Trans. Nonferrous Met. Soc. China 28(2018) 542-555
Impact of silver sulphides on gold cyanidation with polymetal sulphides
Muhammad KHALID,  LARACHI
LARACHI
Department of Chemical Engineering, Laval University, Québec, QC, G1V 0A6, Canada
Received 11 January 2017; accepted 18 May 2017
Abstract:
Gold leaching was influenced in association with silver and polymetal sulphide minerals. A packed bed was adopted to single out the galvanic and passivation effects with four sets of minerals: pyrite-silica, chalcopyrite-silica, sphalerite-silica and stibnite-silica. Pyrargyrite enhanced Au recovery to 77.3% and 51.2% under galvanic and passivation effects from pyrite (vs 74.6% and 15.8%). Pyrargyrite in association with sphalerite also enhanced Au recovery to 6.6% and 51.9% (vs 1.6% and 15.6%) under galvanic and passivation effects from sphalerite. Pyrargyrite associated with chalcopyrite retarded gold recovery to 38.0% and 12.1% (vs 57% and 14.1%) under galvanic and passivation effects. Accumulative silver minerals enhanced Au recovery to 90.6% and 81.1% (vs 74.6% and 15.8%) under galvanic and passivation impacts from pyrite. Silver minerals with sphalerite under galvanic and passivation effects enhanced Au recovery to 71.1% and 80.5% (vs 1.6% and 15.6%). Silver minerals associated with chalcopyrite retarded Au recovery to 10.2% and 4.5% under galvanic and passivation impacts (vs 57% and 14.1%). Stibnite retarded Au dissolution with pyrargyrite and accumulative silver minerals. Pyrargyrite and accumulative silver enhanced gold dissolution for free gold and gold associated with pyrite and sphalerite. Gold dissolution was retarded for gold and silver minerals associated with chalcopyrite and stibnite.
Key words:
silver mineral; gold cyanidation; packed-bed reactor; sulphide mineral; passivation; galvanic interaction;
1 Introduction
Gold leaching in aerated alkaline cyanide slurry has been selected as process route in gold industries for more than hundred years. Efforts have been made to improve gold recovery in the cyanide leaching process which include the optimization of reagents addition, such as cyanide/dissolved oxygen concentration, particle size reduction of the ore and significant control of the operational parameters of the cyanidation process [1-3]. Silver is frequently associated with gold, so during cyanidation process, cyanide and oxygen consumption by the dissolution reactions of gold and silver, are described by Eqs. (1) and (2):
4Au+8CN-+O2+2H2O=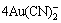 +4OH- (1)
+4OH- (1)
4Ag+8CN-+O2+2H2O= +4OH- (2)
+4OH- (2)
Conductive sulphide minerals comprise a large proportion of gold containing ores, and the effects of these minerals on gold dissolution in aerated cyanide solutions have drawn the interest of many researchers. Earlier studies on the dissolution of gold in cyanide solutions in the presence of sulphide minerals demonstrated that base-metal components, such as copper, iron and zinc, of the sulphide minerals, not only increase significantly the consumption of both cyanide and oxygen, but also have a strong impact on gold leaching kinetics [4-7]. A systematic study was conducted on the kinetics of gold dissolution in the presence of various sulphide minerals, such as pyrite, chalcopyrite, pyrrhotite, arsenopyrite, sphalerite, chalcocite and stibnite, in both air-saturated as well as oxygen-enriched systems. The results demonstrated that the leaching kinetics as well as overall recovery of gold in the presence of polymetal sulphide minerals strongly depends on both the solubility of the sulphide minerals and the cyanide and dissolved oxygen concentration in the solution. Pyrite, chalcopyrite, pyrrhotite and arsenopyrite were found to increase the gold dissolution rate, in an air-enriched as well as oxygen-enriched cyanide solution, while sphalerite, stibnite and chalcocite caused a reduction in the gold dissolution rate [4,5,8-10]. Sulphide minerals are soluble in aerated cyanide solutions to some extent, therefore some sulfur species will be present in the leaching solution as a result of sulphide minerals dissolution. The presence of such species not only results in high reagent consumption but also affects the kinetics and recovery of gold leaching reaction. It was observed that by the addition of trace amounts of sodium sulphide to the cyanide solution, gold leaching was hindered dramatically, which was attributed to the formation of a Au2S passive layer on the gold surface. Strategies like pre-oxidation and lead nitrate (Pb(NO3)2) have been adopted to minimize the effect of sulphide ions [5,11-13].
Gold is often associated with silver, and there are numerous gold deposits where the recovered silver grade exceeds that of gold. Metallic silver dissolves anodically in aqueous cyanide solutions, in a similar manner to gold (Eq. (2)). As far as the leaching kinetics is concerned, metallic silver has been found to dissolve faster than gold [9,14-16]. The presence of silver, in metallic form, alloyed with gold or in dissolved form, has a beneficial effect on gold leaching kinetics. In leaching reaction mechanisms, silver has a de-passivating effect on gold cyanidation via a bimetallic corrosion, which is hindered by the formation of a AuCN(s) film on the gold surface [2,5,9,17,18]. Under typical cyanidation conditions prevailing in gold leaching, the dissolution rate for silver is usually slower than that for gold. This is because, although silver species, such as native silver, chlorargyrite (AgCl), and iodargyrite (AgI), are found in nature and dissolve readily in cyanide, much more common are the less soluble silver minerals [14]. Silver is frequently associated with polymetal sulphides and sulfosalts, the most significant sulphide phase is known as acanthite (Ag2S), while other sliver minerals include pyrargyrite (Ag3SbS3), proustite (Ag3AsS3), aguilarite (Ag4SeS), tennantite Cu6(Cu4(Fe,Zn)2)As4S13 and tetrahedrite (Cu,Fe,Ag,Zn)12Sb4S13 with silver inclusions. As far as the dissolution of these silver minerals is concerned, acanthite tends to dissolve slowly and requires an excess of cyanide, while the other sulfosalts are even more refractory in nature with aerated cyanide solution. The sulphide ion that must be oxidized to release the ionic silver, is known to be the principal cause of refractoriness of the silver sulphide and sulfosalt minerals. This phenomenon differentiates the leaching reaction mechanism of silver sulphides and sulfosalts from those involved in metallic phase leaching [10,16,19-22].
Despite plenty of information available on the kinetics of gold dissolution with silver present in native, alloyed and/or dissolved forms, there are not many studies regarding the effect of prominent silver minerals under separate or accumulative impact and in association with sulphide minerals. A comprehensive study would lead to proper understanding of the effect of prominent silver minerals, such as pyrargyrite (Ag3SbS3) and also the accumulative effect of native silver (Ag), acanthite (Ag2S) and pyrargyrite (Ag3SbS3) on the kinetics of Au dissolution as well as the overall recovery for high silver bearing gold ores. In this work, the manner in which the presence of various silver phases and soluble silver affects gold leaching in free state as well as gold associated with sulphide minerals under galvanic and passivation effects was studied. The case with which the silver metal itself is leached from its bearing mineral species while present within silica and sulphide mineral matrices under direct and indirect impact was analyzed.
2 Experimental
2.1 Materials and reagents
The sulphide-rich ore samples: pyrite (Py), chalcopyrite (Cp), sphalerite (Sp) and stibnite (Sb), depending upon the dominant proportion of the named sulphide mineral therein, tested in this study, were received from Ward’s Natural Science. The chemical as well as mineralogical characterizations of these samples have been given elsewhere [9].
