zinc are shown in Fig. 3.
Figure 3 shows that the effect of NaOH concentration on the leaching rates of lead and zinc can be divided into two stages: 1) When the initial NaOH concentration increases from 0 to 0.2 mol/L, the leaching rate of lead increases significantly, and 2) when the initial NaOH concentration is greater than 0.2 mol/L, the leaching rate of lead changes insignificantly with the increase in NaOH concentration. The leaching rate of zinc initially decreases with increasing NaOH concentration, but remains relatively steady for the concentration above 0.2 mol/L. These behaviors may be caused by sulfates and chloride in the ZOD. NaOH is used to neutralize  (9.6%, mass fraction) and Cl-(2.52%, mass fraction) and further increase the leaching rate of lead, as shown in Fig. 4. Once NaOH concentration surpasses a certain amount, the conditional stability constants for Pb-EDTA and Zn-EDTA complexes will decrease and the amount of zinc present will increase due to the formation of
(9.6%, mass fraction) and Cl-(2.52%, mass fraction) and further increase the leaching rate of lead, as shown in Fig. 4. Once NaOH concentration surpasses a certain amount, the conditional stability constants for Pb-EDTA and Zn-EDTA complexes will decrease and the amount of zinc present will increase due to the formation of  complexes. However, considering that the addition of NaOH has the least effect on the leaching rate of lead according to the largest difference of leaching rates of zinc and lead in Fig. 3, the appropriate NaOH concentration is chosen to be 0.5 mol/L.
complexes. However, considering that the addition of NaOH has the least effect on the leaching rate of lead according to the largest difference of leaching rates of zinc and lead in Fig. 3, the appropriate NaOH concentration is chosen to be 0.5 mol/L.
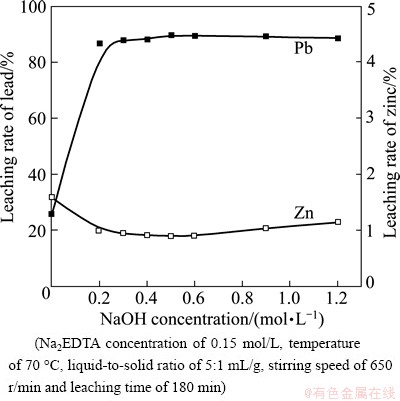
Fig. 3 Effect of NaOH concentration on leaching rates of lead and zinc
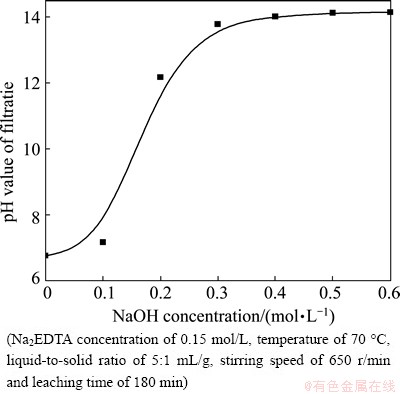
Fig. 4 Effect of NaOH concentration on pH value of filtrate
3.1.4 Effect of temperature
To determine the effect of temperature on the leaching rates of lead and zinc, leaching experiments were conducted at temperatures ranging from 30 to 90 °C. The effect of temperature on the leaching rates of lead and zinc is shown in Fig. 5. The results indicate that the leaching rate of lead increases significantly from 82.54% to 89.98%, when the reaction temperature increases from 30 to 70 °C at 120 min. However, in comparison with the evident variation of the leaching rate of lead, the leaching rate of zinc decreases slightly, from 1.99% (2.48 g/L) to 1.79% (2.23 g/L), within the same temperature variation. The maximum leaching rate of lead is 92.1% at 90 °C for 3 h, and the minimum leaching rate of zinc is 1.79% at 70 °C for 3 h. The results demonstrate that the leaching of lead and zinc is not sensitive to temperature, and considering the effect of temperature on the DC consumption of electrowinning lead, the optimized temperature is chosen to be 70 °C.
