
Crystallization and properties of some CaO-Al2O3-SiO2 system glass-ceramics with Y2O3 addition
ZHENG Wei-hong (郑伟宏), CHENG Jin-shu (程金树), QUAN Jian (全 健)
LOU Xian-chun (楼贤春), LIU Jian (刘 健)
Key Laboratory of Silicate Materials Science and Engineering, Wuhan University of Technology,
Ministry of Education, Wuhan 430070, China
Received 10 April 2006; accepted 25 April 2006
Abstract: The crystallization behavior and mechanical properties of CaO-Al2O3-SiO2 (CAS) system glass-ceramics with addition of Y2O3 were investigated. The optimal sintering temperatures of all heat-treated glasses were altered and the crystallization was accelerated with Y2O3 addition, and only wollastonite as a main crystalline phase was precipitated. The volume fraction of crystalline phase and density were increased with Y2O3 addition. The results suggest that the CAS glass-ceramics would get the lowest sintering temperature and optimal microstructure with the addition of Y2O3 by 3.25 %. The bending strength has a maximum due to the oriented and interlocked wollastonite crystal, which causes crack divert or blunts to limit the further development of the flaw size and increases the surface energy of fracture.Key words:
CaO-Al2O3-SiO2; glass-ceramics; Y2O3 doped; crystallization;
1 IntroductionCalcium aluminum silicate (CAS) glass-ceramics have excellent wear-resistant and chemical durability, and have achieved great industrial and economic importance [1-3]. However, there are still a series of problems at present. Firstly, the high sintering temperature and long sintering time are not energy-saving. Secondly, the poor mechanical properties led to the thickness of products over 10 cm, which made the cost expensive. Therefore reducing sintering temperature and improving mechanical properties are urgent to be solved.
It is reported that rare-earth oxide can be added into ceramics or glass-ceramics as flux to promoted crystallization. For example, HU and LIANG[4] prepared LAS glass ceramics by adding CeO2 as flux and the results showed that addition of 5%(mass fraction, the same below if not mentioned) CeO2 serving as a flux could promote crystallization. The glass transition temperature and the crystallization peak temperature decreased. The transformations of glass to β-quartz and of β-quartz to β-spodumene were also accelerated. YAO and QIU [5] investigated the effect of three kinds of rare-earth oxide (Y2O3, La2O3 and Sm2O3) on the sintering and mechanical properties of alumina ceramics. The results show that rare-earth oxide improves the sintering process and increases the relative densities of alumina ceramics, the flexural strength and fracture toughness reach over 430 MPa and 5.15 MPa?m1/2, respectively.
In this paper, we prepared CAS glass-ceramics doped with Y2O3 and investigated the effects of Y2O3 addition on the crystallization and mechanical properties of glass-ceramics.
2 Experimental
The initial materials were analytical grade reagents SiO2, Al2O3, CaCO3, BaCO3, ZnO, Na2CO3, B2O3 and Y2O3. The detailed compositions of these glasses are given in Table 1[2,3,6-8]. The calculated proportions were thoroughly mixed and melted in Al2O3 crucible in an electrical furnace at about 1 480 ℃ (depending on glass composition) for 2 h. The melts were quenched into glass particles whose diameter were 0.5-6 mm, and were then dried up.
The glass particles were paved into the fireproof
Table 1 Chemical composition of glass-ceramics with different Y2O3 (mass fraction, %)
molds (7 cm×5 cm×1.5 cm) and heat-treated in a silicon-molybdenum furnace. CAS glass-ceramic is a top grade decorative material, which requires excellently flat and bright surface. The flatness and brightness have a direct relation with treated temperature and holding time. In this paper, adopted different sintering temperatures but the same holding time to obtain the same optimal flatness and brightness for the glass-ceramics. The heating temperature was raised from room temperature to optimal sintering temperature and held for 1 h, with a heating rate of 6 ℃/min. The resultant samples were annealed at about 600 ℃ for 30 min.
The crystalline phase of specimen was identified by X-ray diffraction analysis (XRD, D/Max-Radiffractomer, Rigaku, Japan) with Cu Kα radiation. The specimens for microstructural observation were cut from the crystallized glass body. The surface morphologies of specimens, polished and chemically etched in HF acid (3%) at room temperature for 30 s, were examined by scanning electron microscopy (SEM, JSM-5610LV). Density of samples was determined by Archimedes’ immersion method at room temperature. The bending strength was measured by an MTS-810 Ceramic Test System in air with an internal spanning of 30 mm and a crosshead speed of 0.5 mm/min at room temperature. In order to obtain reliable statistical data, at least 10 measurements were made on each sample.
