Trans. Nonferrous Met. Soc. China 27(2017) 2310-2316
Electrochemical synthesis of NbC-Sn composite powder in molten chloride
Hong-mei LI1,2, Qiu-shi SONG1,2, Qian XU1,3, Ying CHEN1,2, Jing-chun MENG1,2
1. School of Metallurgy, Northeastern University, Shenyang 110819, China;
2. Liaoning Key Laboratory for Metallurgical Sensor and Technology, Northeastern University, Shenyang 110819, China
3. State Key Laboratory of Advanced Special Steel, School of Materials Science and Engineering, Shanghai University, Shanghai 200072, China
Received 25 May 2016; accepted 24 October 2016
Abstract:
NbC-Sn composite powder was successfully prepared from SnO2, Nb2O5 and carbon by electrochemical reduction and carbonization in CaCl2-NaCl molten salt at 900 °C. The reaction pathway was investigated by terminating electrochemical experiments for various durations. The influence of carbon on the final products was considered. NbC particles were obtained by leaching the composite with acid. The results showed that the aggregated NbC-Sn composite powdev contained NbC particles about 50-100 nm and Sn particles about 200 nm. SnO2 was reduced to Sn in the sintering process. Nb2O5 was electrochemically reduced to Nb in molten salt, experiencing some intermediate products of calcium niobates and niobium suboxides. Nb metal obtained was converted to NbC with assistance of carbon. The reduction of Nb oxides may be incomplete and Nb3Sn would be formed if carbon is insufficient in the cathodic pellet. NbC with good dispersity is produced by leaching NbC-Sn with HCl.
Key words:
NbC-Sn; electrochemical reduction; molten chloride; calcium niobates; acid leaching;
1 Introduction
Niobium carbide (NbC) is a non-oxide ceramic material primarily used as the starting material for the commercial production of wear-resistant composites and cutting tools due to its attractive properties of high melting point (3610 °C) [1], good thermal stability, excellent chemical inertness, low friction coefficient, high density [2], extreme hardness and satisfactory mechanical toughness [3-5]. In addition, niobium carbide powder is usually added into other metals or alloys as a reinforcing phase to improve mechanical properties of the metallic matrix [6]. However, there are still some challenges for niobium carbide as the hard phase, such as low wettability and poor sinterability. The insufficient features could be enhanced by adding more ductile materials to form a kind of metal-carbide cemented composite [7-10].
There are some methods to prepare niobium carbide, including carburization of niobium oxide to form niobium carbide [11], mechanical alloying of niobium and carbon [12,13], mechanochemical process of NbCl5 and CaC2 [14], and self-propagating combustion of niobium and carbon [15]. Most of these methods employ relatively high temperature or hazardous substances, which make them not very environment friendly. Electrochemical reduction in molten salt is an optional route to prepare metal [16,17], metal alloy [18-21], as well as metallic carbides [22-24]. Particularly, it is favorable for preparation of metal-carbide composite by mixing metallic oxide directly into sintered precursor. Some scientists have reported electrochemical synthesis of the cemented powder in molten salt. ZOU et al [25] studied directly electro-preparation of Ti5Si3/TiC composite from the oxides/C precursor in molten CaCl2. Our group also reported fabrication of Fe-based TiC and Ni-TaC cemented powder previously, in which Fe or Ni was used as a binder to form metal-carbide composite [10,24]. In general, the operating temperature used in the electrochemical process is energy competitive than that used in other metallurgical methods. It is an advantage for generation of dispersive nano-sized carbide particles in metal matrix. But Sn has seldom been reported as metal matrix to support the nano-sized carbide particles in molten salt till now. It should be a little different from Ni, Fe and Si, since the melting point of Sn is obviously lower than the working temperature (231 °C).
In this work, NbC-Sn composite powder was successfully synthesized by electrochemical reduction of Nb2O5 in the presence of SnO2 in molten salt. The possible reaction pathway for electrochemical reduction and carbonization in the melt was investigated. The aim of this work was to prepare NbC-Sn composite powder and dispersived NbC powder in a moderate method.