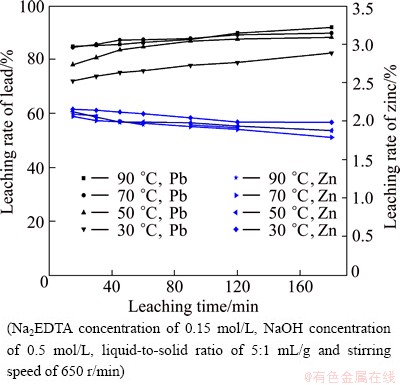
Fig. 5 Effect of temperature on leaching rates of lead and zinc
3.1.5 Integrated leaching experiments
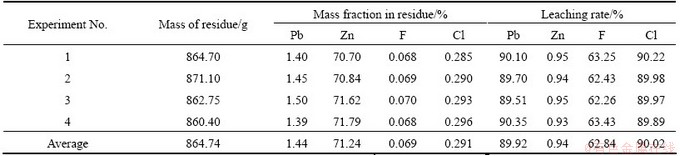
Based on the above mentioned single-factor experiments, four integrated experiments were conducted with a 5 L leaching reagent and the optimized leaching conditions of Na2EDTA concentration of 0.12 mol/L, NaOH concentration of 0.5 mol/L, liquid-to-solid ratio of 5 mL/g, stirring speed of 650 r/min, temperature of 70 °C, and leaching time of 2 h. The results of these integrated experiments are shown in Table 2. It can be seen that the average leaching rates of lead and zinc are 89.92% and 0.94%, respectively. The results are highly repeatable and lead and zinc are easily separable.
3.2 Electrowinning
3.2.1 Effect of H3PO4 addition on anodic current efficiency
The equation modeling parasitic PbO2 formation on the anode during electrowinning is shown as follows:
Pb-EDTA+2H2O-2e=PbO2+2H++H2EDTA (2)
Table 2 Results of integrated experiments
The deposition of PbO2 on the anode has many disadvantages, including impeding the oxygen evolution, increasing the anodic potential, and raising the electrical power consumption. It was reported that phosphorus compounds added to the electrolyte could suppress PbO2 electrodeposition [22]. To examine the effect of H3PO4 addition on the anodic current efficiency, H3PO4 was added at 0, 1.0, 1.25, 1.5, 1.75, 2.0, 3.0 g/L, respectively. The anodic current efficiency (hACE) can be expressed as
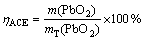 (3)
(3)
where mT(PbO2) represents the theoretical mass of PbO2, and m(PbO2) represents the PbO2 mass deposited on a graphite anode. m(PbO2) can be expressed as
m(PbO2) =AIt
where A represents the electrochemical equivalent of PbO2, I represents current, and t represents electrodeposition time.
The effect of H3PO4 addition on the anodic current efficiency is shown in Fig. 6. Figure 6 shows that in the absence of H3PO4 the anodic current efficiency of the PbO2 electrodeposition is 54%. With the increase of H3PO4 concentration, the anodic current efficiency decreases rapidly and then increases slowly. The optimal H3PO4 concentration is 1.5 g/L.
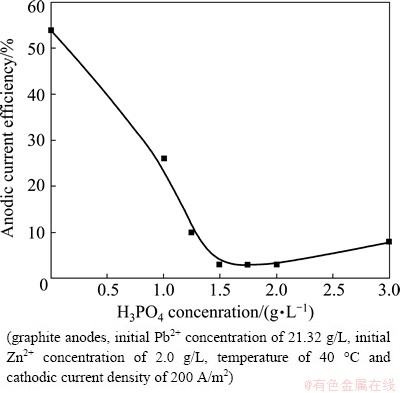
Fig. 6 Effect of H3PO4 addition on anodic current efficiency
3.2.2 Effect of Pb2+ concentration on cathode efficiency
The variation of Pb2+ concentration and cathode efficiency with electrolysis time is shown in Fig. 7. Figure 7 reveals that at high Pb2+ concentrations, the depletion rate of lead is approximately linear and tails off as the concentration of lead decreases to about 5 g/L. After this the concentration decays near exponentially following the well-known expression for a reactor with a diffusion-limited reaction [23]. There is almost no change in zinc concentration. This experiment shows that continuous removal of lead from the electrolyte, down to a concentration of about 5 g/L, is achieved with current efficiency of higher than 93% and lead purity higher than 98%. Below this concentration the current efficiency decreases dramatically. Therefore, the appropriate electrolysis time is determined when the concentration of lead reaches about 5.0 g/L.
