Trans. Nonferrous Met. Soc. China 24(2014) 3810-3817
Electrodeposition of Cu coating with high corrosion resistance on Mg-3.0Nd-0.2Zn-0.4Zr magnesium alloy
Shao-hua WANG1, Xing-wu GUO1,2, Can SUN1, Jia GONG1,2, Li-ming PENG1,2, Wen-jiang DING1,2
1. National Engineering Research Center of Light Alloy Net Forming, Shanghai Jiao Tong University, Shanghai 200240, China;
2. State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200240, China
Received 17 October 2013; accepted 9 November 2014
Abstract:
To improve the corrosion resistance, electrodeposition of Cu coating on Mg-3.0Nd-0.2Zn-0.4Zr (mass fraction, % NZ30K) magnesium alloy via an appropriate pretreatment was investigated. The surface morphologies, compositions and microstructures of the pretreated films and Cu coating were characterized in detail. The results show that the activation film consists of fluoride and phosphates and Zn immersion film forms preferentially on the eutectic compound Mg12Nd phase region. A smooth, uniform and dense Cu coating is successfully obtained. Potentiodynamic polarization tests reveal that Cu coating can greatly improve the corrosion resistance of NZ30K magnesium alloy. Open circuit potential (OCP) and electrochemical impedance spectroscopy (EIS) tests during long-term immersion further demonstrate that Cu coating can provide an effective protection for NZ30K magnesium alloy from corrosion up to ~60 h, due to its dense structure and a stable passive film formed. In addition, Cu coating exhibits good adhesion to substrate as confirmed by thermal shock test.
Key words:
NZ30K magnesium alloy; pretreatment; Cu electrodeposition; corrosion resistance; adhesion;
1 Introduction
As one of the lightest engineering materials, magnesium alloys have demonstrated a wide range of industrial applications, owing to their high specific strength, low density and excellent machinability [1]. However, the poor corrosion resistance has become an obstacle to their widespread application in larger scale [2,3]. Coating of magnesium alloys is the most effective way to improve their corrosion resistance [4,5]. Among them, electrodeposition has been considered as a convenient and versatile process due to its low cost, high efficiency, simple operation and decorative appearance [6-8].
Electrodeposition of Cu coating is often carried out as an under-layer on magnesium alloy to subsequently electroplate other metal coatings (i.e., Ni and Cr). Thus far, electrodeposition of Cu coatings on magnesium alloys via suitable pretreatments has extensively investigated [9-12]. Prior to electrodeposition, an appropriate pretreatment is indispensable to obtain a Cu coating with high corrosion resistance and good adhesion on magnesium alloys, due to their high chemical activity and asymmetrically distributed potentials on the substrate surface [1,13]. ZHANG et al [9] developed a pretreatment including HF pickling-activation and Zn immersion prior to Cu electroplating on AZ91D magnesium alloy. HUANG et al [10,11] proposed galvanostatic etching to electroplate Cu coatings on pure Mg, AZ31 and AZ61 magnesium alloys. More recently, electrodeposition of Cu coatings on Mg–Li magnesium alloy via acid pickling in CrO3 solution containing a small amount of Fe2+ ions and Zn leaching was also studied by YIN et al [12], and the coatings exhibited corrosion potentials in a range from about -1.25 to -0.64 V (vs SCE), implying their imperfect structures. However, most of the researches focused on the commercial Mg-Al magnesium alloys as substrates, and there was limited literature available related to the electro- deposition and corrosion resistance of Cu coatings on Mg-RE magnesium alloys.
Mg-3.0Nd-0.2Zn-0.4Zr (mass fraction, %, NZ30K) alloy is a kind of newly developed Mg-RE magnesium alloys with moderate strength and good toughness in as-cast condition [14,15]. Previous investigations show that NZ30K magnesium alloy owns great potential application in the fields such as automobile, telecommunications, and aerospace. Therefore, it is considerably useful for electroplating Cu coating on NZ30K magnesium alloy via an appropriate pretreatment to improve the corrosion resistance, and thus to spread its practical application.
In this work, a highly corrosion-resistant Cu coating was electrodeposited on NZ30K magnesium alloy via an appropriate pretreatment. The surface morphologies, compositions, and microstructures of the pretreatment films and Cu coating were investigated in detail. The corrosion behavior of Cu coating was evaluated by electrochemical measurements including potentio- dynamic polarization, open circuit potential (OCP) and electrochemical impedance spectroscopy (EIS) in 3.5% NaCl (mass fraction) solution. And also, the adhesion strength of Cu coating to NZ30K substrate was examined by thermal shock test.