2 Experimental
All the starting materials were of analytical grade and commercially available. Nb2O5, SnO2 and carbon powder were mixed with a mole ratio of 2:1:3 (Nb/Sn/C) in ethylalcohol, and subsequently ball milled for 1 h. Approximately 1 g of the mixture powder was pressed under a uniaxial pressure of 10 MPa, followed by a sintering process at 1000 °C for 3 h in argon atmosphere. A eutectic mixture of CaCl2 and NaCl with mass ratio of 7:3 was dehydrated at 300 °C for more than 24 h, which was then elevated to the targeted temperature. The schematic illustration of the electrolytic cell is given in Fig. 1. The vessel was sealed and argon gas was flushed into the reactor to clean the air and vapour. Then, the reactor was heated up to 900 °C at a rate of 3 °C/min. Subsequently, pre-electrolysis was carried out between two graphite rods at 2.5 V for 0.5 h to remove possible impurities existing in the melt [26-28]. The sintered pellet was used as the cathode and a high density graphite rod of 13 mm in diameter and 70 mm in length served as the anodes. A constant voltage of 3 V was applied between the electrodes for certain durations immediately after they were immersed into the melt using a WYJ 40A 15V power supply. This voltage provides sufficient electrochemical driving force for deoxidation of niobium oxides, avoiding continuous decomposition of CaCl2 and NaCl. All the experiments were performed under high-purity argon. The sample obtained were removed from the reactor, ultrasonically washed with distilled water and ethylalcohol in sequence, and then dried at 60 °C. Phase composition and morphology were examined by a D/Max-2500PC X-ray diffractometer (XRD) with Cu Kα radiation and a JSM-6360L V scanning electron microscope (SEM) in combination with energy-dispersive X-ray (EDS).
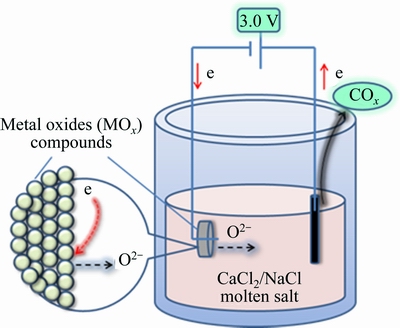
Fig. 1 Schematic illustration of electrolytic cell
3 Results and discussion
3.1 Analysis of sintered pellet
The typical XRD pattern of Nb2O5, SnO2 and carbon powder with a mole ratio of 2:1:3 (Nb/Sn/C) mixture sintered at 1000 °C for 3 h is shown in Fig. 2(a). Characteristic peaks of NbO2, Nb2O5 and Sn can be detected. This indicates that SnO2 is completely reduced to Sn by carbon in the sintering process, as expressed in Eq. (1). The Sn retrieved from SnO2 is beneficial for formation of metal-carbide composite in comparison to Sn metal directly mixed into the precursor. It is difficult to crush Sn particles into small pieces because of their good ductility. So, it is not easy to disperse Sn uniformly in the sintered pellet. Furthermore, the loss of Sn is obvious during sintering procedure if Sn metal was added directly into the precursor. The melting point of Sn is 231 °C, while the sintering process was performed at 1000 °C. So, the metal would flow out of the pellet if too much Sn aggregated together in the pellet, as illustrated in Fig. 2(d). Nevertheless, Sn was almost homogeneously mixed without segregation in the pellet if Sn oxide was applied, and the phenomenon of Sn loss is not obvious in Fig. 2(c). Moreover, some Nb2O5 was reduced into NbO2 in the sintering process. In our previous study [4], it was found that Nb2O5 maintained to be inert with the presence of carbon at 1000 °C, though reaction between the two chemicals is thermo- dynamically favorable (Eq. (2)). However, reduction of Nb2O5 occurred once SnO2 was mixed into the precursor. This phenomenon is probably ascribed to CO released from carbothermal reduction of SnO2 (Eq. (1)), which would participate the reduction of Nb2O5 (Eq. (3)). This promotes the transformation from Nb2O5 to NbO2 in the sintered process.