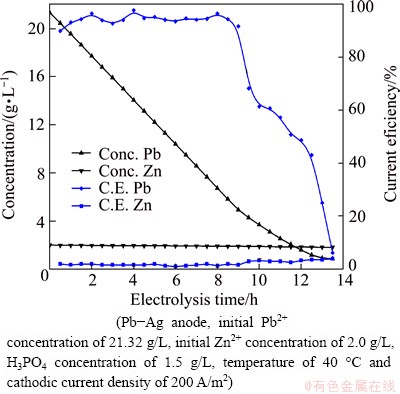
Fig. 7 Variation of metal ion concentration and current efficiency with electrolysis time
3.2.3 Effect of current density on cathode efficiency
The variation in cathodic current density during lead electrowinning was studied in the range from 180 to 220 A/m2 and its effects on the cell voltage, power consumption and current efficiency are shown in Figs. 8 and 9, respectively. The results indicate that both cell voltage and power consumption during lead electrowinning increase with the increase of current density. The increase in cell voltage may be attributed to the increase in both cathodic and anodic polarizations at high current density. When the Pb2+ concentration decreases from 21.32 to 5.0 g/L, the average cathode efficiencies at current densities of 180, 200 and 220 A/m2 are 96.92%, 94.28% and 90.03%, respectively. 200 A/m2 was chosen as the optimal current density for lower power consumption and higher productivity.
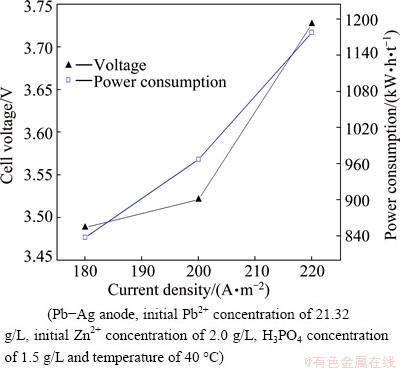
Fig. 8 Effect of current density on cell voltage and power consumption
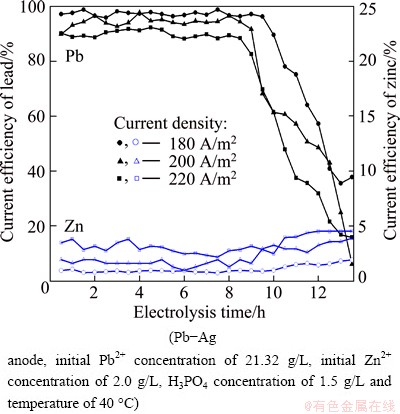
Fig. 9 Effect of current density on current efficiencies
3.2.4 Effect of temperature on cathode efficiency
The electrodeposition of lead from solutions containing Pb2+ and Zn2+ was studied at 30, 40, 50 and 60 °C, respectively. The effects of temperature on the cell voltage, power consumption, and cathode efficiency are shown in Figs. 10 and 11, respectively. From Fig. 10, it can be seen that cell voltage decreases with increasing the bath temperature. A significant decrease in power consumption is observed with an increase in temperature. Power consumption, however, decreases almost linearly with increasing bath temperature. Current efficiency holds at about 94% in the studied temperature range. High temperatures are found to improve the quality of deposition due to lower current efficiencies of zinc. These results show that the most effective temperature is 60 °C, which has the benefit of preparing the sample for electrolysis directly after leaching.