2 Experimental
2.1 Materials and solutions
As-cast NZ30K magnesium alloy (3.02% Nd, 0.26% Zn, 0.41% Zr and Mg balance, mass fraction) was used as the substrate. Specimens were cut into disks with dimension of d12 mm×5 mm, mechanically polished using 1200 grit SiC papers, cleaned with deionized water, and then degreased ultrasonically in acetone for 10 min. All the chemicals prepared for the pretreatment and electroplating solutions were of analytical reagent grade and used as received without further purification.
2.2 Pretreatments and electrodeposition of Cu coating
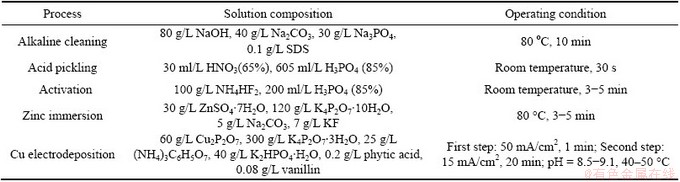
The pretreatment steps of NZ30K magnesium alloy were as follows: alkaline cleaning, acid pickling, activation, Zn immersion, and the specimens were rinsed with deionized water after each step. Electrodeposition of Cu coating on the Zn-pretreated NZ30K substrate was carried out using a pulse power supplier, by a two-step galvanostatic method including a strike by 50 mA/cm2 for 1 min, immediately followed by 15 mA/cm2 for 20 min. Two parallel Cu plates were used as anodic electrodes to ensure a homogeneous electrical field around the cathodic electrode. The distance between anodic electrode and cathodic electrode was 20 mm. The detailed process flow, solution compositions and operating conditions are listed in Table 1.
2.3 Characterization
The surface and cross-section morphologies of NZ30K substrate after different pretreatment steps and Cu electrodeposition were observed using field emission scanning electron microscope (FE-SEM, SIRION 200, FEI, America), coupled with energy-dispersive X-ray spectrometer (EDX, INCA, Oxford, UK). The crystalline structures were determined by X-ray diffraction (XRD, D/MAX 2000 V) with a Cu Kα target. XRD was executed in the range of 2θ=20-80° with scanning speed of 4 (°)/min and step of 0.01°. The corrosion resistance of Cu coating was evaluated by electrochemical measurements including open circuit potential (OCP), potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) using a PARSTAT 2273 advanced electrochemical system from Princeton Applied Research. All the electrochemical tests were preformed in 3.5% NaCl solution and carried out at (25±2) °C. A three-electrode electrochemical cell was used, with the specimen as working electrode, a saturated calomel electrode (SCE) as reference electrode and a platinum plate as counter electrode. The specimen area exposed to test solution was 0.5 cm2. Prior to potentiodynamic polarization, the specimen was immersed in the electrolyte for 1 h, allowing the system to be stabilized. The scan rate of potentiodynamic polarization curves was 1 mV/s. EIS tests were conducted at OCP and the measuring frequency was ranged from 105 Hz down to 10–1 Hz, with a sinusoidal signal amplitude of 10 mV. The adhesion strength of Cu coating to NZ30K substrate was examined by thermal shock method according to ASTM B33-04. The specimen was annealed at 150 °C for 1 h, and then quenched in room temperature water. The procedure was recycled for 10 times. Then the specimens were examined for blistering, crinkle and/or breaking off.
Table 1 Process flow, solution compositions and operating condition of pretreatments and Cu electrodeposition on NZ30K substrate
3 Results and discussion
3.1 Pretreatment of NZ30K magnesium alloy
The microstructure of the as-cast NZ30K substrate is mainly composed of α-Mg matrix with an average grain size about 40-50 μm and the eutectic compound Mg12Nd distributes along the α-Mg dendrite boundaries, as shown in Fig. 1(a). EDX analysis in Table 2 reveals that Zn mainly exists in Mg12Nd phase, and Zr is added to refine the grain size of NZ30K magnesium alloy [16]. Such microstructural heterogeneity leads to potential diversity on the substrate, and has an adverse effect on Cu electrodeposition. Hence, an appropriate pretreatment is indispensable to obtain a Cu coating with high corrosion resistance and good adhesion on NZ30K substrate.