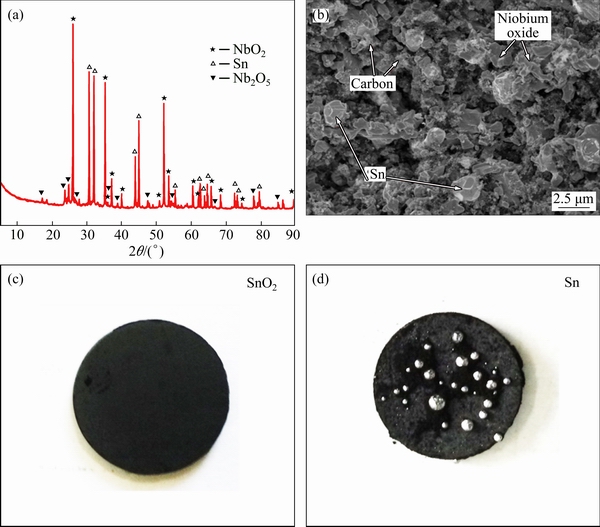
Fig. 2 XRD pattern (a) and SEM image (b) of Nb2O5/SnO2/C composite pellet sintered at 1000 °C for 3 h, photos of sample with SnO2 (c) and Sn (d) sintered for 3 h
Figure 2(b) shows the SEM image of the composite pellet sintered at 1000 °C for 3 h. The particles of niobium oxide and Sn present different morphologies, and both the sizes are about 1-2 μm. In addition, the carbon additive is about 100 nm in size with an uncrystallized structure, which should be favourable for the formation of carbide [4]. A lot of cavities exist in the pellet, which may result from the evolution of CO gas from carbothermal reduction of SnO2.
SnO2+2C=Sn+2CO(g)
△GΘ(1000 °C)=-130.086 kJ/mol (1)
Nb2O5+C=2NbO2+CO(g)
△GΘ(1000 °C)=-10.267 kJ/mol (2)
Nb2O5+CO(g)=2NbO2+CO2 (g)
△GΘ(1000 °C)=41.959 kJ/mol (3)
3.2 Phase identification of reduced samples
A series of interrupted experiments were carried out to investigate the reduction pathway. Figure 3 demonstrates the XRD patterns of the reduced samples with electrolyzing durations ranging from 0.5 to 9 h. After 0.5 h of reduction, the phases of NbC, Sn and Ca3Nb2O8 were indexed, while the diffraction peaks of NbO2 and Nb2O5 disappeared. This implies that niobium oxides including NbO2 and Nb2O5 were electrochemically reduced to Nb metal, and carbonization of the reduced Nb subsequently took place. Meanwhile, Nb2O5 without undergoing electro-reduction would combine CaO to generate Ca3Nb2O8. Intercalation of CaO into structure of Nb2O5 makes Ca3Nb2O8 a more stable phase, which would experience a relatively tough reduction process. When the reaction time was prolonged, prevalent niobium ions were continuously reduced. Correspondingly, Ca2+ and O2- ions were released into the melt from the structure of Ca3Nb2O8. This would cause phase evolution to the less intercalation phase of Ca2Nb2O7 in the pellet, which became the most dominant phase after 3 h of reduction. With proceeding of the electro-reduction, the diffraction peaks of calcium niobates decreased gradually, while the intensity of NbC peaks increased reversely. Once the duration reached 6 h, the diffraction peaks of calcium niobates completely disappeared, and the pellet is composed of Sn and NbC. This suggests that electrochemical conversion of niobium oxides to niobium metal was achieved. The trace of reaction among Sn metal, intermediate and final products has not been detected from XRD pattern. The phases in the cathodic pellet remained without variation even if the electrolysis time was long.