The samples were ground and sorted to remove particles coarser than 106 μm and finer than 53 μm. Consequently, the same granulometric fraction was used for the different ores, providing a uniform total area per unit-mass for all the cyanidation experiments. Pure gold (P80=39 μm, 99.998%), pure silver (P80=26 μm, 99.9%, Alfa Aesar, USA), pure silver sulphide (99.9% metals basis, Alfa Aesar, USA) and pyrargyrite mineral (Mineralogical Research Co.), were used in the present study. Solution used in all cyanidation experiments was prepared with distilled water. The reagents such as, sodium cyanide, NaCN (98%, Sigma-Aldrich, Canada), sodium hydroxide, NaOH (Fisher Scientific, Canada) and boric acid, H3BO3 (99.5%, Sigma-Aldrich, Canada), used in the present study, were all certified analytical grade.
2.2 Equipment and procedures
In the present study, two scenarios were taken into account. Firstly, the effect of pyrargyrite (Ag3SbS3) on gold dissolution was addressed in combination with sulphide minerals. Two arrangements for each sulphide mineral (X=Py, Cp, Sp, Sb) were tested in a packed-bed reactor (PBR) using one of the X powders, both the gold and pyrargyrite mineral dispersed within the sulphidic mineral (X), as well as silica layer (Fig. 1). Secondly, the accumulative impact of the prominent silver minerals such as, metallic silver (Ag), acanthite (Ag2S) and pyrargyrite (Ag3SbS3), on gold leaching behaviour under direct as well indirect contact with the sulphidic (X) minerals was taken into account as well. The notations α, β, γ will designate metallic silver (Ag), acanthite (Ag2S) and pyrargyrite (Ag3SbS3), respectively, unless otherwise specified.
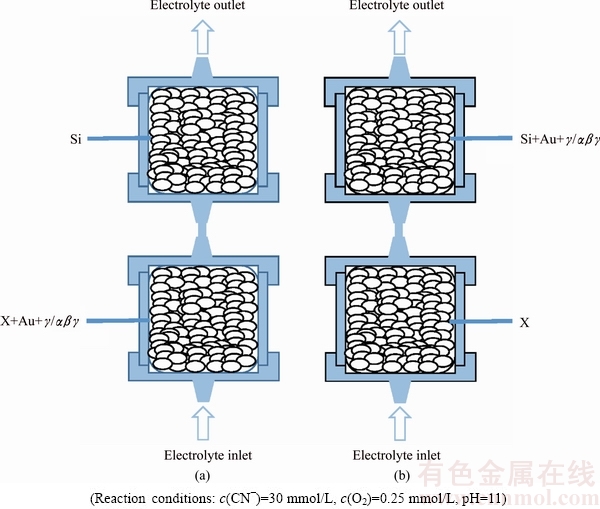
Fig. 1 Gold (Au) and silver minerals (γ/αβγ) dispersion pattern within sulphide (X) layer (a) and within quartz layer (b)
The leaching pattern of pure gold under direct influence of the sulphidic minerals was investigated by dispersion of 50 mg of Au powder in 4 g of one of the above “X” sulphide minerals. The galvanic interactions among Au as well as sulphide mineral particles were enabled via this arrangement. Conversely, the dispersion of Au powder within the inert silica layer thus will be segregated from the above sulphide minerals under the passivation effect from the segregated sulphide layer. Mineral and silica phases were electrically disconnected by packing these in separate reactors connected in series. The notation “||” depicts the inter-reactor separation in the ||X||silica|| syntax. Detailed studies on the leaching behaviour of these sulphides have been elaborated elsewhere [9]. In the present case, the total recovery of gold would be mentioned in comparison with this study.
First, the effect of silver minerals on gold dissolution was assessed with each of the above sulphide minerals in a two-layer ||X||silica|| configuration by seeding, one at a time with 50 mg Au powder along with 25 mg of γ, in one of the sulphide minerals (X = Py, Cp, Sp, or Sb) or the silica layers (Fig. 1). The influence of Ag3SbS3, on gold recovery by the distribution of gold and pyrargyrite within different mineral phases of a synthetic ore was studied using the PBR. The impact of various mineral associations on gold leaching in the presence of pyrargyrite has been addressed by placing Au, X and γ altogether within the same layer of the packed-bed reactor. This arrangement enables the Au-X-γ ternary galvanic interactions among all the constituents of the ore bodies. On the other hand, the indirect impact from the segregated sulphide layer as well as the direct contact with Au-γ was assessed in two-layer ||X||silica|| configurations by seeding Au and pyrargyrite either in X (X = Py, Cp, Sp, or Sb) layer or in the silica layer (Fig. 1). The modalities adopted in the present study have been elaborated such as ||X+Au+γ||silica|| and ||X||silica+Au+γ||, where Au and γ subscripts represent the seeded layer while “X” represents one of the above sulphide mineral layer.
The accumulative impact of the silver minerals in combination with the sulphide minerals was investigated by placing Au particles and the silver minerals α, β, γ in a similar way of two-layer configuration as explained above, i.e., ||X||silica||. The study was performed by the dispersion of all the precious metals within the sulphide as well as silica layers, like in the ||X+Au+αβγ||silica|| and ||X||silica+Au+αβγ|| two-layer systems. The accumulative effect of silver minerals on gold dissolution was assessed by seeding, one at a time with 50 mg Au powder along with 25 mg of each of the respective silver minerals α, β, and γ, in one of the sulphide minerals (X= Py, Cp, Sp, or Sb) or the silica layers (Fig. 1). Close contact among all the precious metals and sulphide particles results in a global galvanic environment while the dispersion of precious metals within the silica particles segregated from the sulphide layer would be under the passivation impact of the species resulting from the interaction of sulphide particles with the aerated cyanide solution.
Preparation of the feed solution for the cyanidation tests involved the dissolution of sodium cyanide (30 mmol/L CN-) in a sodium hydroxide solution buffered to pH 11 by the introduction of boric acid solution and stored in a 250 mL magnetically-stirred glass container. The dissolved oxygen concentration was maintained (~8.5 mg/L O2 at 25 °C) by continuous aeration of the solution with air-bubbling through a sparger for 6 h duration. This aerated cyanide solution was continuously recirculated through the PBR in closed loop by using a peristaltic pump at constant flow rate of 10.4 mL/min. Furthermore, the experimental set-up has been described in detail elsewhere [9]. The dissolved oxygen concentration was monitored by means of a dissolved oxygen probe (FOXY-AL300 model from Ocean Optics), while pH of the feed solution was maintained at 11±0.01 and was monitored by using an Oakton 1000 series pH-meter.
In case of Au and pyrargyrite (γ), the benchmark tests were performed by dispersing 50 mg of each precious metal Au and γ within the silica layer which filled the whole PBR working section. On the other hand, the benchmark test for accumulative silver minerals, such as silver metal, acanthite and pyrargyrite (αβγ), was performed by taking 50 mg of each silver mineral α, β, γ and dispersing those altogether within the silica layer which filled the whole PBR. The leaching patterns shown by Au and Ag were referred to as Au-curve and Ag-curve, respectively, in all leaching charts.
The chemical speciation of the dissolved precious metals present within the leach solution was performed by collecting small aliquots from the container at regular time intervals using a syringe after filtering with a particles filter VWR 0.45 μm. The analysis of the leach solution was performed by using a Perkin Elmer AA-800 atomic absorption spectrometer (AAS). The leaching kinetics as well as overall recovery for the precious metals was monitored by estimating the percentage of respective dissolved metal in comparison with the initially dispersed precious metals.