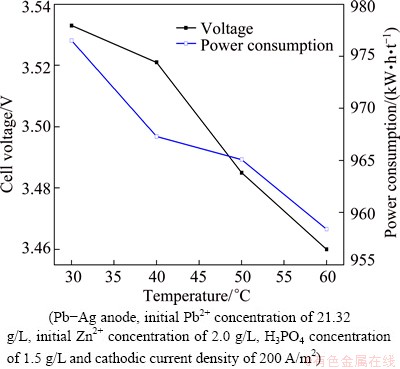
Fig. 10 Effect of temperature on cell voltage and power consumption
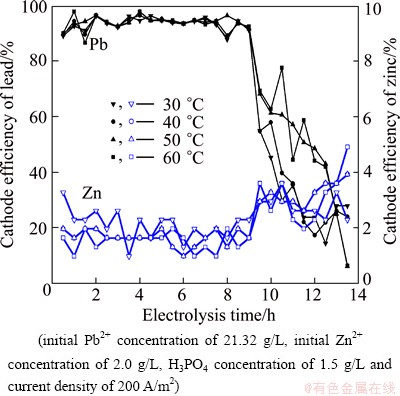
Fig. 11 Effect of temperature on cathode efficiencies of lead and zinc
3.3 Cycling experiments
In order to reduce the chemical reagent consumption and to decrease the production of spent electrolytes containing lead, NaOH and Na2EDTA, the spent electrolyte should be reused to leach fresh ZOD. Based on the previously mentioned experiments, the optimal leaching conditions were chosen as follows:
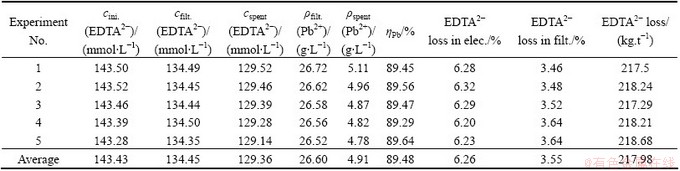
Table 3 Leaching and electrodeposition results after 5 cycles
Na2EDTA concentration 0.12 mol/L, NaOH concentration 0.5 mol/L, liquid-to-solid ratio 5:1 mL/g, stirring speed 650 r/min, temperature 70 °C, leaching time 120 min. In the cycling experiments, the spent electrolyte with a certain dose of Na2EDTA and NaOH was used to leach fresh ZOD. The optimal electrodeposition conditions were chosen as follows: temperature 60 °C, current density 200 A/m2, H3PO4 concentration 1.5 g/L, and lead ion concentration higher than 5 g/L. During the electrolysis, the pH value decreases due to the H+ generation, as modeled in Eq. (4):
Me(EDTA)+2OH-=Me(s)+EDTA2-+O2+2H+ (4)
The pH drop problem could be overcome with the addition of solid NaOH. EDTA2- loss will come from two sources: absorbed in the leaching residues and consumed in the anodic reaction. After each cycle, EDTA2- loss in the leaching and electrodeposition period was calculated. The leaching and electrodeposition results after 5 cycles are shown in Table 3.
The average leaching rates lead, zinc, fluoride and chloride are 89.48%, 0.87%, 56.84% and 84.02%, respectively. The increased concentrations of fluoride and chloride in the leaching solution after five cycles are 0.989 and 20.38 g/L, respectively.
About 0.218 kg of Na2EDTA and 0.958 kW·h of direct current (DC) were consumed for per kilogram of lead powder. EDTA2- is mostly absorbed by residues during the ZOD leaching [24]. Some EDTA2- decomposed at the lead electrowinning stage, in which EDTA2- was anodically oxidized into CO2, formaldehyde and ethylendiamine [25].
4 Conclusions
1) According to the results of single-factor experiments, the optimal leaching conditions were obtained as follows: Na2EDTA concentrations 0.12 mol/L, NaOH concentration 0.5 mol/L, temperature 70 °C and time 120 min. Under these optimized conditions, the average leaching rates of lead and zinc are about 90% and 0.94 %, respectively.
2) Continuous removal of lead from the electrolyte, down to around 5 g/L, is accompanied by an average current efficiency of higher than 94% and lead purity higher than 98% under the conditions of Pb-Ag anode, H3PO4 concentration of 1.5 g/L, temperature of 60 °C and current density of 200 A/m2. Lead is recovered from the filtrate relatively efficiently (>95%), mostly through the electrodeposition on the cathode.
3) After running for five cycles, about 0.218 kg of Na2EDTA and 0.958 kW·h of direct current were consumed per kilogram of lead powder. Fluoride and chloride are removed with 56.84% and 84.02%, respectively, which is convenient for the further recovery of zinc. Most EDTA2- is absorbed into the ZnO residues, though some EDTA2- decomposes on the anode.
