Article ID: 1003-6326(2005)05-1172-06
Synthesis and characterization of CoFe2O4 nanoparticles
XIAO Xu-xian(肖旭贤)1, HUANG Ke-long(黄可龙)1,
YAN Jian-hui(阎建辉)1,2, HE Qiong-qiong(何琼琼)3
(1.School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Department of Chemical Engineering, Hunan Institute of Science and Technology, Yueyang 414000, China;
3. School of Basic Medical Sciences, Central South University, Changsha 410078, China)
Abstract:
The reverse microemulsion composition consisting of 37.0% cyclohexane, 26.0% surfactant (TX-10 and AEO9), 13.0% n-pentanol and 24.0% aqueous phase was investigated and chosen for the preparation of cobalt ferrite nanoparticles. Then silicon dioxide was coated onto the surface of the magnetite nanoparticles. The two kinds of nanoparticles were characterized by means of X-ray diffractometry(XRD), scanning electron microscopy (SEM), infrared spectroscopy (IR), and energy dispersion spectrometry (SEM-EDS). The SEM results indicate that both nanoparticles have narrow size distribution, less agglomeration and are in the size range of 10-60nm. XRD patterns show that there is not any peak detected except for the peaks of CoFe2O4, and imply that the coated silicon dioxide is amorphous. IR absorption spectra of the samples show the characteristic bands of Si—O—Si group and Fe—O group. SEM-EDS indicates that the molar ratio of Fe to Si is 96.11∶3.89. These results prove that a thin film of SiO2 is coated on the surface of the magnetite nanoparticles. And the characterization of cobalt ferrite nanoparticles prepared by conventional precipitation method are compared.
Key words:
CoFe2O4; nanoparticle; microemulsion; silicon dioxide; coating CLC number: TQ138.1;
Document code: A
1 INTRODUCTION
Recently, the nanoparticles in the size range of 1-100nm have been received increasing attention because of their unusual physical and chemical properties compared with larger particles[1, 2]. Various nanoparticles have been prepared by different kinds of methods, such as coprecipitation method[3], shock wave treatment[4], sol-gel process[5], hydrothermal process[6] and microemulsion approach[7-10] . In comparison, microemulsion system is more attractive than other processing routes, so various organic or inorganic nanoparticles have been prepared via microemulsion approach. For example, Wang et al[11] reported the preparation of α-Fe nanoparticles by a microemulsion system consisting of saturated Fe2+ solution, isopropanol and PVP; Chen et al[1] synthesized colloidal gold nanoparticles by a reverse microemulsion system consisting of CTAB, n-pentanl, hexane and water; Caponetli et al[2] prepared CdS nanoparticles by a microemulsion system containing water, AOT and n-heptane.
Ferrite nanoparticles are the promising materials for extensive investigation and application because of their excellent catalytic activity and magnetic characteristics. At present, several synthesis methods of CoFe2O4 nanoparticles have been developed, such as chemical coprecipitation method[12], sol-gel process[13] and microemulsion approach[14]. For example, Xu et al[15] synthesized nano-particles by combustion method; Shah and Pillai[14] prepared the precursor of CoFe2O4 by microemulsion approach, and these precursors were then calcined to prepare CoFe2O4 nanoparticles. Ferrite nanoparticles have been applied to biomedical research for the last decade. Although ferrite nanoparticles have good magnetic characteristics, their poor biocompatibility restricts their application. While SiO2 has good biocompatibility. In this paper, we attempted to synthesize CoFe2O4 nanoparticles using a new reverse microemulsion system consisting of water(or brine), TX-10, AEO9, cyclohexane and n-pentanol. TX-10 and AEO9 were used as a new mixed surfactant with the mass ratio of 1∶1. And then we prepared silica-coated CoFe2O4 with good biocompatibility and magnetic characteristics by coating process. The characterization of uncoated CoFe2O4 and SiO2-coated CoFe2O4 nanoparticles were investigated using XRD, SEM, IR and EDS techniques, and the characterization of CoFe2O4 nanoparticles prepared by conventional precipitation method was also compared.