3 Results and discussion
3.1 Benchmark tests for gold and pyrargyrite leaching
Gold as well as the pyrargyrite dissolution tests were performed by their dispersion among silica particles, respectively, and their dissolution patterns were termed as benchmark test curves because of the inert chemical as well as electrochemical nature of the silica particles. Figure 2(a) represents the benchmark leaching curves for Au and Ag3SbS3 particles. The dissolution of pure Au particles with pure aerated cyanide solution, while dispersed within silica matrix, resulted in Au recovery of 15% after 6 h of cyanidation. This low dissolution of pure gold in pure aerated cyanide solution was due to the formation of a passivation layer on the surface of gold particles. Passivation of gold surface under certain cyanidation conditions has been confirmed experimentally due to the formation of insoluble sodium aurocyanide film on the surface of gold [23-25].

Fig. 2 Gold and pyrargyrite (γ) dissolution (a) and effect of pyrargyrite (γ) on gold dissolution (b) within quartz layer
In the case of Ag3SbS3, Ag recovery from this silver bearing-mineral dispersed in silica was estimated to be 2% after 6 h contacting with aerated cyanide solution as illustrated in Fig. 2(a). Pyrargyrite is a well-known refractory silver ore leading to very poor silver extractions (often ≤10%) in cyanide leaching [26]. Numerous pre-treatment strategies as well as oxidants have been used to enhance silver recovery from pyrargyrite [27].
The influence of Ag3SbS3 on the dissolution of Au particles, while both Au and Ag3SbS3 dispersed within the silica layer, was investigated and the leaching pattern for Au as well as Ag is shown in Fig. 2(b). Association of Ag3SbS3 with Au particles resulted in retardation of the Au dissolution within aerated cyanide solution. Au recovery was estimated to be 8.6% as compared to 15% of the benchmark test, while the Ag recovery was found to be 1.9% after 6 h of cyanidation and it almost followed the benchmark pattern of leaching for Ag from Ag3SbS3 (Fig. 2(b)).
3.2 Effect of pyrargyrite on Au leaching with pyrite- silica system
The influence of pyrargyrite on the kinetics as well as overall recovery of gold dissolution under galvanic and passivation impacts from the pyrite mineral layer is elaborated in Fig. 3. Sulphide minerals associated with gold play an important role during gold cyanidation. Close contact of gold and sulphide mineral particles gives rise to the development of galvanic interactions among the ore constituents. Gold as well as sulphide minerals undergo the phenomenon of galvanic corrosion when gold and a conductive mineral are brought into contact in the same corrosive environment, acting as two dissimilar electrodes [8,28]. Gold particles under the influence of galvanic contact with pyrite, resulted in Au dissolution of 74.6%, reducing to 15.8% under passivation from the segregated pyrite layer after 6 h of cyanidation (Table 1).
Close association of Au, Ag3SbS3 and pyrite mineral on Au dissolution was evaluated by mixing Au particles with Ag3SbS3 and pyrite mineral particles and packing altogether within the same PBR. On the other hand, the passivation effect of the pyrite mineral layer on Au dissolution in cyanide solution was assessed by placing both Au and Ag3SbS3 in the silica layer being segregated in the PBR from the pyrite mineral. The electrochemical contacts, i.e., ternary galvanic contacts, among all the constituents were enabled by placing Au, Ag3SbS3 and pyrite within the same layer. Dispersing Au and Ag3SbS3 among the silica particles, segregated from the pyrite layer, gave rise to binary galvanic contacts between Au and Ag3SbS3.
The presence of Au, Ag3SbS3 and pyrite particles in close contact with one another has resulted in Au recovery of 77.3% (Fig. 3(a)). Addition of Ag3SbS3 along with Au within the pyrite layer enhanced Au dissolution to 77.3% as compared to 74.6%, for Au within pyrite alone after 6 h of cyanidation. Ag3SbS3 enhanced the leaching kinetics as well as the overall recovery of gold for the pyrite mineral, while it was observed to retard the dissolution of gold present with the silica particles (Fig. 2(b)).
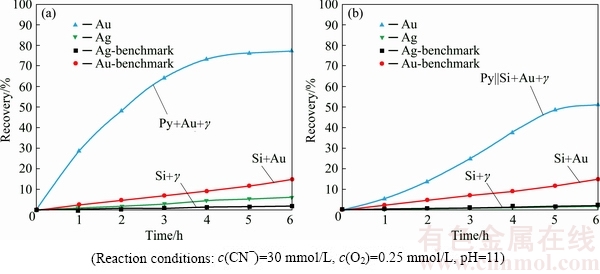
Fig. 3 Gold dissolution with pyrite and pyrargyrite, Py+Au+γ||Si (a) and pyrite and pyrargyrite, Py||Si+Au+γ (b)
Table 1 Effect of silver sulphide on gold dissolution with conductive sulphides [10]
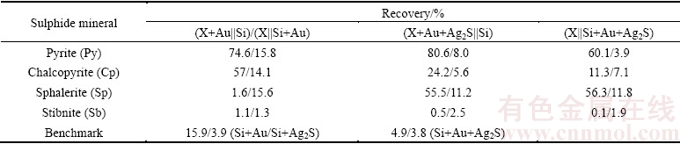
Although pyrargyrite addition has increased Au recovery for the pyrite-silica system, this increase was observed to be less as in case of acanthite present with gold for the same system. This could be attributed to the amount of silver dissolved during the cyanidation experiment which was higher in case of acanthite mineral than pyrargyrite mineral (Table 1). Similarly, silver recovery from the dissolution of Ag3SbS3 was estimated to be 6.1% as shown in Fig. 3(a), as compared to 2% in case of the benchmark test (Fig. 2(a)), after 6 h of cyanidation. The difficulty in the dissolution of pyrargyrite in aerated cyanide solution is related to the presence of stable pyramidal 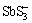 group in sulfosalt silver minerals [29].
group in sulfosalt silver minerals [29].
The presence of pyrargyrite along with gold among silica particles segregated by the pyrite mineral layer enhanced Au dissolution as well (Fig. 2(b)). Au recovery was estimated to be 51.2% with both Au and Ag3SbS3 present in the silica layer segregated by the pyrite layer. It was observed that Ag3SbS3 enhanced gold dissolution while Au particles were under indirect influence from the pyrite mineral particles. The increase in Au recovery was found to be less as compared to Au recovery for the Ag2S counterpart (Table 1). In this case, the enhancement in Au recovery is influenced by the amount of dissolved silver in the aerated cyanide solution (Fig. 3(b), Table 1). Ag recovery from Ag3SbS3 mineral particles present among silica particles and segregated from the pyrite particles was estimated to be 1.8% after 6 h of cyanidation (Fig. 3(b)). Au dissolution was enhanced by the introduction of pyrargyrite mineral under direct as well as indirect impact from the pyrite mineral particles. This shows that the silver recovered by the dissolution of pyrargyrite, acted in a similar way as that of the silver present in dissolved form in the aerated cyanide solution [30].
3.3 Effect of pyrargyrite on Au leaching with chalcopyrite-silica system
Pyrargyrite mineral influenced Au dissolution in the same manner as for acanthite mineral in a combination of the chalcopyrite-silica system in an aerated cyanide solution. The effects of Ag3SbS3 on the leaching kinetics as well as overall gold recovery in an aerated cyanide solution are shown in Fig. 4. The accumulative impact of pyrargyrite and chalcopyrite minerals on Au dissolution, while the micro-electrical contacts were enabled among all the constituents, resulted in Au dissolution of 38.03% (Fig. 4(a)), as compared to 57% for Au particles under galvanic impact from the chalcopyrite mineral particles (Table 1). The Ag recovered with this arrangement of mineral system was estimated to be 2.9% (Fig. 4(a)). The sulphides of silver like pyrargyrite are termed as refractory in the leaching process and the structural, electronic, and bonding properties of sulfosalts mainly determine their reactivity and the extent of their reaction in cyanide or other leaching systems [29].