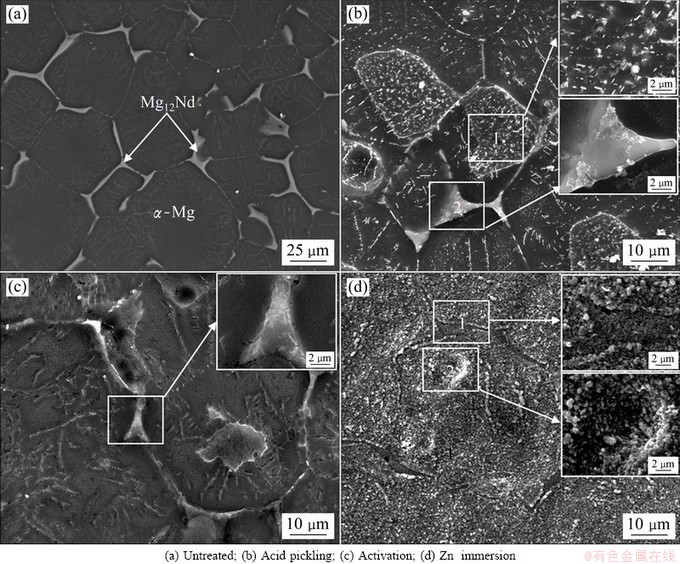
Fig. 1 SEM images of NZ30K substrate after different pretreatment steps
Table 2 EDX results of NZ30K magnesium alloy after different pretreatment steps (mass fraction, %)
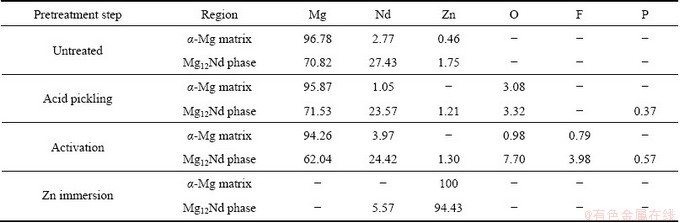
After acid pickling, both α-Mg matrix and Mg12Nd phase are uniformly corroded, and some cavities are observed along the boundaries between the α-Mg matrix and Mg12Nd phase, thus producing a relatively rough surface (Fig. 1(b)). The formation of these cavities can be attributed to the fierce galvanic corrosion between the α-Mg matrix and Mg12Nd phase when the substrate is immersed in pickling solution. Such rough surface is benefitial for improvement of the adhesion strength between the subsequent coatings to substrate, due to the enhanced mechanical interlocking effect. Moreover, numerous irregular agglomerates are randomly distributed on the surface of α-Mg matrix (spot 1) and Mg12Nd phase (spot 2). The presence of P and O elements in EDX analysis (Table 2) indicates that insoluble Mg3(PO)4 is formed loosely on the surface of NZ30K substrate during acid pickling. After activation, the corrosion products formed via acid pickling are removed in activation solution, and an activation film is formed on the surface of NZ30K substrate (Fig. 1(c)). It is obvious that most surface Mg12Nd phase is covered by irregular clusters (the inset in Fig. 1(c)), which is considerably different from that on α-Mg matrix. EDX further confirms that the F and O contents on Mg12Nd phase are significantly higher than those on α-Mg matrix, suggesting that the activation film consisting of MgF2, NdF3, magnesium oxides/hydroxides and phosphates is preferentially formed on Mg12Nd phase. After Zn immersion, a smooth, uniform and compact Zn film is formed on the entire surface of NZ30K substrate (Fig. 1(d)). The insets with higher magnification and EDX analysis further indicate that there is no obvious diversity of film compactness on Mg12Nd phase and α-Mg matrix. Such uniform and dense Zn film could equalize the potential diversity distribution on the substrate, and effectively decrease the potential difference between the magnesium alloy and the subsequent Cu coating. However, it should be noted that a significant diversity and preferential reduction of Zn film on Mg12Nd phase are observed when Zn immersion time is shorter than 3 min (not presented here). Hence, a suitable Zn immersion time should be controlled to prepare a Cu coating with good adhesion and high corrosion resistance.