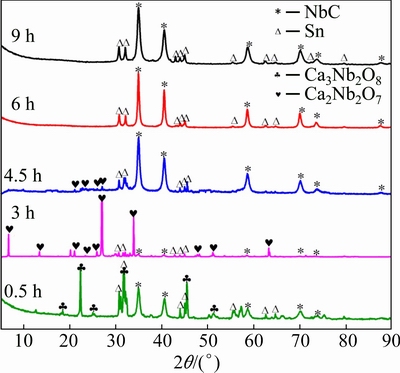
Fig. 3 XRD patterns of samples electrochemically reduced from Nb2O5/SnO2/carbon composite pellets under 3.0 V at 900 °C for various durations
According to these results presented above, the possible pathway for electrochemical reduction of Sn-NbC could be predicted. Some Nb2O5 and all NbO2 were reduced gradually to metallic niobium under the driving force of voltage once the electrolysis started, as presented in Eqs. (4) and (5). Though NbO was not seen in the XRD patterns, it is reasonable to predict its existence as an intermediate product since it is observed usually during direct deoxidation of Nb2O5. Carbothermal reduction of Nb suboxides such as NbO2 and NbO is possible, but it is obviously less predominant because of the relatively low working temperature, in comparison with electrochemical reduction from our previous study on electro-synthesis of niobium carbide in molten salt. Carbon powder would react with the reduced Nb to generate NbC (Eq. (6)). Meantime, Nb2O5 without experience of electro-deoxidation combined CaO in the melt to form a series of calcium niobates (Eq. (7)), which are more difficult to recover in contrast to Nb2O5. Calcium niobates were decomposed gradually to niobium with the proceeding of electrolysis, and NbC was obtained as the final product (Eq. (8)). Sn metal in the pellet maintained to be inert without phase transformation throughout the electrochemical process. On the anode, the ionized O2- was oxidized to evolve CO/CO2 gas with participation of carbon.
NbO+C=Nb+CO(g) (4)
Nb2O5+2e=2NbO2+O2- (5)
Nb+C=NbC (6)
Nb2O5+xCaO=Nb2O5·xCaO, x=2, 3 (7)
Nb2O5·xCaO+2C+10e=2NbC+5O2-+xCaO, x=2, 3 (8)
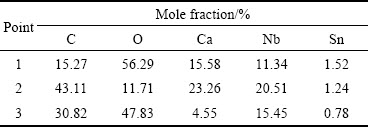
The morphologies of products reduced for different reaction time are illustrated in Fig. 4, and EDS analyses for the regions marked in the images are shown in Table 1. Some particles present in Fig. 4(a) have angular shape, whose size is about 3 μm on average. The particles should be mainly composed of Ca3Nb2O8 since the Ca to Nb mole ratio in region ‘1’ is about 1.37 : 1. This means that CaO began to penetrate into Nb2O5 to form calcium niobates.
After 3 h of reduction, calcium niobates became the most predominant phase in the cathodic pellet, as displayed in Figs. 4(b)-(d). Particles more than 50 μm were observed across the sectional region of the whole pellet, especially on the surface area of the cathode. This indicates that intercalation of CaO into the cathode is the most notable process, which makes the particles grow up to be big ones [29]. The concentration of CaO is relatively high on surface area of the pellet, where the formation of calcium niobates is remarkable. This observation is in agreement with phase determination in the XRD pattern.
When the pellet was electrolyzed for 4.5 h, the large niobates particles were broken up to relatively small particles with angular shape, as can be seen in Fig. 4(e). The mole ratio of Ca to Nb is about 1.1:1, and O content has been decreased from 56.3% to 11.7% (mole fraction). This implies that calcium niobate was further electrochemically decomposed. When the reduction time was longer than 6 h, the cathodic pellet basically contained NbC-Sn particles. Calcium niobates were almost consumed up except some tough particles, which also decreased with reaction duration, as shown in Figs. 4(e)-(g). In point 3, the Ca to Nb mole ratio is 0.29:1. This means that calcium ions enter into the molten salt with the reduction of calcium niobate. For comparison, it took about 13.5 h from Nb2O5 to the final product of NbC during direct preparation of niobium carbide. The efficiency enhancement for reduction may be attributed to the existence of Sn in the pellet. Our experiment introduced an excellent conductive phase to accelerate electro-deoxidation of the pentavalent niobium compounds.
3.3 Influence of carbon amount and preparation of dispersive NbC
The influence of carbon content on the final products was further investigated by varying the mole ratio of Nb, C and Sn in the precursor. Figure 5 gives XRD patterns of the pellets with various compositions electro-chemically reduced at 3.0 V for 12 h. Phase composition of the electrolytic products varied with initial mole ratio of the elements. If carbon is insufficient in the pellet (the mole ratio of the Nb/Sn/C is 2:1:1), some NbO was found besides NbC in the electrolytic product. This signifies that carbon takes a positive role on reduction of niobium oxide. Additionally, Nb tended to combine Sn to form Nb3Sn without carbon, and Sn phase can hardly be observed. But phases in the products changed little if excessive carbon (Nb/Sn/C of 2:1:2 to 2:1:4) was added into the precursor. Carbon excluded in carbonization would exist in the product, which was not documented in XRD patterns because of its uncrystallized structure.