4) Using alkaline EDTA solution to process ZOD has the advantages of a shorter flowsheet, higher efficiency, better stability and better selectivity on lead.
References
[1] CHEN S M, CHENG D K, LI Y H, ZHOU J M. Study of comprehensive recovery of high-contents as Waltz zinc oxide [J]. Non-ferrous Mining and Metallurgy, 2001, 17(5): 29-31. (in Chinese)
[2] GUAN Ya-jun. Technical study on producing electrolytic zinc from secondary zinc oxide of lead fuming furnace [J]. China Nonferrous Metallurgy, 2006, 6(8): 32-35. (in Chinese)
[3] CHABOT S S, JAMES S E. Treatment of secondary zinc oxides for use in an electrolytic zinc plant [C]//Lead-Zinc 2000. DUTRIZAC J E, GONZALEZ J A, HENKE D M, JAMES S E, SIEGMUND A H J. Washington D.C.: TMS (The Minerals, Metals Materials Society), 2000: 739-750.
[4] DORONIN I E, SVYAZHIN A G. Commercial methods of recycling dust from steelmaking [J]. Metallurgist, 2011, 54(9-10): 673-681.
[5] LIN M. Alkaline leaching of metal melting industry wastes and separation of zinc and lead in the leach solution [J]. Journal of Environmental Sciences, 2000, 12(4): 452-457.
[6] OUSTADAKIS P, TSAKIRIDIS P E, KATSIAPI A, AGATZINI-LEONARDOU S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part I: Characterization and leaching by diluted sulphuric acid [J]. Journal of Hazardous Materials, 2010, 179(1): 1-7.
[7] CRUELLS M, ROCA A, NUNEZ C. Electric-arc furnace flue dusts: Characterization and leaching with sulfuric-acid [J]. Hydrometallurgy, 1992, 31(3): 213-231.
[8] ELGERSMA F, KAMST G F, WITKAMP G J, van ROSMALEN G M. Acidic dissolution of zinc ferrite [J]. Hydrometallurgy, 1992, 29(1): 173-189.
[9] RAGHAVAN R, MOHANAN P K, SWARNKAR S R. Hydrometallurgical processing of lead-bearing materials for the recovery of lead and silver as lead concentrate and lead metal [J]. Hydrometallurgy, 2000, 58(2): 103-116.
[10] RASHCHI F, DASHTI A, ARABPOUR-YAZDI M, ABDIZADEH H. Anglesite flotation: A study for lead recovery from zinc leach residue [J]. Minerals Engineering, 2005, 18(2): 205-212.
[11] TURAN M D,  Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75(1): 169-176.
Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75(1): 169-176.
[12] ARAI K, TOGURAI J M. Leaching of lead sulfate in sodium-carbonate solution [J]. Hydrometallurgy, 1984, 12(1): 49-59.
[13] NAGIB S, INOUE K. Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching [J]. Hydrometallurgy, 2000, 56(3): 269-292.
[14] HIROYOSHI N, KISHIMOTO N, TSUNEKAWA M, HIRAJIMA T, MISHIMA H. Recovery of metals from melting furnace fly ash by ammonia/chloride leaching [C]//Proceedings of the 29th Spring Annual Meeting of The Mining and Materials Processing Institute of Japan. 2004: 31-32. (in Japanese)
[15] BLAYLOCK M J, SALT D E, DUSHENKOV S, ZAKHAROVA O, GUSSAMAN C, KAPULNIK Y, ENSLEY B D, RASKIN I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents [J]. Environmental Science and Technology, 1997, 31(3): 860-865.
[16] WU J, HSU F C, CUNNINGHAM S D. Chelate-assisted Pb phytoextraction: Pb availability, uptake and translocation constraints [J]. Environmental Science and Technology, 1999, 33(11): 1898-1904.
[17] PETERS R W. Chelating extraction of heavy metals from contaminated soils [J]. Journal of Hazardous Materials, 1999, 66(1): 151-210.
[18] PETER R W, SHEM L D. Use of chelating agents for remediation of heavy metal contaminated soil [C]//Environmental Remediation- Removing Organic and Metal Ion Pollutants, Symp. Series, Washington D C: American Chemical Society, 1992: 70-84.