Figure 2 presents the typical XRD patterns of NZ30K substrate after different pretreatments and Cu electrodeposition. Figure 2(a) reveals that NZ30K substrate is mainly composed of α-Mg matrix and Mg12Nd phase. After acid pickling (Fig. 2(b)), the intensities of the diffraction peaks corresponding to α-Mg matrix become obviously weaker than those of the untreated NZ30K substrate (Fig. 2(a)), due to the preferential dissolution of α-Mg matrix. After activation (Fig. 2(c)), the diffraction peaks of NZ30K substrate become further weakened, but no peaks ascribed to MgF2 and/or NdF3 are detected, possibly owing to its amorphous structure. After Zn immersion (Fig. 2(d)), four new characteristic diffraction peaks for Zn appear, indicating that a Zn film is formed. After Cu electrodeposition (Fig. 2(e)), the diffraction peaks corresponding to NZ30K substrate almost disappear and three characteristic diffraction peaks for Cu are observed, indicating that a Cu coating is electrodeposited successfully on the surface of NZ30K substrate.
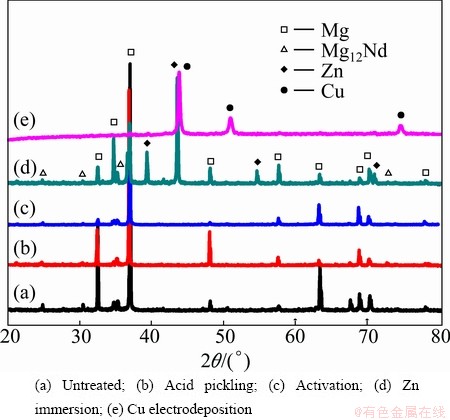
Fig. 2 XRD patterns of NZ30K substrate after different steps
3.2 Electrodeposition of Cu coating
A Cu coating with bright red-brown appearance and metallic luster is electrodeposited on NZ30K substrate via the appropriate pretreatments, as shown in Fig. 3(a). SEM image (Fig. 3(b)) shows that the Cu coating is uniform, dense, with numerous nodular structures. Figure 3(c) indicates that there are no visible holes and defects on the cross-section and no obvious boundary or interface between the Cu coating and Zn film. This suggests a good adhesion of Cu coating to NZ30K substrate. Thermal shock test shows that no blistering, crinkle, breaking off are observed on the surface of Cu coating after 10 cycles (the inset in Fig. 3(d)), further strongly indicating the good adhesion. It should be mentioned that a strike by high deposition current density (50 mA/cm2) for a short time (1 min) could effectively decrease the replacement reaction between Zn film and Cu2+ ions in the plating bath, which is benefitial for improving the adhesion of the subsequent Cu coating to substrate. The composition distribution of Mg, Zn and Cu elements along the line from Cu coating to substrate are also given in Fig. 3(d). The average thickness of Cu coating and Zn film is about 8.5 μm and 3.5 μm, respectively.
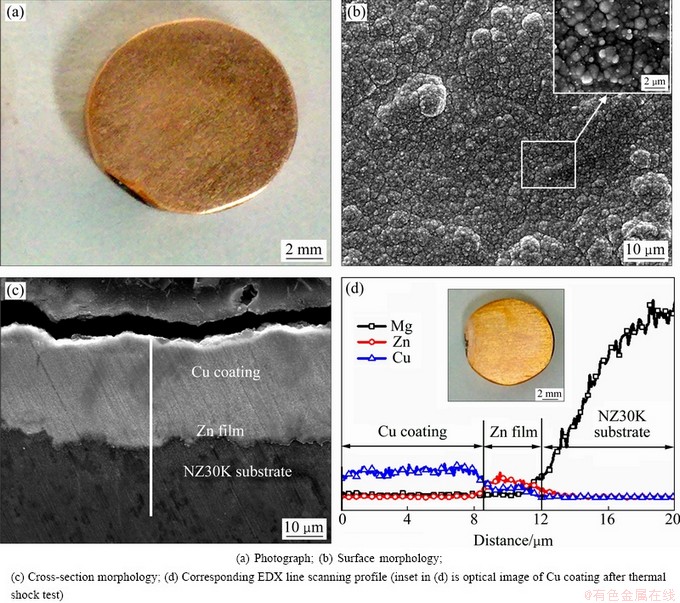
Fig. 3 Photograph and micrograph of Cu coating electrodeposited on NZ30K substrate
3.3 Corrosion behaviors of Cu coating
3.3.1 Potentiodynamic polarization
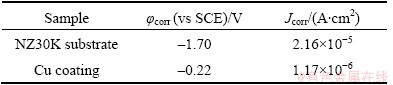
Figure 4 shows the typical potentiodynamic polarization curves of NZ30K substrate and Cu coating in 3.5% NaCl solution. Corrosion characteristics including corrosion potential (φcorr) and corrosion current density (Jcorr) are derived from the potentiodynamic polarization curves by Tafel extrapolation, and are summarized in Table 3. It is clear that Cu coating can greatly improve the corrosion resistance of NZ30K magnesium alloy, with an ennoblement of φcorr from -1.70 to -0.22 V (vs SCE) and an obvious decrease of Jcorr from 2.16×10-5 to 1.17×10-6 A/cm2, due to its dense and pore-free structure.