2 EXPERIMENTAL
2.1 Chemical reagents
Fe(NO3)3·9H2O(99.0% pure), Co(NO3)2(99.0% pure), cyclohexane (99.0% pure), n-pentanol (99.0% pure) and ammonia (>36%, NH3) were produced by the Second Chemical Reagent Plant of Shanghai, China. Methanol(99.0% pure) and acetone(99.0% pure) were purchased from the Chemical Reagent Plant of Hunan Normal University. TX-10 (99.0%, technical grade) and AEO9(99%, technical grade)were supplied by Hunan Licheng Groups and double-distilled water was used to prepare the samples.
2.2 Microemulsion composition
In order to prepare reverse microemulsions, a high-purity cyclohexane was used as the continuous oil phase; n-pentanol was used as cosurfactant; mixture of AEO9 and TX-10(mass ratio of 1∶1) was used as surfactant; and an aqueous solution was used as the dispersd phase. The composition was indicated in Table 1.Two microemulsions (Ⅰand Ⅱ) with identical compositions but different aqueous phases were prepared. The aqueous phase in microemulsionⅠwas a mixture of cobalt(Ⅱ) nitrate and ferric nitrate solution with the molar ratio of 1∶2. The aqueous phase in microemulsion Ⅱ was the precipitation agent, ammonia, with a sufficient concentration for the precipitating of Co2+ and Fe3+ ions. In order to identify the oil-continuous compositions of these two reverse microemulsions, we determined the two partial ternary phase diagram (at room temperature) with the mixed nitrate solution or ammonia solution as aqueous phase. The oil-continuous microemulsions were checked by the measurement of electrical conductivity.
Table 1 Composition of microemulsion system
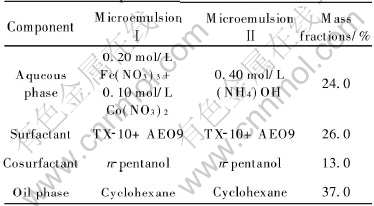
2.3 Nanoparticle preparation
CoFe2O4 nanoparticles were prepared via two processing methods: 1) the conventional precipitation method and 2) the microemulsion method. In the precipitation method, aqueous NH3 solution (24%, mass fraction) was added to 0.10mol/L Co2+ and 0.20mol/L Fe3+ solution, then the system was refluxed at 85℃ for 5h. Finally CoFe2O4 nanosized product was dried under vacuum at 60-70℃.
For the microemulsion method, these two reverse microemulsions (Ⅰand Ⅱ ) were mixed under constant stirring. Within the nano-sized aqueous droplets of the reverse microemulsion, the reaction of Co2+ and Fe3+ ions with OH- anions happened and the cobalt-iron hydroxide precipitate formed. The process of the reaction was controlled by the pH value through modulating the amounts of ammonia reverse microemulsion. After 10min, the system was warmed to 95℃, refluxed for 5h, and the CoFe2O4 nanoparticles appeared. Being naturally cooled to room temperature, the CoFe2O4 nanoparticles were separated from the reaction media by centrifugation and washed with absolute ethanol and distilled water for several times. Finally, CoFe2O4 nanosized product was dried under vacuum at 60-70℃ for 7h.
The CoFe2O4 powders were added into the glycol solution, and then the water glass solution was added into the mixed solution. The pH value of the solution was adjusted to about 10 using 1mol/L HCl. The mass fraction of CoFe2O4 was 7%. The gelation time was longer than 2h with the temperature of 80℃. After being coated, the particles were filtered and washed using double-distilled water and then dried at 120℃ for 3h.
2.4 Characterization
All products were characterized using X-ray diffractometry (CuKα, Philips PW1729). The powders were also characterized for particle/agglomeration size distribution using laser scattering technique (Horiba LA-910). Observation of crystallites by scanning electron microscopy (SEM) was performed on LEO 1430 VP SEM. The element composition of the coated particle was analyzed by energy dispersion spectrometry (SEM-EDS, KYKY-2800). Infrared spectroscopy (IR, Nicolet, 750) of the samples was used to study the chemical bonds between uncoated CoFe2O4 and SiO2 coated CoFe2O4 powders.