Figure 6(a) demonstrates the SEM image of the cathode pellet with the Nb/Sn/C mole ratio of 2:1:3 after electrochemical reduction at 3.0 V for 12 h. Obviously, the typical Nb2O5·xCaO particles disappeared. The cathodic pellet is comprised of spherical Nb particles and Sn particles with angular shape. NbC and Sn particles aggregate closely with respective size 50-100 nm and 200 nm.
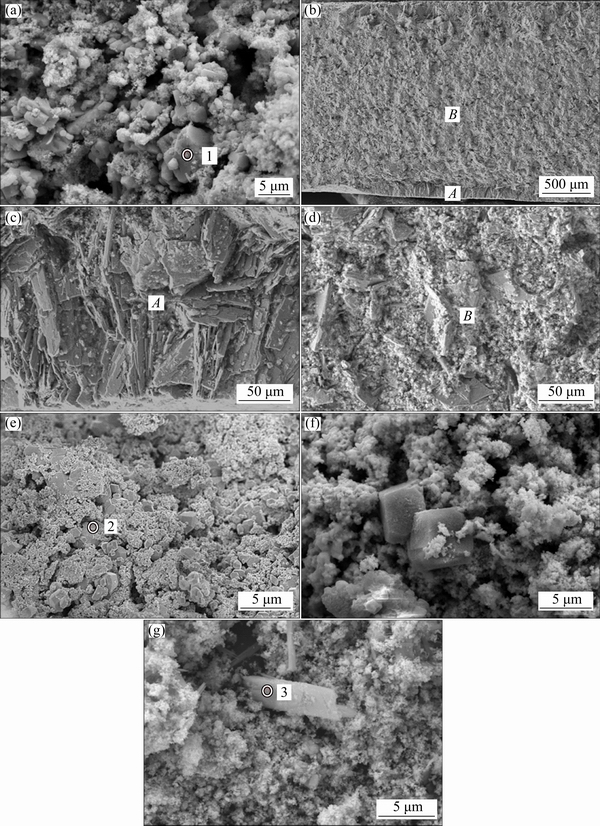
Fig. 4 SEM images of samples electrochemically reduced for 0.5 h (a), 3 h (b), enlarged images (c, d) of areas A and B in (b), 4.5 h (e), 6 h (f), 9 h (g) under 3.0 V at 900 °C
Table 1 EDS analyses of points 1, 2, 3 marked in Fig. 4
Subsequently, the NbC-Sn composite was leached by 18% HCl aqueous solution at 25 °C for 20 h to remove Sn metal. Figure 6(b) shows the XRD pattern for the leached product. It can be seen that Sn was eliminated in the leaching treatment, while NbC maintained inertness. In contrast to the aggregated morphology of Sn-NbC, carbide obtained is much more dispersive and porous, as illustrated in Fig. 6(c). The production is ascribed to the existence of Sn metal in electro-reduction of niobium oxide, since it can hinder interconnection of NbC particles to generate aggregated ones.