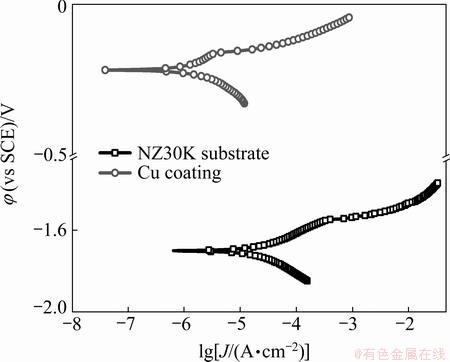
Fig. 4 Potentiodynamic polarization curves of NZ30K substrate and Cu coating in 3.5% NaCl solution
Table 3 Corrosion characteristics of NZ30K substrate and Cu coating summarized from potentiodynamic polarization curves in 3.5% NaCl solution
3.3.2 Long-term immersion test
To further evaluate the long-term corrosion resistance and corrosion mechanism of Cu coating, the changes in OCP and EIS characteristics as a function of immersion time were also carried out in 3.5% NaCl solution.
Figure 5 shows the change in the OCP of Cu coating as a function of immersion time in 3.5% NaCl solution. During the initial 60 h immersion, the OCP maintains steadily in a range from -0.22 to -0.23 V (vs SCE), suggesting that Cu coating can effectively serve as a barrier layer to prevent NZ30K substrate from corrosion. However, the OCP decreases slightly to about -0.39 V after 72 h immersion, then drops dramatically to about -0.80 V after 74 h immersion, and finally to about -1.31 V after 76 h immersion. This indicates that Cu coating starts to deteriorate after 60 h immersion, and totally breaks down after 72 h immersion, owing to the severe galvanic corrosion between Cu coating and NZ30K substrate. A visible deep corrosion pit is observed on the surface of Cu coating after 76 h immersion. Hence, Cu coating can effectively protect NZ30K substrate from corrosion for 60 h in 3.5% NaCl solution.
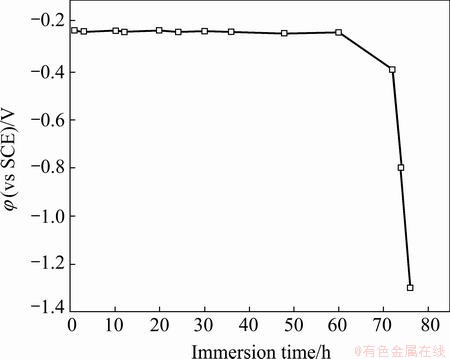
Fig. 5 Change in OCPs of Cu coating as a function of immersion time in 3.5% NaCl solution
Figure 6 shows the EIS plots and fitting results of Cu coating with different immersion time in 3.5% NaCl solution. During the initial 24 h immersion, the Nyquist plots in Fig. 6(a) are characterized with two capacitive loops with gradually increased diameter, and meanwhile the Bode plots in Fig. 6(b) display smoothly higher |Z|f→0 and broader phase degree in the middle frequency region with the increase in immersion time. This suggests that a passive film (corrosion products of Cu coating) is formed on the surface of Cu coating during the initial immersion period. It has been reported that a passive film consisting of CuCl and/or Cu2O is formed on the surface when Cu is immersed in neutral and weakly acidic media containing Cl- ions [17,18]. During 24-60 h immersion, the shape and diameter of EIS plots hardly change, indicating that the passive film is relatively stable during this immersion period and could provide corrosion protection for NZ30K substrate. However, with further prolonging immersion time, the diameter of capacitive loop in Nyquist plots and |Z|f→0 in Bode plots starts to shrink, implying that the passive film begins to deteriorate and the electrolyte penetrates into the Cu coating to interact with magnesium alloy substrate. The diameter of capacitive loop is only about 85 Ω·cm2 after 76 h immersion, demonstrating a total breakdown of Cu coating, owing to the fierce galvanic corrosion between Cu coating and NZ30K substrate. Such changes in EIS data are in good agreement with the analysis of OCP-time curve in Fig. 5.