3 RESULTS AND DISCUSSION
The partial quasi-ternary phase diagrams of the reverse microemulsion systems of TX-10, AEO9, n-pentanol, cyclohexane and Brine at 30℃ are shown in Figs.1(a) and (b), where S (surfactant) and C (cosurfactant) represent the total mass of TX-10, AEO9 and n-pentanol, O is the mass of oil phase and W is the mass of brine. Two aqueous solutions are not the same in two figures: one is mixed nitrates of 0.10mol/L Co(NO3)2 and 0.20mol/L Fe(NO3)3 in Fig.1(a) and the other aqueous solution is 0.4mol/L ammonia solution in Fig.1(b). But the oil, surfactant and cosurfactant used are the same in two figures. The reverse microemulsion region is represented by the shaded area in two figures. The two phase diagrams are similar and the reverse microemulsion region is widened with increasing mixed surfactant/oil phase ratio.
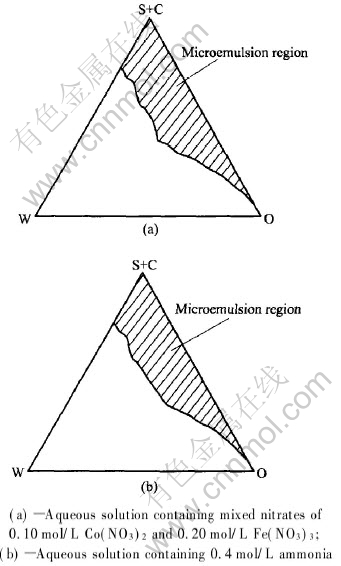
Fig.1 Partial phase diagram established for ternary system comprising coclohexane,
TX-10+AEO9, n-pentanol and different aqueous solutions at room temperature
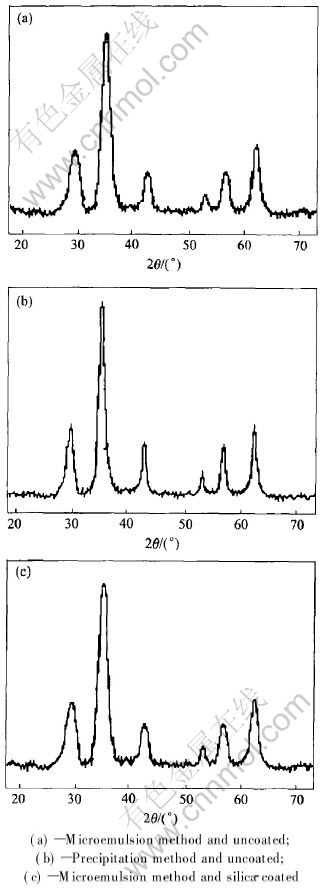
Fig.2 X-ray diffraction patterns of nanoparticles prepared by different methods
Figs.2(a) and (b) show the XRD patterns for the particles prepared with the microemulsion and precipitation method, respectively. It is evident that these powders contain only spinel ferrite. CoFe2O4 can be formed by two methods and all the peaks in the two patterns match well with JCPDS card. No impurity peaks can be found on the XRD patterns. However, it can be observed that the peaks for the particles obtained by the micro-emulsion method have broader and weaker intensity patterns than those by the precipitation method. This phenomenon is not resulted from lower crystallinity or amorphous structure. Fig.2(c) shows the X-ray diffraction pattern of the silicon dioxide-coated CoFe2O4 which is similar to that in Fig.2(a). No diffraction peak characteristic of crystalline silica is observed, whereas the six characteristic diffraction peaks of CoFe2O4 are examined by X-ray diffractometry. This result implies that the coated silicon dioxide is amorphous.
The SEM images of the particles prepared by the microemulsion and precipitation method are shown respectively in Figs.3(a) and (b). It is obvious from these figures that the sizes and morphologies of the particles show little difference. The particles prepared by the microemulsion method are spherical and regular (Fig.3(a)). Their diameters are in the range of 10-50nm and narrow particle size distribution is shown in Fig.4(a).Whereas the particles prepared by precipitation method are less regular and some are agglomerated(Fig.3(b)). Their diameters are in the range of 30-70nm and a very broad particle size distribution is shown in Fig.4(b). Fig.3(c) shows the SEM image of the magnetite nanoparticles prepared by the microemulsion and coated with silicon dioxide, which shows that most of the particles are spherical with an average diameter of 35nm. The distribution of particle diameters is shown in Fig.4(c).