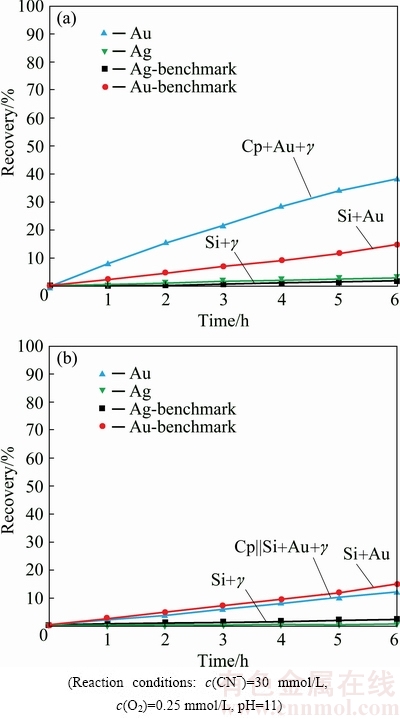
Fig. 4 Gold dissolution with chalcopyrite and pyrargyrite, Cp+Au+γ||Si (a) and with chalcopyrite and pyrargyrite, Cp||Si+Au+γ (b)
Addition of Ag2S to Au particles present among chalcopyrite particles, reduced Au dissolution to 24% after 6 h of cyanidation, while Ag recovered with this arrangement of the minerals as well as precious metal particles was estimated to be 5.6% (Table 1). This means that retardation in Au dissolution is directly related to the amount of Ag present in dissolved state in the aerated cyanide leach solution. The greater the amount of dissolved Ag in the leach solution, the greater the retardation observed in the kinetics as well as overall recovery of Au particles present in combination with chalcopyrite-silica system. Figure 4(a) also represents the kinetics and recovery of Ag from the dissolution of Ag3SbS3, while present in an arrangement where the galvanic contacts were enabled among all the mineral particles, and the Ag recovery was estimated to be 2.9% after the same duration of cyanidation.
Figure 4(b) demonstrates the leaching curves of Au and Ag while both Au and Ag3SbS3 were put altogether within the silica layer and segregated from the chalcopyrite mineral layer. Au recovery was estimated to be 12.1%, as compared to 15% for the benchmark test after 6 h of cyanidation. This means that the influence of Ag3SbS3 was proved to be retarded under the indirect effect from the chalcopyrite layer as well. The extent to which Au dissolution was retarded in this case was also observed to be lower as compared to Au dissolution for the same mineral configuration but with acanthite mineral (Table 1).
Ag3SbS3 mineral particles dispersed along with Au particles within the silica layer and segregated from the chalcopyrite mineral layer resulted in Ag recovery of 0.5% after 6 h of cyanidation (Fig. 4(b)). This demonstrates that a small reduction in Au recovery was due to little dissolution of pyrargyrite mineral in the cyanide leach solution. Overall, the association of Ag3SbS3 particles with Au particles, was found to have a net deceleration impact on Au dissolution, irrespective of the presence within the same or segregated layer of the Sb-bearing mineral. As much as the silver is dissolved in the cyanide solution with chalcopyrite, it will form an in-situ Ag2S passive film on the gold surface and retard the net dissolution of gold [9].
3.4 Effect of pyrargyrite on Au leaching with sphalerite-silica system
Association of pyrargyrite mineral influenced Au dissolution while in combination with sphalerite for which a two-layer mineral system arrangement was adopted, such as placement of Au and Ag3SbS3 in sphalerite as well as silica layers. Figure 5 illustrates the dissolution pattern of Au particles under the influence of Ag3SbS3 and in direct as well as indirect impact from the sphalerite mineral layer. The presence of Ag3SbS3 and Au particles within a direct contact with sphalerite enhanced the Au dissolution from 1.6% to 6.6%, while silver recovered by dissolution of Ag3SbS3 was estimated to be 1.1%, after 6 h of cyanidation (Fig. 5(a), Table 1). Addition of Ag3SbS3 to Au particles present in close contact with sphalerite mineral enhanced Au dissolution by 5% as compared to Au particles present with the sphalerite particles only (Table 1).
Although Au recovery was enhanced with addition of Ag3SbS3, for Au particles present with the sphalerite particles, it was not as high as that in case of Ag2S mineral for the same set of mineral arrangement, i.e., 55.5% (Table 1) after 6 h of cyanidation. This could be easily understood by the presence of little amount of Ag in dissolved form as a result of the dissolution of Ag3SbS3, i.e., 1.1% as compared to 11.2% for Ag2S (Table 1). This means that the presence of this amount of dissolved silver in the leach solution was not enough to overcome the poor electrical conductivity from the sphalerite particles [8-10].
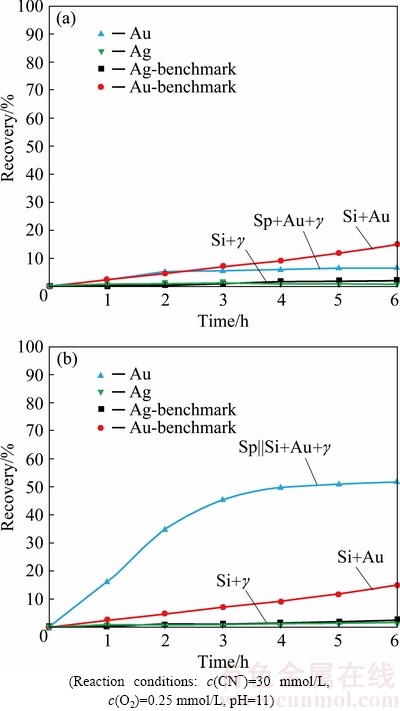
Fig. 5 Gold dissolution with sphalerite and pyrargyrite, Sp+Au+γ||Si (a) and with sphalerite and pyrargyrite, Sp||Si+Au+γ (b)
The leaching pattern as well as the overall Au recovery, while Au and Ag3SbS3 particles present within the silica particles and segregated from the sphalerite layer, is depicted in Fig. 5(b). The Au particles with the stated arrangement resulted in Au dissolution of 52%, as compared to 15.6% without addition of Ag3SbS3. The Ag recovered from the dissolution of Ag3SbS3 was measured to be 1.8% after 6 h of cyanidation. In this scenario, Au dissolution was significantly enhanced even with the smaller extent of Ag present in dissolved form (Fig. 5(b)).
Au dissolution was enhanced by 36.4% with addition of Ag3SbS3, segregated by the sphalerite layer as compared to Au present within silica layer and segregated from the Sp layer. From this, it is clear that even a little amount of Ag present within the cyanide leach solution would enhance Au dissolution remarkably while it is not contact with the poorly conducting sphalerite particles [9]. Pyrargyrite has been identified to have an increasing impact on Au dissolution in combination of sphalerite mineral under galvanic as well as passivation effect from the sulphide mineral layer.
3.5 Effect of pyrargyrite on Au leaching with stibnite-silica system
The effect of pyrargyrite on Au dissolution in combination to the stibnite mineral was investigated as well (Fig. 6). Stibnite has been reported to be very refractory regarding Au dissolution even present in a very small quantity within gold bearing ores [8,9]. Dissolution of Au particles was investigated by their placement within the stibnite as well as silica layer (Table 1). Figure 6(a) represents the Au leaching pattern as well recovery under the influence of Ag3SbS3 and in close connection with stibnite particles after 6 h of cyanidation. Adding Ag3SbS3 to the Au particles in connection with the stibnite particles, reduced Au recovery to 0.5% during the stipulated cyanidation time.