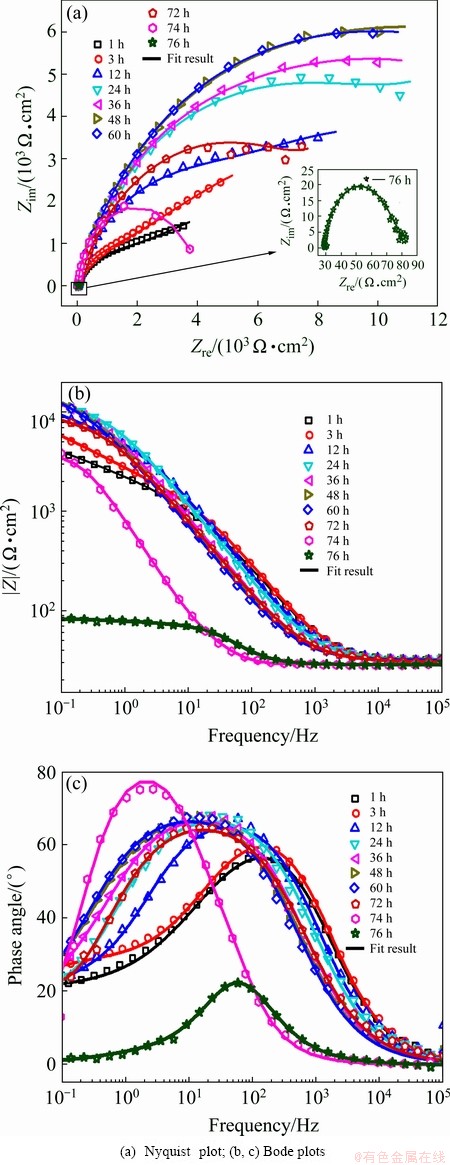
Fig. 6 EIS plots and fitting results of Cu coating with different immersion time in 3.5% NaCl solution
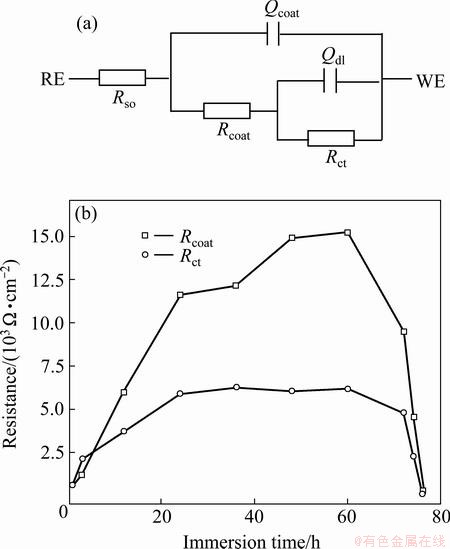
Fig. 7 Equivalent circuit proposed for fitting of EIS plots in Fig. 6(a) and change in Rcoat and Rct as a function of immersion time in 3.5% NaCl solution (b)
An equivalent circuit in Fig. 7(a) is proposed for fitting of EIS plots to further understand the corrosion mechanism of Cu coating on the surface of NZ30K substrate. Rs refers to the solution resistance, Qcoat and Qdl are used instead of the capacitance of coating and the double layer capacitance originated from the electrochemical corrosion activity at alloy surface, respectively. Rcoat represents the resistance of the coating, and Rct is the charge transfer resistance representing the resistance of electron transfer during electrochemical reaction course. A good correspondence between the fitted and measured spectra is obtained, as shown in Fig. 6. The corrosion process of Cu coating can be also described by the changes in the Rcoat and Rct parameters as a function of immersion time, as depicted in Fig. 7(b). Both Rcoat and Rct gradually increase in the initial 24 h immersion due to a growth of passive film formed by the anodic dissolution of Cu coating, then maintain relatively steady during 24-60 h immersion, and finally decrease rapidly after 72 h immersion, owing to the breakdown of Cu coating and galvanic corrosion between Cu coating and NZ30K substrate. These results further confirm that the Cu coating can provide a long-term corrosion protection performance for up to 60 h, which is also consistent with the analysis of OCP-time curve in Fig. 5.