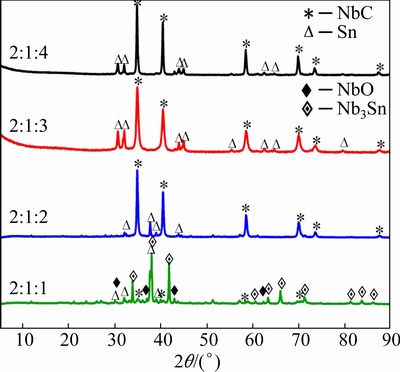
Fig. 5 XRD patterns of samples electrochemically reduced from Nb2O5/SnO2/carbon composite pellets with different mole ratios of Nb/Sn/C under 3.0 V for 12 h at 900 °C
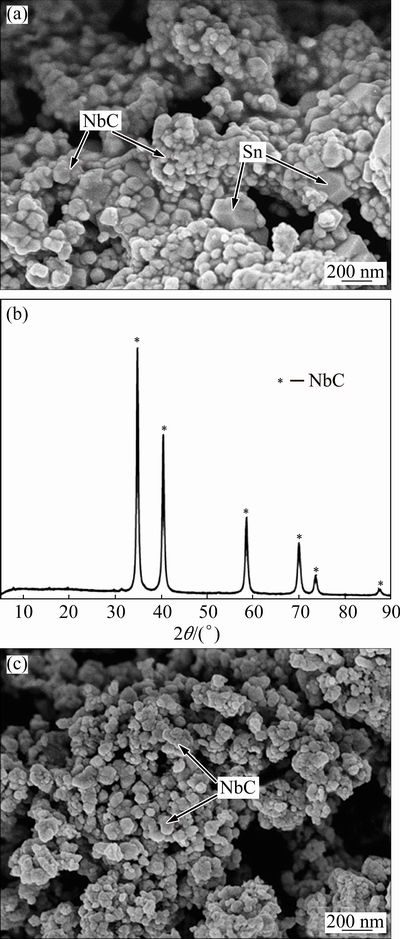
Fig. 6 SEM image of sample (Nb/Sn/C mole ratio=2:1:3) electrochemically reduced under 3.0 V for 12 h at 900 °C (a), XRD pattern (b) and SEM image of sample leached by 18% HCl (c)
4 Conclusions
1) NbC-Sn composite powder is successfully synthesized by carbothermal reduction of SnO2, electrochemical reduction of Nb2O5 and carbonization in CaCl2-NaCl molten salt. The product is composed of aggregated particles, including NbC particles about 50-100 nm and Sn particles about 200 nm. Dispersive NbC particles about 50 nm in size are obtained by treatment of the product with HCl.
2) Niobium oxides are electrochemically reduced gradually to metallic niobium, and subsequently converted to NbC with the assistance of carbon. Meanwhile, Nb2O5 without electro-deoxidation combines CaO in the melt to form a series of calcium niobates. Consequently, niobates are electrically converted to Nb and final product of NbC.
3) SnO2 is thermally reduced to Sn metal by carbon in the sintering process. It is favorable for dispersive residence of Sn metal in the precursor and formation of NbC-Sn composite. Sn metal in the pellet is inert throughout the electrochemical process. The presence of Sn enhances the efficiency of electrochemical reduction of niobium oxides.
References
[1] XIAO Dai-hong, HE Yue-hui, LUO Wei-hong, SONG Min. Effect of VC and NbC additions on microstructure and properties of ultrafine WC-10Co cemented carbides [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(6): 1520-1525.
[2] LI Y Y, XIAO Z Y, NGAI T L, XIA W, CHEN W P. Warm compacted NbC participate reinforced iron-base composite II: Microstructure and properties [J]. Transactions of Nonferrous Metals Society of China, 2002, 12(4): 664-668.
[3] KWON H, KIM W, KIM J. Stability domains of NbC and Nb (CN) during carbothermal reduction of niobium oxide [J]. Journal of the American Ceramic Society, 2015, 98(1): 315-319.
[4] SONG Qiu-shi, XU Qian, MENG Jing-chun, LOU Tai-ping, NING Zhi-qiang, QI Yang, YU Kai. Preparation of niobium carbide powder by electrochemical reduction in molten salt [J]. Journal of Alloys and Compounds, 2015, 647: 245-251.
[5] GROVE D E, GUPTA U, CASTLEMANJR A W. Effect of hydrocarbons on the morphology of synthesized niobium carbide nanoparticles [J]. Langmuir, 2010, 26(21): 16517-16521.
[6] MEDEIROS F F P, DASILVA A G P , DESOUZA C P. Synthesis of niobium carbide at low temperature and its use in hardmetal [J]. Powder Technology, 2002, 126(2): 155-160.
[7] AHN S, KANG S. Formation of core/rim structures in Ti(C,N)- WC-Ni cermets via a dissolution and precipitation process [J]. Journal of the American Ceramic Society, 2000, 83(6): 1489-1494.
[8] KIM S, MIN K, KANG S. Rim structure in Ti(C0.7N0.3)-WC-Ni cermets [J]. Journal of the American Ceramic Society, 2003, 86(10): 1761-1766.
[9] KWON H, KANG S. Microstructure and mechanical properties of TiC-WC-(Ti,W)C-Ni cermets [J]. Materials Science and Engineering A, 2009, 520(1): 75-79.