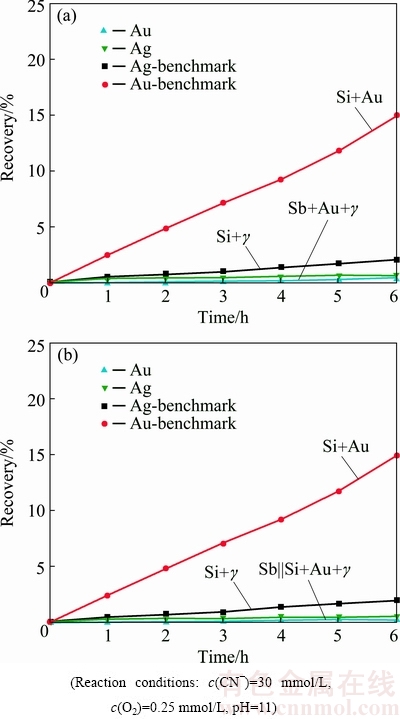
Fig. 6 Gold dissolution with stibnite and pyrargyrite, Sb+Au+γ||Si (a) and with stibnite and pyrargyrite, Sb||Si+Au+γ (b)
The addition of Ag3SbS3 reduced the Au recovery to 0.5%, as compared to 1.1% for the Au particles present within the stibnite layer only (Table 1). Ag recovered by the dissolution of Ag3SbS3 while present within the stibnite layer was measured to be 0.6%, after 6 h of contact with the aerated cyanide solution (Fig. 6(a)).
Au dissolution was investigated by placement of Au and Ag3SbS3 particles within the silica layer and segregating it from the stibnite layer as well (Fig. 6(b)). Au and Ag recoveries were found to be 0.38% and 0.56%, respectively after 6 h of cyanidation. Association of Ag3SbS3 with Au particles in segregated connection with stibnite mineral has been found to be retarding to Au dissolution. The influence of Ag3SbS3 on Au dissolution under direct as well as indirect impact from the stibnite mineral layer was observed to be retarding to Au dissolution severely. This severe reduction in Au recovery could be attributed to the formation of a tight antimony oxide (Sb2O5) as well as silver sulphide layers, resulted by the interaction of stibnite and pyrargyrite with the aerated cyanide solution. These findings are also in coherence with the findings in case of metallic silver as well as acanthite on Au dissolution with the stibnite mineral [9,10].
3.6 Benchmark tests for gold and silver minerals leaching
The benchmark test for the dissolution of pure gold in pure aerated cyanide solution was performed in the same way as explained above. In case of the benchmark dissolution test for the silver minerals, 50 mg of each of the three silver mineral constituents, namely, metallic silver (Ag), acanthite (Ag2S) and pyrargyrite (Ag3SbS3), designated as α, β and γ, was used. These silver minerals were dispersed all together within the silica layer which filled the whole PBR. The benchmark leaching pattern for the accumulative silver minerals is illustrated in Fig. 7(a), which also bears the Au benchmark leaching curve. Within 6 h, a fraction of 15% of gold leached out mirroring dissolution of 38% of silver minerals. The higher recovery of silver as compared to gold, is because of the presence of metallic silver which dissolves much more readily in aerated cyanide solution [8,9]. Dissolution of the other two counter parts, i.e., acanthite and pyrargyrite, has been reported to be notoriously slow in conventional cyanidation [10,27].
The prominent silver mineral acanthite (Ag2S) tends to dissolve slowly requiring an excess of cyanide with the sulfosalt-like pyrargyrite even more refractory. Retardation in silver extraction from Ag2S could be attributed to its low solubility [22]. The accumulative impact of the prominent silver minerals, such as metallic silver (α), acanthite (β) and pyrargyrite (γ), on Au dissolution was investigated as well (Fig. 7(b)). Au dissolution was 69.9% after 6 h of cyanidation as compared to 15% for the Au benchmark test, irrespective of the retardation impact of acanthite and pyrargyrite on Au dissolution within the silica layer [10]. This indicates that the presence of silver in the metallic form enhanced the Au dissolution remarkably, irrespective of the presence of retarding factors like acanthite as well as pyrargyrite minerals. In case of acanthite as well as pyrargyrite minerals, the decrease in Au dissolution could be attributed to the formation of passive films on the surface of gold particles in the cyanide media [10,31].
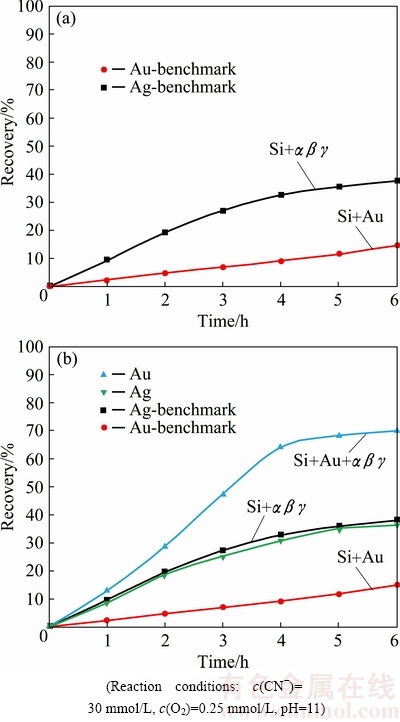
Fig. 7 Gold and silver minerals dissolution within quartz layer (a) and effect of silver minerals on gold (Au+αβγ) dissolution within quartz layer (b)
Species like AuCNads were held responsible for the oxidation as well as reduction of gold particles and also adsorbed species on gold surface such as AuCN·AuOH, Au(OH)(CN)-, Au(OH)(CN)3- and Au(OH) were reported to be responsible for the poor dissolution of impurity-free gold in pure aerated cyanide solutions [2,10]. Therefore, silver present in the metallic form was found to overcome the surface obstructing species resulted from the dissolution of acanthite and pyrargyrite in aerated cyanide solution. The surface passive film, such as silver sulphide, was not easy to dissolve with standard cyanidation conditions as it is clear from the dissolution pattern of acanthite [10].
3.7 Effect of Ag, Ag2S and Ag3SbS3 on Au leaching with pyrite-silica system
The accumulative impact of the most prominent silver minerals, such as metallic silver (Ag, α), acanthite (Ag2S, β) and pyrargyrite (Ag3SbS3, γ) was investigated on Au dissolution in direct as well as indirect contact with pyrite mineral layer. The influence of silver minerals on Au dissolution in combination to pyrite mineral was also investigated (Fig. 8).