It is known that the electrodeposited Cu coating cannot provide long-term corrosion protection for magnesium alloy due to the existence of pinholes/defects, and thus is often used as an under-layer to electroplate the subsequent metal coatings, i.e., Ni and Cr. However, in the present work, a highly corrosion-resistant Cu coating is successfully electrodeposited on NZ30K magnesium alloy via an appropriate pretreatment, and could effectively protect NZ30K magnesium alloy from corrosion for 60 h in 3.5% NaCl solution, based on the above OCP and EIS characteristics during long-term immersion tests.
4 Conclusions
1) In the pretreatment process, both activation film containing MgF2, NdF3 and/or Mg3(PO)4, and Zn immersion film are formed preferentially on Mg12Nd phase. A uniform and compact Zn immersion film is covered on the entire surface of NZ30K magnesium alloy via an appropriate pretreatment.
2) A smooth, uniform and dense Cu coating with good adhesion to substrate is electrodeposited on NZ30K magnesium alloy, as confirmed by SEM observations and thermal shock test.
3) Potentiodynamic polarization tests in 3.5% NaCl solution indicate that Cu coating can greatly improve the corrosion resistance of NZ30K magnesium alloy, due to its dense and pore-free structure.
4) OCP and EIS characteristics during long-term immersion demonstrate that the Cu coating can protect NZ30K magnesium alloy from corrosion for 60 h. And the corrosion process of Cu coating can be described as follow: (1) a passive film forms in the initial 24 h immersion; (2) the coating is relatively stable during 24-60 h immersion; (3) the coating starts to deteriorate after 60 h immersion, and a visible through-going corrosion pit is observed after 76 h immersion.
Acknowledgments
The helpful assistance to coating analysis from the Instrumental Analysis Center of Shanghai Jiao Tong University (SJTU) is gratefully acknowledged.
References
[1] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys—A critical review [J]. Journal of Alloys and Compounds, 2002, 336(1-2): 88-113.
[2] SONG G L, ATRENS A. Corrosion mechanisms of magnesium alloys [J]. Advanced Engineering Materials, 1999, 1(1): 11-33.
[3] ATRENS A, SONG G L, CAO F Y, SHI Z M, BOWEN P K. Advances in Mg corrosion and research suggestions [J]. Journal of Magnesium and Alloys, 2013, 1(3): 177-200.
[4] ZHANG J F, YAN C W, WANG F H. Electrodeposition of Al-Mn alloy on AZ31B magnesium alloy in molten salts [J]. Applied Surface Science, 2009, 255(9): 4926-4932.
[5] ZHANG J F, ZHANG W, YAN C W, DU K Q, WANG F H. Corrosion behaviors of Zn/Al-Mn alloy composite coatings deposited on magnesium alloy AZ31B (Mg-Al-Zn) [J]. Electrochim Acta, 2009, 55(2): 560-571.
[6] WU H, ZHAO G L, MU J W, LI X S, HE Y. Effects of ultrasonic dispersion on structure of electrodeposited Ni coating on AZ91D magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: s703-s707.
[7] YANG H Y, GUO X W, CHEN X B, BIRBILIS N. A homogenisation pre-treatment for adherent and corrosion-resistant Ni electroplated coatings on Mg-alloy AZ91D [J]. Corrosion Science, 2014, 79: 41-49.
[8] HAJIALI FINI M, AMADEH A. Improvement of wear and corrosion resistance of AZ91 magnesium alloy by applying Ni-SiC nanocomposite coating via pulse electrodeposition [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(10): 2914-2922.
[9] ZHANG S, CAO F H, CHANG L R, ZHENG J J, ZHANG Z, ZHANG J Q, CAO C N. Electrodeposition of high corrosion resistance Cu/Ni-P coating on AZ91D magnesium alloy [J]. Applied Surface Science, 2011, 257(21): 9213-9220.
[10] HUANG C A, LIN C K, YEH Y H. Increasing the wear and corrosion resistance of magnesium alloy (AZ91D) with electrodeposition from eco-friendly copper- and trivalent chromium-plating baths [J]. Surface and Coatings Technology, 2010, 205(1): 139-145.
[11] HUANG C A, WANG T H, WEIRICH T, NEUBERT V. A pretreatment with galvanostatic etching for copper electrodeposition on pure magnesium and magnesium alloys in an alkaline copper-sulfate bath [J]. Electrochimica Acta, 2008, 53(24): 7235-7241.