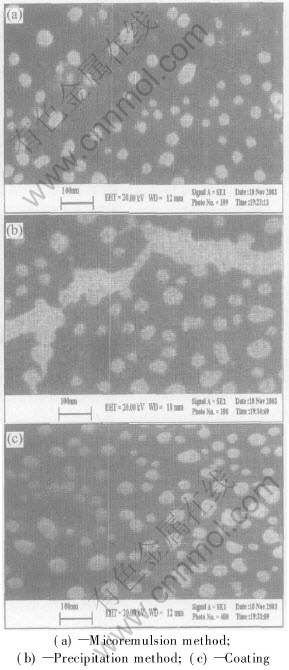
Fig.3 SEM micrographs of nanoparticles prepared by different methods
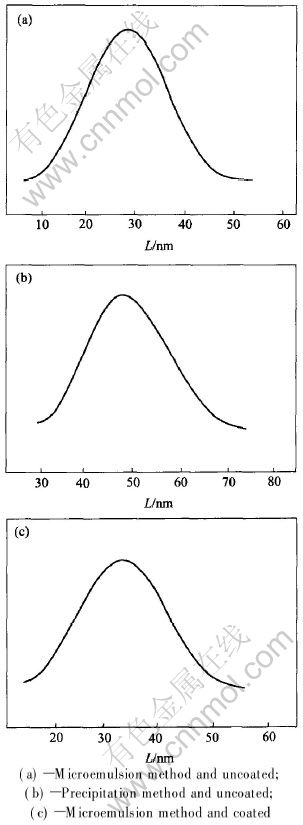
Fig.4 Size distribution of nanoparticles prepared by different methods
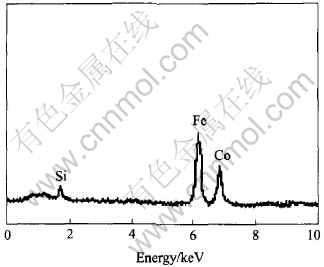
Fig.5 SEM-EDS elemental analysis of SiO2-coated CoFe2O4 nanoparticles
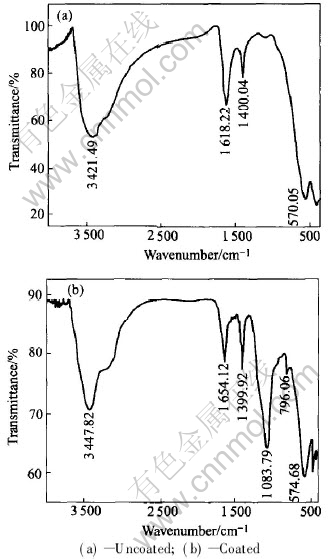
Fig.6 IR spectra of samples
EDS is used to identify the elements of the powder. Fig.5 shows a typical SEM-EDS elemental analysis result of silica-coated CoFe2O4 nanoparticles. Si element peak at 1.76keV can be seen from the figure. The molar ratio of Fe to Si is 96.11∶3.89. We can conclude that a thin film of silicon dioxide is coated on the surface of CoFe2O4 particles.
IR spectroscopy analysis is also used to evaluate the presence of silicon dioxide on the surface of CoFe2O4 nanoparticles. Figs.6(a) and (b) show the IR spectra of uncoated and coated CoFe2O4 nanoparticles respectively. Three absorption peaks appear in the spectra, and they are related to the characteristic vibrational transverse optical modes of Si—O—Si chemical bond. The band which appears at about 453.17cm-1 is the rocking vibrational mode; the 796.06cm-1 absorption band corresponds to the bending vibration; and the band near about 1083.79cm-1 corresponds to an asymmetric stretching vibration of Si—O—Si bond. The characteristic absorption bands (at 574.68 and 413.14cm-1) of the Fe—O bond of SiO2-coated CoFe2O4 compared with those of uncoated CoFe2O4 (at 570.05 and 410.11cm-1) are also found. In addition, in Figs.6(a) and (b) the absorption bands near 3400 and 1630cm-1 refer to the vibration of remainder H2O in the samples. And there also exists the contribution of adsorbed CO2 for the band near 1400cm-1 in Figs.6(a) and (b). From these results of IR spectra and SEM-EDS, it is proven that the CoFe2O4 nanoparticles can be coated with silicon dioxide.