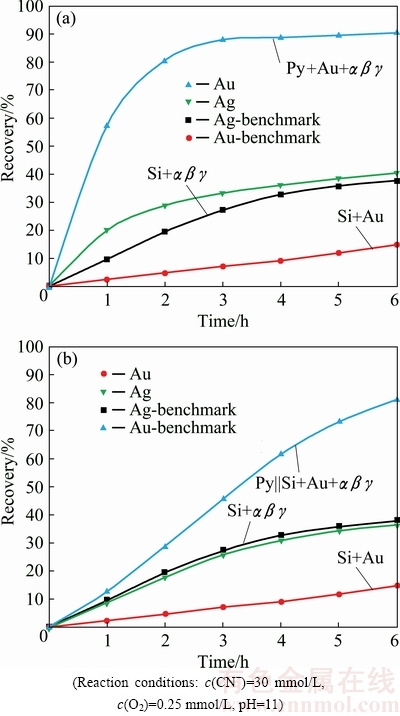
Fig. 8 Gold dissolution with pyrite and silver minerals αβγ, Py+Au+αβγ||Si (a) and with pyrite and silver minerals, Py||Si+Au+αβγ (b)
The presence of αβγ along with gold within the pyrite mineral layer resulted in Au recovery of 90.6%, while dissolution of silver minerals resulted in Ag recovery of 40.4% after 6 h of cyanidation (Fig. 8(a)). Au recovery was enhanced from 74.6% to 90.6%, with a net increase of 16%, by introducing αβγ to Au particles as compared to Au particles within the pyrite layer (Table 1). Placement of the silver minerals, Au and pyrite mineral particles within the same PBR gave rise to an arrangement, where all the constituents are under a state of global galvanic contacts resulting from close contact of the ore constructing constituents. Au and Ag recoveries represent a significant enhancement in precious metal recovery because of the establishment of permanent galvanic contacts.
The other set of arrangement made for these minerals placement followed the dispersion of silver minerals and Au within the silica layer contiguous to pyrite mineral layer. The precious metals leaching data with this type of minerals placement are shown in Fig. 8(b). Precious metal Au and Ag recoveries were 81.1% and 36.5%, respectively. The association of Au particles with αβγ particles within the silica layer, where the galvanic interactions were enabled among Au and αβγ particles and at the same time these minerals were under the passivation effect from the segregated pyrite mineral layer, resulted in a remarkable promotion in the dissolution of the precious metals. Like the presence of acanthite [10] and pyrargyrite, the presence of metallic silver along with two early mentioned analogues was found to enhance Au dissolution. Interestingly, as shown in Fig. 8, the increase in leaching kinetics as well as overall recovery of Au is significantly higher with the presence of metallic silver as compared to acanthite and pyrargyrite. It could be attributed to the role of silver as a bi-metallic corrosion, allowing oxygen reduction to occur on the silver surface in addition to the oxygen reduction taking place on the sulphide mineral surface [9].
3.8 Effect of Ag, Ag2S and Ag3SbS3 on Au leaching with chalcopyrite-silica system
Gold dissolution kinetics as well as overall recovery in close contact and/or segregation from the chalcopyrite mineral layer is summarized in Table 1. The influence of silver minerals on Au dissolution in contact with the chalcopyrite mineral is shown in Fig. 9.
Figure 9(a) shows Au recovery as a result of global galvanic contacts among the mineral and precious metal constituents due to the dispersion of silver minerals and Au particles within the chalcopyrite mineral particles. The precious metal recoveries for Au and Ag were 10.2% and 14.2%, respectively. The presence of silver minerals along with gold in direct combination with chalcopyrite particles resulted in a pronounced retardation in gold dissolution, with just 10.2% of Au recovery with αβγ as compared to 57% with Au present within chalcopyrite particles alone (Table 1). The non-silver sulphide mineral, such as chalcopyrite, was found to have a retarding effect on the leaching kinetics of silver sulphide in ferricyanide–cyanide system [30].
Acanthite and pyrargyrite were also observed to have a retarding effect while gold particles present under direct and indirect influence from the chalcopyrite mineral particles. It is obvious from the results discussed above and Table 1 that silver present in metallic form has retarded gold dissolution significantly as compared to silver minerals such as acanthite and pyrargyrite.

Fig. 9 Gold dissolution with chalcopyrite and silver minerals, Cp+Au+αβγ||Si (a) and with chalcopyrite and silver minerals, Cp||Si+Au+αβγ (b)
Chalcopyrite layer in segregation with respect to the silica layer hosting the silver minerals and Au particles prompted a passivation effect from the species resulting from the dissolution of chalcopyrite. With this set of arrangements for the various mineral constituents, Au and Ag recoveries were 4.5% and 13.1%, respectively, after 6 h of cyanidation. Au recovery was found to be lesser, even with the presence of galvanic contacts among the silver minerals and Au particles. This means that these galvanic interactions were not enough to overcome the retardation effect from the dissolved species of the segregated chalcopyrite mineral layer. Combination of silver minerals and chalcopyrite was observed to be retarding to Au dissolution irrespective of its presence in direct or indirect contact with Au particles. The silver minerals present in any combination with the chalcopyrite resulted in an in-situ production of silver sulphide film on the surface of Au particles and this film could be held responsible for the poor dissolution of Au particles [9,10].
3.9 Effect of Ag, Ag2S and Ag3SbS3 on Au leaching with sphalerite-silica system
The gold powder dispersed within the sphalerite layer as well as within the silica layer and segregated from the sphalerite mineral has been studied and Au recovery is given in Table 1. The impact of silver minerals on Au dissolution was studied by dispersion of αβγ and Au within the same sphalerite layer.
Under the global galvanic impact from ore constituents, Au and Ag recoveries were estimated to be 71.1% and 26.03%, respectively (Fig. 10(a)). The association of silver minerals with Au particles enhanced Au recovery from 1.6% (Table 1) to 71.1%, with a net increase of 69.5% in Au recovery with respect to Au present within the sphalerite layer. The results show that the association of αβγ with Au particles, while present within the sphalerite particles has overcome the poor electrical conductivity of sphalerite, with a remarkable increase in Au recovery (Fig. 10(a)).

Fig. 10 Gold dissolution with sphalerite and silver minerals, Sp+Au+αβγ||Si (a) and with sphalerite and silver minerals, Sp||Si+Au+αβγ (b)
The indirect influence from sphalerite mineral on Au dissolution was investigated by simultaneous dispersion of Au and silver minerals within a silica layer segregated from sphalerite. The precious metal recoveries for Au and Ag are shown in Fig. 10(b). The Au recovery was 80.5% while Ag recovered after 6 h of cyanidation was 36.5%. Similarly, Au recovered after the same period for Au particles dispersed within silica and segregated from sphalerite layer was 15.6% (Table 1). The results demonstrated that Au leaching kinetics and recovery were significantly enhanced, i.e., ~65% increase in Au recovery, with addition of silver minerals, while Ag recovery almost followed benchmark pattern.
Addition of silver minerals to Au particles for the sphalerite-silica system has been observed to have an accelerating effect on Au dissolution in a similar fashion as for Ag2S and Ag3SbS3. However, the presence of metallic silver made a tremendous contribution to Au dissolution as compared to Ag2S [10] and Ag3SbS3. Silver metal present as pure metal, alloyed or dissolved state, enhances Au dissolution significantly [9,10,17,32].
3.10 Effect of Ag, Ag2S and Ag3SbS3 on Au leaching with stibnite-silica system
Stibnite showed an intensive retarding effect towards gold dissolution [8-10,33]. Au recovery in aerated cyanide solution for gold associated with stibnite amounted to 1.1% (Table 1). The effect of silver minerals αβγ on Au dissolution in direct contact or indirect association with stibnite is illustrated in Fig. 11.
Figure 11(a) exposes the dissolution of Au particles while under global impact of galvanic interactions from silver minerals and stibnite. Recoveries of Au and Ag after 6 h of cyanidation were 0.5% and 2.1%, respectively. Au recovery for the stibnite-silica system was reduced from 1.1% to 0.5% with the addition of silver minerals. Ag recovery from the dissolution of silver minerals was also found to be retarded. Addition of silver minerals has further retarded the dissolution of Au embedded in the stibnite layer.