[10] SUN Lin, SONG Qiu-shi, XU Qian, NING Zhi-qiang, LU Xiong-gang. The electrochemical synthesis of TiC reinforced Fe based composite powder from titanium-rich slag [J]. New Journal of Chemistry, 2015, 39(6): 4391-4397.
[11] KIM H S, BUGLI G,  D M. Preparation and characterization of niobium carbide and carbonitride [J]. Journal of Solid State Chemistry, 1999, 142(1): 100-107.
D M. Preparation and characterization of niobium carbide and carbonitride [J]. Journal of Solid State Chemistry, 1999, 142(1): 100-107.
[12] MARQUES M T, LIVRAMENTO V, CORREIA J B, ALMEIDA A, VILAR R. Production of copper-niobium carbide nanocomposite powders via mechanical alloying [J]. Materials Science and Engineering A, 2005, 399(1): 382-386.
[13] SHIRI S G, ABACHI P, POURAZARANG K, RAHVARD M M. Preparation of in-situ Cu/NbC nanocomposite and its functionally graded behavior for electrical contact applications [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(3): 863-872.
[14] NEWELL J D, PATANKAR S N. Room temperature synthesis of nanocrystalline NbC powder [J]. Materials Letters, 2009, 63(1): 81-83.
[15] TSUCHIDA T, AZUMA Y. Synthesis of niobium carbide and nitride in air from mechanically activated Nb-C powder mixtures [J]. Journal of Materials Chemistry, 1997, 7(11): 2265-2268.
[16] WENG Qi-gang, LI Rui-di, YUAN Tie-chui, LI Jian, He Yue-hui. Valence states, impurities and electrocrystallization behaviors during molten salt electrorefining for preparation of high-purity titanium powder from sponge titanium [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2): 553-560.
[17] CAI Zhuo-fei, ZHANG Zhi-mei, GUO Zhan-cheng, TANG Hui-qing. Direct electrochemical reduction of solid vanadium oxide to metal vanadium at low temperature in molten CaCl2-NaCl [J]. International Journal of Minerals, Metallurgy, and Materials, 2012, 19(6): 499-505.
[18] WANG Shi-dong, LI Quan, YE Xiu-shen, SUN Qing-guo, WU Zhi-jian. Effect of oxide and fluoride addition on electrolytic preparation of Mg-La alloy in chloride molten salt [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(10): 3104-3111.
[19] SUN Xiu-yun, LU Gui-min, FAN Shu-di. Electrochemical mechanism of electrolysis codeposition of Mg-Sr alloy in molten salt [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(5): 1629-1634.
[20] CAO Peng, ZHANG Mi-lin, HAN Wei, YAN Yong-de, CHEN Li-jun. Electrodeposition of quarternary Mg-Zn-Li-Ca alloys from molten salts [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(3): 861-865.
[21] ZHANG Mi-lin, CAO Peng, HAN Wei, YAN Yong-de, CHEN Li-jun. Preparation of Mg-Li-La alloys by electrolysis in molten salt [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 16-22.
[22] DAI Lei, LU Yuan, WANG Xian-yu, ZHU Jing, LI Yue-hua, WANG Ling. Production of nano-sized chromium carbide powders from Cr2O3/C precursors by direct electrochemical reduction in molten calcium chloride [J]. International Journal of Refractory Metals and Hard Materials, 2015, 51: 153-159.
[23] DAI Lei, WANG Xian-yu, ZHOU Hui-zhu, YU Yao, ZHU Jing, LI Yue-hua, WANG Ling. Direct electrochemical synthesis of zirconium carbide from zirconia/C precursors in molten calcium chloride [J]. Ceramics International, 2015, 41(3): 4182-4188.
[24] SONG Qiu-shi, XU Qian, DING Ren-yi, MENG Jing-chun, NING Zhi-qiang, LOU Tai-ping, QI Yang, YU Kai. Electrochemical fabrication of multicore-shell Ni-TaC composite particles in molten salt [J]. Journal of the Electrochemical Society, 2016, 163(3): E49-E53.
[25] ZOU Xing-li, LU Xiong-gang, ZHOU Zhong-fu, LI Chong-he. Direct electrosynthesis of Ti5Si3/TiC composites from their oxides/C precursors in molten calcium chloride [J]. Electrochemistry Communications, 2012, 21: 9-13.