3 Theoretical analysis
Glass-ceramics may be considered composite materials made up of different crystalline phases (ci) and a residual glass (g). The volume of a glass-ceramics, Vgc, is practically an additive function of the volumes of the vitreous and crystal phases present[9].
![]() (1)
(1)
As a consequence, the density of the glass-ceramic can be related to the percentage and densities of the different phases by the formula as
![]() (2)
(2)
where ρgc is the density of the glass-ceramic, ρg is the density of the residual glass phase and xci and ρci are the mass percent and the density of the crystal phase i, respectively.
In this glass-ceramic, only wollastonite was precipitated. So Eqn.(2) can be described as
![]() (3)
(3)
where ρw is the density of wollastonite. Under real conditions, only a portion of the parent glass is transformed to a crystal phase. It is assumed that the volume of the parent glass, Vg, consists of two parts: Vgt with the same composition as the crystal phase which is to be formed and Vgr with the same composition as the parent glass, Vg.
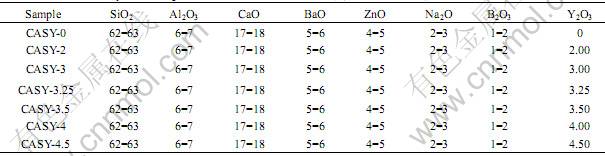
So the percentage crystal phase (x) can be obtained through
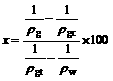 (4)
(4)
And the volume fraction of crystalline phase (Vf) can be determined by
![]() (5)
(5)
Fig.1 shows the XRD patterns of samples thermally treated at different temperatures. The main reflection at 2θ=30.033? might be attributed to β-CaSiO3 (a=10.10, b=11.05, c=7.305, α=99.53, β=100.56, γ=83.44). Additional reflections attributable to β-CaSiO3 also occur at 23.230?, 25.362?, 26.923? and 28.938?. These reflections were accorded with No.42-0547 JCPDS card. The main phases of four samples were all wollastonite despite of different Y2O3 contents.
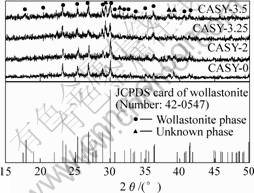
Fig. 1 XRD patterns of samples thermally treated at different temperatures
Fig.2 (a) shows the SEM micrograph of CASY-0 with crystals having size of about 6 ?m. The micrograph of CASY-2 is shown in Fig.2(b). The crystals are significantly larger than those precipitated in CASY-0, which indicates that the addition of Y2O3 by 2 % can enhance crystal growth. As shown in Fig.2(c), the microstructure changes further in CASY-3.25 with Y2O3 3.25 % addition. The size of crystals becomes larger.
ionally, needle-like shaped wollastonite oriente and interlock, with equal size, can be discerned. It can be seen from Fig.2(d) that the crystals continues to grow and the oriented and interlocked microstructure changes.
4.2 Mechanical properties
Fig.3 shows the bending strength of the samples investigated. The values show that the rupture strength increases from 84 MPa to 87.85 MPa with 2% Y2O3. Increasing the addition of Y2O3 to 3.25% results in a further improvement of mechanical properties. Here, the rupture strength of 110 MPa is obtained. The addition of Y2O3 over 3.25 %, however, results in a decrease of mechanical properties. Fig.4 shows that the density increases from 2.5 to 2.89 g/cm3 when the addition of Y2O3 increases from 0 to 4.5%.
5 Discussion
The volume fraction of crystal (Vf) can be figured out by Eqn.5. The values of ρgt and ρw are available in by MARZURIN[10], which was 2.87 and 2.92 g/cm3, respectively. The densities of the glasses (ρg) were measured by experiments. The values of Vf and ρg are shown in Fig.5.