Association of αβγ on Au dissolution was investigated under indirect impact of stibnite mineral particles by placing stibnite in a separate layer. Au and Ag dissolutions are exemplified in Fig. 11(b). Recoveries of Au and Ag were 0.3% and 1.6%, respectively, after 6 h of cyanidation. Au recovery was retarded from 1.32% for Au within silica and segregated from stibnite to 0.3% for Au along with αβγ within silica and under segregation from stibnite layer. Au recovery was severely influenced by the stibnite mineral either in direct or indirect contact.
The dissolution of stibnite resulted in the formation of antimony oxides (Sb2O5) layer on the surface of Au particles which was responsible for the retardation in Au dissolution. Addition of silver minerals has further retarded the dissolution of gold because of the formation of Ag2S layer on the surface of gold particles in addition to the presence of antimony oxide layer [10,33].
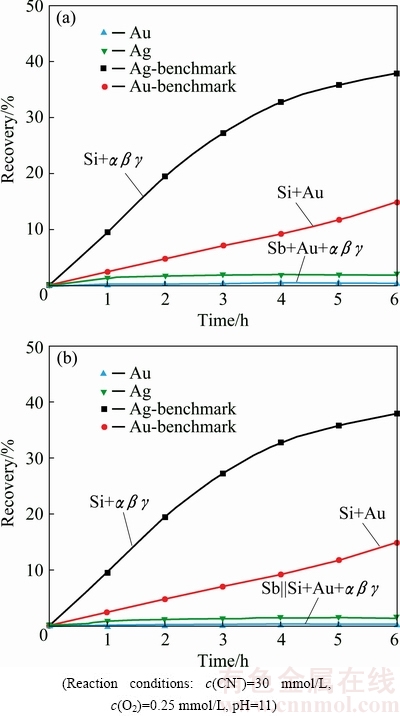
Fig. 11 Gold dissolution with stibnite and silver minerals, Sb+Au+αβγ||Si (a) and with stibnite and silver minerals, Sb||Si+Au+αβγ
4 Conclusions
1) The effect of prominent silver minerals, such as metallic silver, acanthite and pyrargyrite, was investigated in terms of leaching kinetics and overall recovery of free gold and gold associated with conductive sulphide minerals. A packed-bed reactor strategy was adopted to investigate the Au and Ag dissolution under direct as well as indirect impact from the sulphide minerals. Four sets of mineral systems were established: pyrite-silica, chalcopyrite-silica, sphalerite-silica and stibnite-silica systems.
2) The impact of pyrargyrite mineral on Au dissolution was investigated for free gold, i.e., gold dispersed within silica layer, and gold associated with sulphides. Pyrargyrite was found to be retarding for free gold, which was attributed to the formation of surface obstructing species due to the dissolution of pyrargyrite mineral. For sulphide-associated gold, the addition of pyrargyrite enhanced the leaching kinetics and overall Au recovery for the pyrite-silica as well as sphalerite-silica systems, while both Au and Ag3SbS3, being under the galvanic as well as passivation impact from the respective sulphide mineral particles. The chalcopyrite-silica and stibnite-silica systems were observed to have a retarding effect on Au dissolution in the presence of pyrargyrite mineral, irrespective of being under galvanic or passivation impact from the sulphide mineral.
3) The accumulative impact of the silver minerals (metallic silver, acanthite and pyrargyrite) was investigated for the free gold as well as gold associated with the sulphides in a similar manner. The presence of silver minerals with free gold enhanced significantly the kinetics and recovery of gold. On the other hand, for the gold associated with the sulphides, association of silver minerals with Au particles enhanced the leaching kinetics as well as overall recovery for the pyrite-silica and sphalerite-silica systems, under direct and indirect impact from the respective sulphides. The presence of silver minerals retarded the Au dissolution severely both under galvanic and passivation effects, for the chalcopyrite-silica and stibnite-silica systems. This retardation could be attributed to the formation of silver sulphide layer on the surface of gold particles upon addition of silver minerals.
Acknowledgements
Financial support from the Natural Sciences and Engineering Research Council through its Cooperative Research & Development grants program and from the supporting partners Agnico Eagle, Barrick, Camiro, Corem, Glencore, IamGold, Niobec and Teck is gratefully acknowledged. The authors are also very thankful to Prof. Brian Hart from Department of Earth Sciences (University of Western Ontario), and Dr. Patrick Laflamme from Corem for encouraging discussion on gold leaching. The author (MK) would like to thank Mr. Olivier Gravel for his guidance and support.
References
[1] MARSDEN J O, HOUSE C I. Chemistry of gold extraction [M]. 2nd ed. Littleton, Colorado: Society for Mining, Metallurgy, and Exploration (SME), 2006.
[2] SENANAYAKE G. A review of effects of silver, lead, sulfide and carbonaceous matter on gold cyanidation and mechanistic interpretation [J]. Hydrometallurgy, 2008, 90: 46-73.
[3] BAS A D, SAFIZADEH F, GHALI E, CHOI Y. Leaching and electrochemical dissolution of gold in the presence of iron oxide minerals associated with roasted gold ore [J]. Hydrometallurgy, 2016, 166: 143-153.
[4] LIU G Q, YEN W T. Effects of sulphide minerals and dissolved oxygen on the gold and silver dissolution in cyanide solution [J]. Minerals Engineering, 1995, 8: 111-123.
[5] DAI X, JEFFREY M I. The effect of sulfide minerals on the leaching of gold in aerated cyanide solutions [J]. Hydrometallurgy, 2006, 82: 118-125.
[6] AZIZI A, PETRE C F, LARACHI F. Leveraging strategies to increase gold cyanidation in the presence of sulfide minerals—Packed bed electrochemical reactor approach [J]. Hydrometallurgy, 2012, 111-112: 73-81.
[7] AZIZI A, PETRE C F, ASSIMA G P, LARACHI F. The role of multi-sulfidic mineral binary and ternary galvanic interactions in gold cyanidation in a multi-layer packed-bed electrochemical reactor [J]. Hydrometallurgy, 2012, 113-114: 51-59.
[8] AZIZI A, PETRE C F, OLSEN C, LARACHI F. Untangling galvanic and passivation phenomena induced by sulfide minerals on precious metal leaching using a new packed-bed electrochemical cyanidation reactor [J]. Hydrometallurgy, 2011, 107: 101-111.
[9] KHALID M, LARACHI F. Effect of silver on gold cyanidation in mixed and segregated sulphidic minerals [J]. The Canadian Journal of Chemical Engineering, 2017, 95: 698-707.
[10] KHALID M, LARACHI F, ADNOT A. Impact of silver sulfide on gold cyanidation with conductive sulfide minerals [J]. The Canadian Journal of Chemical Engineering, 2017, 95: 1875-1884.
[11] LORENZEN L, van DEVENTER J S J. The mechanism of leaching of gold from refractory ores [J]. Minerals Engineering, 1992, 5: 1377-1387.
[12] AZIZI A, PETRE C F, OLSEN C, LARACHI F. Electrochemical behavior of gold cyanidation in the presence of a sulfide-rich industrial ore versus its major constitutive sulfide minerals [J]. Hydrometallurgy, 2010, 101: 108-119.
[13] AZIZI A, OLSEN C, LARACHI F. Efficient strategies to enhance gold leaching during cyanidation of multi-sulfidic ores [J]. The Canadian Journal of Chemical Engineering, 2014, 92: 1687-1692.
[14] HISKEY J B, SANCHEZ V M. Mechanistic and kinetic aspects of silver dissolution in cyanide solutions [J]. Journal of Applied Electrochemistry, 1990, 20: 479–487.
[15] SENANAYAKE G. The cyanidation of silver metal: Review of kinetics and reaction mechanism [J]. Hydrometallurgy, 2006, 81: 75-85.