[19] AGER P, MARSHALL W D. The removal of metals and release of EDTA from pulp wash water [J]. Journal of Wood Chemistry and Technology, 2001, 21(4): 413-425.
[20] POCIECHA M, LESTAN D, DOMEN L. Electrochemical EDTA recycling with sacrificial Al anode for remediation of Pb contaminated soil [J]. Environmental Pollution, 2010, 158(8): 2710-2715.
[21] HAMANO T, MITSUHASHI Y, KOJMA N, AOKI N, SHIBATA M, ITO Y, OJI Y. Sensitive spectrophotometric method for the determination of ethyl-enediaminetetraacetic acid in foods [J]. Analyst, 1993, 118(7): 909-912.
[22] TSVETAN D, STEPHAN R. Processes during the electrorefining and electrowinning of lead [J]. Hydrometallurgy, 1996, 40(3): 277-291.
[23] SCOTT K. A consideration of circulating bed electrodes for the recovery of metal from dilute solutions [J]. Journal of Applied Electrochemistry, 1988, 18(4): 504-510.
[24] NOWACK B, SIGG L. Adsorption of EDTA and metal-EDTA complexes to goethite [J]. Journal of Colloid and Interface Science, 1996, 177(1): 106-121.
[25] JOHNSON J W, JIANG H W, HANNA S B, JAMES W J. Anodic oxidation of ethylenediaminetetraacetic acid on Pt in acid sulphate solution [J]. Journal of the Electrochemical Society, 1972, 119(5): 574-580.
用碱性Na2EDTA溶液从次氧化锌烟灰中选择性回收铅
刘 青,杨声海,陈永明,何 静,薛浩天
中南大学 冶金与环境学院,长沙 410083
摘 要:采用碱性Na2EDTA溶液从次氧化锌烟灰中回收铅。探讨温度、浸出时间、Na2EDTA浓度和起始NaOH浓度对铅、锌浸出率的影响。得到最优实验条件如下:液固比5:1 mL/g、搅拌速度650 r/min、Na2EDTA浓度0.12 mol/L、NaOH初始浓度0.5 mol/L、温度70 °C、浸出时间120 min。在最优实验条件下,铅、锌、氟和氯的平均浸出率分别为89.92%、0.94%、62.84% 和90.02%。浸出液用于电沉积铅粉。在温度为60 °C、电流密度为200 A/m2、H3PO4 浓度为1.5 g/L、铅离子浓度不低于5 g/L时,电沉积铅粉平均电流效率大约为93%,阴极铅纯度高于98%。电沉积1 kg铅粉大约消耗0.218 kg Na2EDTA和0.958 kW·h电能。
关键词:铅;次氧化锌;碱性Na2EDTA;电沉积;浸出
(Edited by Wei-ping CHEN)
Foundation item: Project (50974138) supported by the National Natural Science Foundation of China; Project (2010ssxt158) supported by Graduate Student Innovation Foundation of Central South University, China
Corresponding author: Sheng-hai YANG; Tel: +86-731-88830470; E-mail: 75894838@163.com
DOI: 10.1016/S1003-6326(14)63177-0
Abstract: The selective recovery of lead from the zinc oxide dust using an alkaline Na2EDTA solution was investigated. The effects of temperature, leaching time, Na2EDTA concentration and initial NaOH concentration on the leaching rates of lead and zinc were studied. The following optimized leaching conditions were obtained: liquid-to-solid ratio 5:1 mL/g, stirring speed 650 r/min, Na2EDTA concentration 0.12 mol/L, initial NaOH concentration 0.5 mol/L, leaching temperature 70 °C, leaching time 120 min. Under the optimized conditions, the average leaching rates of lead, zinc, fluoride and chloride are 89.92%, 0.94%, 62.84% and 90.02%, respectively. The filtrate was used to electrowin lead powders. The average current efficiency of electrowinning is about 93% and lead content is higher than 98% under the conditions of temperature of 60 °C, current density of 200 A/m2, H3PO4 concentration of 1.5 g/L, and lead ion concentration of above 5 g/L. The consumption of Na2EDTA and the direct current are about respectively 0.218 kg and 0.958 kW·h for per kilogram of lead powder.