Fig.5 shows that the addition of Y2O3 increasing from 0 to 4.5 % leads to the volume fraction of crystalline phase rising from 37% to 57%. According to YAO and QIU[5], the distribution of rare earth ion atgrain boundary is non-equilibrium thermodynamics. The content of rare earth at grain boundary is higher than that in crystalline phase. Therefore, Y3+ ions cluster together around the surface of glassy grain. Additionally,
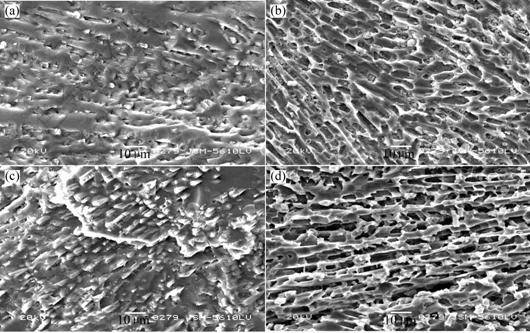
Fig.2 SEM micrographs of samples: (a)—CASY-0; (b)—CASY-2; (c)—CASY-3.25; (d)—CASY-3.5
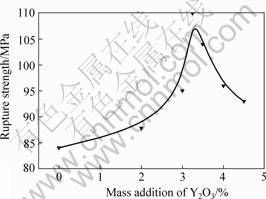
Fig.3 Relationship between rupture strength and Y2O3 content
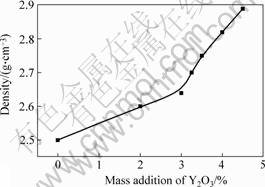
Fig. 4 Relationship between density and Y2O3 content
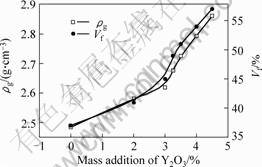
Fig.5 Relationship between ρg and Vf and addition of Y2O3
Y3+ ions are high field intensity cations and can destroy the network of glass, which results in the decrease of viscosity. The surface of glassy grain could form liquid phase at a lower temperature and the crystallization is accelerated.
The oriented and interlocking wollastonite crystals
were precipitated with the addition of Y2O3 by 3.25%. The high content of crystalline phase and interlocking structure cause cracks to divert or deflect away from the initial direction. Crack deflection becomes more effective in limiting the flaw sizes within the materials, which increases the absorption of energy released upon crack propagation. Such energy-absorbing fracture propagation must increase the effective surface energy of fracture. When the absorption of energy was great enough, crack could be blunted and its fracture mode appeared to be mainly intergranular, thus strength increased [11]. While the flake diameter and aspect ratio exceed the critical values, as the addition of Y2O3 was over 3.5 %, the bending strength decreased because the orientated and interlocked microstructure was destroyed.
References[1] WANG H D, CHENG J S, ZHAO Q, YUAN J. Influence of ZnO and BaO contents on sintering and crystallization of glass-ceramic decorative materials[J]. Journal of Wuhan University of Technology(Mater Sci Ed), 1996, 11(2): 22-27.
[2] TULYAGANOV D U. Development of glass-ceramics by sintering and crystallization of fine powder of calcium-magnesium-aluminosilicate glass [J]. Ceramics International, 2002, 28(5): 515-520.
[3] H?LAND W, BEALL G. Glass-Ceramic Technology[M]. American Ceramic Society, 2002. 55-56.
[4] HU A M, LIANG K M. Phase transformations of Li2O-Al2O3-SiO2 glasses with CeO2 addition [J]. Ceramics International, 2005, 31: 11-14.
[5] YAO Yi-jun, QIU Tai. Effect of Y2O3, La2O3, Sm2O3 on behaviors of alumina ceramics[J]. Journal of the Chinese Rare Earth Society, 2005(23): 158-161.(in Chinese)
[6] CIOFFI R, PERNICE P, ARONNE A, CATAUROG M Q. glass-ceramics from fly ash with added Li2O[J]. Journal of the European Ceramic Society, 1994, 13(2): 143-148.
[7] ALIZADEH P, MARGHUSSIAN V K. Effect of nucleating agents on the crystallization behaviour and microstructure of SiO2-CaO-MgO (Na2O) glass-ceramics[J]. Journal of European Ceramic Society, 2000, 20: 775-782.
[8] MARGHUSIAN V K, DAYI Niaki M H. Effects of composition changes on the crystallization behaviour and properties of SiO2-Al2O3-CaO-MgO (Fe2O3-Na2O-K2O) glass-ceramics[J]. Journal of the European Ceramic Society, 1995, 15: 343-348.
[9] KARAMANOV A, PELINO M. Evaluation of the degree of crystallization in glass-ceramics by density measurements [J]. Journal of the European Ceramic Society, 1999, 19: 649-654.
[10] MAZURIN O V. The Glass Properties Handbook(Vols 1-5)[M]. Nauka, Leningard, 1975-1989.(in Russian)
[11] FANG Ping-an, WU Zhao-ping. Effect of microstructural evolution on the mechanical properties of lepidolite based glass-ceramics [J]. Journal of the European Ceramic Society, 2002, 22: 1381-1385.
Corresponding author: ZHENG Wei-hong; Tel: +86-27-62624330; Fax:+86-27-87860801; E-mail: guiguiss@126.com