4 CONCLUSIONS
A new reverse microemulsion system consisting of TX-10, AEO9, cyclohexane n-pentanol and wate(brine) was studied and successfully used to synthesize CoFe2O4 nanoparticles. We have prepared SiO2-coated CoFe2O4 with good biocompatibility and magnetic characteristics by coating process. Then, the difference among microstructures, crystallogram, and IR spectra of uncoated and silica-coated products prepared from reverse micoemulsion method was investigated. The characterization of CoFe2O4 nanoparticles prepared by conventional precipitation method was also valued. These results are utilized to prove the formation of silicon dioxide thin films on the surface of nanoparticles.
REFERENCES
[1]CHEN F X, XU G Q, HOR T S A. Preparation and assembly of colloidal gold nano-particles in CTAB-stabilized reverse microemulsion [J]. Materials Letters, 2003, 57(21): 3282-3286.
[2]Caponetti E, Pedone L, Chillura-Martino D, et al. Synthesis, size control, and passivation of CdS nanoparticles in water/AOT/n-heptane microemulsions [J]. Mater Sci Eng C, 2003, 23(4): 531-539.
[3]Macek J, Marinek M. Formation of nickel and zirconia nanocomposites by the coprecipitation method [J]. Nanostructured Mater, 1999, 12(1-4): 499-520.
[4]XU Kan, LIU Jian-Jun, XU Tao, et al. Synthesis nanometer-sized zinc ferrite by shock wave treat [J]. J Inorg Mater, 1997,12(5): 759-762.
[5]Koteswara R K, Banu T, Vithal M, et al. Preparation and characterization of bulk and nano particles of La2Zr2O7 and Nd2Zr2O7 by sol-gel method [J]. Mater Lett, 2002, 54(2-3): 205-210.
[6]XIA Chang-tai, SHI Er-wei, ZHONG Wei-zhuo, et al. Hydrothermal synthesis of BaTiO3 nano/microcrystals [J]. Journal of Crystal Growth, 1996, 166(1-4): 961-966.
[7]Porta F, Prati L, Rossi M, et al. Synthesis of Au(0) nanoparticles from W/O microemulsions [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 211(1): 43 - 48.
[8]QI Li-min, MA Ji-ming, CHENG Hu-min, et al. Preparation of BaSO4 nanoparticles in non-ionic W/O microemulsions [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1996, 108: 117-126.
[9]QIU Sun-qing, DONG Jun-xiu, CHEN Guo-xu. Preparation of Cu nanoparticles from water-in-oil micro-emulsions [J]. Journal of Colloid and Interface Science, 1999, 216: 230-234.
[10]Calandra P, Goffredi M, Liveri V. Study of the growth of ZnS nanoparticles in Water/AOT/n-heptane microemulsions by UV-absorption spectroscopy [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999, 160(1): 9-13.
[11]WANG C Y, JIONG W Q, ZHOU Y, et al. Synthesis of α-Fe ultrafine particles in a saturated salt solution/isopropanol/PVP microemulsion and their structural characterization [J]. Materials Research Bulletin, 2000, 35: 53-58.
[12]CHE Ren-chao, LI Yong-qing, CHEN Zhao-hui, et al. Research of chemical preparation process and magnetic property of co-ferrite fine powder [J]. Journal of Functional Materials, 1999, 30(6): 615-616.
[13]SHI Xiao-bo, LI Chun-gen, WANG De-xian. The preparation and the catalytic activity of CoFe2O4 nanoparticles [J]. Chemistry, 2000, 8: 544-546.
[14]Shah D O, Pillai V. Synthesis of high-coercivity cobalt ferrite particles using water-in-oil microemulsions [J]. Journal of Magnetism and Magnetic Materials, 1996, 163: 243-248.
[15]XU Zhi-gan, CHENG Fu-xian, ZHOU Biao, et al. Synthesis of CoFe2O4 nanoparticles by combustion method and its characterization [J]. Chinese Science Bulletin, 2000, 45(17): 1837-1841.
Foundation item: Project(2001AA218011) supported by the Hi-tech Research and Development Program of China
Received date: 2004-11-26; Accepted date:2005-06-20
Correspondence: HUANG Ke-long, Professor, PhD; Tel: +86-731-8879850; E-mail: klhuang@mail.csu.edu.cn