[26] SUN Yi, ZHANG Mi-lin, HAN Wei, LI Mei, YANG Yu-sheng, YAN Yong-de, ZHANG Meng. Electrochemical preparation of Mg-Li-Al-Er alloys by co-reduction in molten chloride [J]. Acta Metallurgica Sinica (English Letters), 2013, 26(4): 455-460.
[27] ZOU Xing-li, LU Xiong-gang, LI Chong-he. Electrochemical extraction of Fe-Ti-Si alloys direct from Ti bearing compound ores [J]. Mineral Processing and Extractive Metallurgy, 2011, 120(2): 118-124.
[28] LIU Mei-feng, LU Shi-gang, KAN Su-rong, LI Guo-xun. Effect of electrolysis voltage on electrochemical reduction of titanium oxide to titanium in molten calcium chloride [J]. Rare Metals, 2007, 26(6): 547-551.
[29] SONG Qiu-shi, XU Qian, TAO Ran, KANG Xue. Cathodic phase transformations during direct electrolytic reduction of Nb2O5 in a CaCl2-NaCl-CaO melt [J]. International Journal of Electrochemical Science, 2012, 7(1): 272-281.
熔盐电化学合成NbC-Sn复合粉末
李红梅1,2,宋秋实1,2,许 茜1,3,陈 莹1,2,孟靖淳1,2
1. 东北大学 冶金学院,沈阳 110819;
2. 东北大学 辽宁省冶金传感器及技术重点实验室,沈阳 110819;
3. 上海大学 省部共建高品质特殊钢冶金与制备国家重点实验室,材料科学与工程学院,上海 200072
摘 要:研究在900 °C 的CaCl2-NaCl熔融盐体系中,以SnO2、Nb2O5 和碳粉为前驱体,使用电化学还原和原位碳化的方法,成功制备出NbC-Sn复合粉体材料。通过对不同反应阶段的产物进行分析,研究反应过程机理。考察前驱体中碳粉物质的量变化对最终产物的影响,并通过酸浸NbC-Sn粉体的方式制备NbC。研究表明通过该方法制备出尺寸分别为50~100 nm和200 nm的NbC和Sn颗粒,两者紧密地聚集在一起。SnO2在烧结过程中被碳还原为金属Sn,Nb2O5在熔盐中被逐步电化学还原为金属Nb,并与碳反应生成NbC。其中,历经了铌酸盐的形成与分解、低价铌氧化物的形成与进一步还原等过程。当阴极片中的碳不足时,会造成铌氧化物还原不完全并形成Nb3Sn。复合粉体材料通过HCl水溶液浸出,能够获得分散性很好的NbC粉体材料。
关键词:NbC-Sn;电化学还原;氯化物熔盐;铌酸钙;酸浸
(Edited by Xiang-qun LI)
Foundation item: Projects (51404057, 50874026) supported by the National Natural Science Foundation of China; Project (N150204014) supported by Fundamental Research Funds for the Central Universities, China
Corresponding authors: Qian XU; Tel: +86-21-56338244; E-mail: qianxu@shu.edu.cn;
Qiu-shi SONG; Tel: +86-24-83687731; E-mail: songqs@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(17)60257-7
Abstract: NbC-Sn composite powder was successfully prepared from SnO2, Nb2O5 and carbon by electrochemical reduction and carbonization in CaCl2-NaCl molten salt at 900 °C. The reaction pathway was investigated by terminating electrochemical experiments for various durations. The influence of carbon on the final products was considered. NbC particles were obtained by leaching the composite with acid. The results showed that the aggregated NbC-Sn composite powdev contained NbC particles about 50-100 nm and Sn particles about 200 nm. SnO2 was reduced to Sn in the sintering process. Nb2O5 was electrochemically reduced to Nb in molten salt, experiencing some intermediate products of calcium niobates and niobium suboxides. Nb metal obtained was converted to NbC with assistance of carbon. The reduction of Nb oxides may be incomplete and Nb3Sn would be formed if carbon is insufficient in the cathodic pellet. NbC with good dispersity is produced by leaching NbC-Sn with HCl.