[12] YIN T T, WU R Z, LENG Z, DU G J, GUO X Y, ZHANG M L, ZHANG J H. The process of electroplating with Cu on the surface of Mg-Li alloy [J]. Surface and Coatings Technology, 2013, 225: 119-125.
[13] ZHU Y P, YU G, HU B N, LEI X P, YI H B, ZHANG J. Electrochemical behaviors of the magnesium alloy substrates in various pretreatment solutions [J]. Applied Surface Science, 2010, 256(9): 2988-2994.
[14] FU P H, PENG L M, JIANG Ha Y, CHANG J W, ZHAI C Q, Effects of heat treatments on the microstructures and mechanical properties of Mg-3Nd-0.2Zn-0.4Zr (wt.%) alloy [J]. Materials Science and Engineering A, 2008, 486(1-2): 183-192.
[15] Li Z M, FU P H, PENG L M, WANG Y X, JIANG H Y, WU G H. Comparison of high cycle fatigue behaviors of Mg-3Nd-0.2Zn-Zr alloy prepared by different casting processes [J]. Materials Science and Engineering A, 2013, 579: 170-179.
[16] CHANG J W, PENG L M, GUO X W, ATRENS A, FU P H, DING W J. Comparison of the corrosion behaviour in 5% NaCl solution of Mg alloys NZ30K and AZ91D [J]. Journal of Applied Electrochemistry, 2008, 38(2): 207-214.
[17] TRABANELLI G, ZUCCHI F, BRUNORO G, BOLOGNESI G P. Photopotential measurements in the study of surface layers in metal corrosion, inhibition and passivation phenomena [J]. Thin Solid Films, 1972, 13(1): 131-142.
[18] MODESTOV A D, ZHOU G D, GE H H, LOO B H. A study by voltammetry and the photocurrent response method of copper electrode behavior in acidic and alkaline solutions containing chloride ions [J]. Journal of Electroanalytical Chemistry, 1995, 380 (1-2): 63-68.
Mg-3.0Nd-0.2Zn-0.4Zr镁合金表面电沉积Cu镀层及其耐腐蚀性能
王少华1,郭兴伍1,2,孙 灿1,龚 佳1,2,彭立明1,2,丁文江1,2
1. 上海交通大学 轻合金精密成型国家工程研究中心,上海 200240;
2. 上海交通大学 金属基复合材料国家重点实验室,上海 200240
摘 要:研究Mg-3.0Nd-0.2Zn-0.4Zr(NZ30K)镁合金上电沉积Cu镀层的前处理过程及耐腐蚀行为。研究结果表明:活化膜和浸锌层均优先在Mg12Nd共晶相表面沉积。Cu镀层能够为镁基体提供长达60 h的防护作用,这主要归因于其致密的镀层结构及浸泡过程中形成较稳定的钝化膜。热震试验证明镀层具有良好的结合力。
关键词:NZ30K镁合金;前处理;电沉积Cu;腐蚀性能;结合力
(Edited by Yun-bin HE)
Foundation item: Project (51371116) supported by the National Natural Science Foundation of China; Project supported by the Foundation of Open Research Topic in State Key Laboratory of Metal Matrix Composite, China
Corresponding author: Xing-wu GUO; Tel: +86-21-54745091; E-mail: xingwuguo@sjtu.edu.cn
DOI: 10.1016/S1003-6326(14)63537-8
Abstract: To improve the corrosion resistance, electrodeposition of Cu coating on Mg-3.0Nd-0.2Zn-0.4Zr (mass fraction, % NZ30K) magnesium alloy via an appropriate pretreatment was investigated. The surface morphologies, compositions and microstructures of the pretreated films and Cu coating were characterized in detail. The results show that the activation film consists of fluoride and phosphates and Zn immersion film forms preferentially on the eutectic compound Mg12Nd phase region. A smooth, uniform and dense Cu coating is successfully obtained. Potentiodynamic polarization tests reveal that Cu coating can greatly improve the corrosion resistance of NZ30K magnesium alloy. Open circuit potential (OCP) and electrochemical impedance spectroscopy (EIS) tests during long-term immersion further demonstrate that Cu coating can provide an effective protection for NZ30K magnesium alloy from corrosion up to ~60 h, due to its dense structure and a stable passive film formed. In addition, Cu coating exhibits good adhesion to substrate as confirmed by thermal shock test.