[16] LIN H K, OLESON J L, WALSH D E. Behavior of gold and silver in various processing circuits at the Fort Knox Mine [J]. Minerals and Metallurgical Processing, 2010, 27: 219-223.
[17] JEFFREY M I, RITCHIE I M. The leaching of gold in cyanide solutions in the presence of impurities II: The effect of silver [J]. Journal of The Electrochemical Society, 2000, 147: 3272-3276.
[18] DAI X, BREUER P L. Leaching and electrochemistry of gold, silver and gold–silver alloys in cyanide solutions: Effect of oxidant and lead(II) ions [J]. Hydrometallurgy, 2013, 133: 139-148.
[19] LUNA-SANCHEZ R M, LAPIDUS G T. Cyanidation kinetics of silver sulphide [J]. Hydrometallurgy, 2000, 56: 171–188.
[20] PARGA J R, VALENZUELA J L, CEPEDA T F. Pressure cyanide leaching for precious metals recovery [J]. The Journal of The Minerals, Metals & Materials Society, 2007, 59: 43-47.
[21] RAJALA J, DESCHENES G. Extraction of gold and silver at the Kupol Mill using CELP [C]//World Gold Conference 2009. Johannesburg: The Southern African Institute of Mining and Metallurgy, 2009: 35-42.
[22] CELEP O, BAS A D, YAZICI E Y, ALP I, DEVECI H. Improvement of silver extraction by ultrafine grinding prior to cyanide leaching of the plant tailings of a refractory silver ore [J]. Mineral Processing and Extractive Metallurgy Review, 2015, 36: 227-236.
[23] CATHRO K J, KOCH D F A. The anodic dissolution of gold in cyanide solutions [J]. Journal of Electrochemical Society, 1964, 111: 1416-1420.
[24] THURGOOD C P, KIRK D W, FOULKES F R, GRAYDON W F. Activation energies of anodic gold reactions in aqueous alkaline cyanide [J]. Journal of the Electrochemical Society, 1981, 128: 1680-1685.
[25] WADSWORTH M E, ZHU X, THOMPSON J S, PEREIRA C J. Gold dissolution and activation in cyanide solution [J]. Hydrometallurgy, 2000, 57: 1-11.
[26] CELEP O, ALP I, PAKTUNC D, THIBAULT Y. Implementation of sodium hydroxide pretreatment for refractory antimonial gold and silver ores [J]. Hydrometallurgy, 2011, 108: 109-114.
[27] RODRIGUEZ-RODRIGUEZ C, NAVA-ALONSO F, URIBE- SALAS A, VINALS J. Pyrargyrite (Ag3SbS3): Silver and antimony dissolution by ozone oxidation in acid media [J]. Hydrometallurgy, 2016, 164: 15–23.
[28] AGHAMIRIAN M M, YEN W T. Mechanisms of galvanic interactions between gold and sulfide minerals in cyanide solution [J]. Minerals Engineering, 2005, 18: 393-407.
[29] MELENDEZ A M, GONZALEZ I, ARROYO R. An approach to the reactivity of isomorphous proustite (Ag3AsS3) and pyrargyrite (Ag3SbS3) in cyanide solutions [J]. ECS Transactions, 2010, 28: 191-199.
[30] XIE D, DREISINGER D B. Leaching of silver sulfide with ferricyanide–cyanide solution [J]. Hydrometallurgy, 2007, 88: 98-108.
[31] BAS A D, SAFIZADEH F, ZHANG W, GHALI E, CHOI Y. Active and passive behaviors of gold in cyanide solutions [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 3442-3453.
[32] WADSWORTH M E, ZHU X. Kinetics of enhanced gold dissolution: Activation by dissolved silver [J]. International Journal of Mineral Processing, 2003, 72: 301-310.
[33] GUO H, DESCHENES G, PRATT A, FULTON M, LASTRA R. Leaching kinetics and mechanisms of surface reactions during cyanidation of gold in the presence of pyrite and stibnite [J]. Minerals and Metallurgical Processing, 2005, 22: 89-95.
银硫化物对多金属硫化矿氰化浸金的影响
Muhammad KHALID,  LARACHI
LARACHI
Department of Chemical Engineering, Laval University, Québec, QC, G1V 0A6, Canada
摘 要:浸金会受到伴生银和多金属硫化矿的影响。采用填充床反应器研究4组矿物系统:黄铁矿-二氧化硅、黄铜矿-二氧化硅、闪锌矿-二氧化硅和辉锑矿-二氧化硅电极的电偶和钝化效果。结果表明,在电偶和钝化作用下,硫锑银矿(Ag3SbS3)使黄铁矿中Au的浸出率分别提高到77.3%和51.2%(相对于74.6%和15.8%);在电偶和钝化作用下,闪锌矿+硫锑银矿也使金的浸出率分别提高到6.6%和51.9%(相对于1.6%和15.6%);黄铜矿+硫锑银矿使金的浸出率分别降低到38.0%和12.1%(相对于57%和14.1%);在电偶和钝化作用下,添加银矿使黄铁矿中Au的浸出率分别提高到90.6%和81.1%(相对于74.6%和15.8%);添加银矿物和闪锌矿使Au浸出率分别提高至71.1%和80.5%(相对于1.6%和15.6%);添加银矿物和黄铜矿,金的浸出率分别降低至10.2%和4.5%(相对于57%和14.1%)。硫锑银矿和辉锑矿在一起会阻碍金的溶解。硫锑银矿和添加银矿增强游离金与黄铁矿和闪锌矿伴生金的溶解,黄铜矿和辉锑矿中伴生金银阻碍了金的溶解。
关键词:银矿;氰化浸金;填充床反应器;硫化矿;钝化;电偶相互作用
(Edited by Xiang-qun LI)
Corresponding author:  LARACHI; Tel: +1-418-6563566; Fax: +1-418-6565993; E-mail: Faical.Larachi@gch.ulaval.ca
LARACHI; Tel: +1-418-6563566; Fax: +1-418-6565993; E-mail: Faical.Larachi@gch.ulaval.ca
DOI: 10.1016/S1003-6326(18)64687-4
Abstract: Gold leaching was influenced in association with silver and polymetal sulphide minerals. A packed bed was adopted to single out the galvanic and passivation effects with four sets of minerals: pyrite-silica, chalcopyrite-silica, sphalerite-silica and stibnite-silica. Pyrargyrite enhanced Au recovery to 77.3% and 51.2% under galvanic and passivation effects from pyrite (vs 74.6% and 15.8%). Pyrargyrite in association with sphalerite also enhanced Au recovery to 6.6% and 51.9% (vs 1.6% and 15.6%) under galvanic and passivation effects from sphalerite. Pyrargyrite associated with chalcopyrite retarded gold recovery to 38.0% and 12.1% (vs 57% and 14.1%) under galvanic and passivation effects. Accumulative silver minerals enhanced Au recovery to 90.6% and 81.1% (vs 74.6% and 15.8%) under galvanic and passivation impacts from pyrite. Silver minerals with sphalerite under galvanic and passivation effects enhanced Au recovery to 71.1% and 80.5% (vs 1.6% and 15.6%). Silver minerals associated with chalcopyrite retarded Au recovery to 10.2% and 4.5% under galvanic and passivation impacts (vs 57% and 14.1%). Stibnite retarded Au dissolution with pyrargyrite and accumulative silver minerals. Pyrargyrite and accumulative silver enhanced gold dissolution for free gold and gold associated with pyrite and sphalerite. Gold dissolution was retarded for gold and silver minerals associated with chalcopyrite and stibnite.


