二段中和法处理酸性矿山废水
郑雅杰1, 2,彭映林1, 2,李长虹2
(1. 中南大学 冶金科学与工程学院,湖南 长沙,410083;
2. 中南大学 云浮研究院,广东 云浮,527300)
摘要:采用石灰与氢氧化钠二段中和法处理酸性矿山废水。研究结果表明:用石灰调节废水pH至5时,Fe,Mn,Zn的去除率分别为14.14%,5.94%和13.91%;采用氢氧化钠二段中和后,当废水pH为10.20,曝气流量为50 mL/min,反应时间为20 min时,废水中铁、锰、锌去除率均达到99.7%以上,其废水中TFe,Mn2+和Zn2+残留质量浓度分别为80,810,30 μg/L,均低于国家污水综合排放标准(GB 8978—1996)。石灰一段中和渣为石膏(CaSO4·2H2O);氢氧化钠二段中和渣为锰锌铁氧体(Fe2Mn0.5Zn0.5O4·nH2O)和四氧化三铁(Fe3O4);石灰与氢氧化钠二段中和法与石灰中和法相比较,二段中和渣量少,二段中和渣具有综合利用价值。
关键词:
中图分类号:X703.1 文献标志码:A 文章编号:1672-7207(2011)05-1215-05
Treatment of acid mine drainage by two-step neutralization
ZHENG Ya-jie1, 2, PENG Ying-lin1, 2, LI Chang-hong2
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Yunfu Institute of Central South University, Yunfu 527300, China)
Abstract: The acid mine drainage was treated by two-step neutralization method using lime and sodium hydroxide. The results show that the removal rates of total iron (TFe), Mn and Zn are 14.14%, 5.94% and 13.91%, respectively, when the pH of the wastewater is adjusted to about 5 with lime. In the second step, using sodium hydroxide as neutralizing agent, when pH is 10.20, airflow is 50 mL/min, reaction time is 20 min, the removal rates of TFe, Mn and Zn are all up to 99.7%, and the residual contents of TFe, Mn and Zn are 80, 810 and 30 μg/L, respectively in wastewater, which are below the Chinese standards of wastewater discharge (GB 8978—1996). The first-step slag product treated by lime neutralizing is gypsum (CaSO4·2H2O). The second-step slag products treated by sodium hydroxide neutralizing are Fe2Mn0.5Zn0.5O4·nH2O and Fe3O4. Compared with lime neutralization method, the slag products in two-step neutralization method are much less and have comprehensive utilization value.
Key words: acid mine drainage; two-step neutralization; gypsum; ferrite
在矿山开采、矿石运输、选矿、废石排放及尾矿贮存等过程中,还原性硫化矿物在空气、水和细菌作用下被氧化后产生酸性矿山废水。酸性矿山废水水量大,pH低,铁含量高,并含有多种重金属离子,如锰、锌、铜等,如果直接排放,将对水体产生严重污染,甚至破坏生态环境[1-4]。目前,国内外处理这类废水的方法很多,如化学沉淀法[5-6]、吸附法[7-8]、生物法[9]等。其中应用较多的是中和沉淀法,常见的中和剂有石灰、石灰石、苏打、苛性碱等。采用石灰石作为中和剂具有成本低、渣含水量较低并易于脱水等优点,但反应速度慢,因此,常常与石灰串联使用。用石灰和石灰石处理酸性矿山废水适应性强,但渣量大,不利于有价金属的回收,且易造成二次污染[10-11]。近年来,以氢氧化钠中和剂,利用铁氧体法处理重金属废水得到了广泛研究[12-14]。铁氧体法处理重金属废水效果好,设备简单,渣量少,不易造成二次污染,但是,处理成本较高。为了降低处理成本以及减少固体废弃物的排放,本文作者采用石灰与氢氧化钠二段中和法处理酸性矿山废水。
1 实验
1.1 实验步骤
酸性矿山废水水质(质量分数)如表1所示,其pH为1.0。取500 mL废水于烧杯中,搅拌,加入石灰乳液,调节pH至一定值后过滤,在滤液中加氢氧化钠溶液调节pH,并控制终点pH,曝气流量为50 mL/min,反应20 min后过滤。取滤液分析其中铁锰锌浓度,中和渣在60 ℃烘干后进行X线荧光分析(XRF)和X线衍射(XRD)分析。
表1 酸性矿山废水水质
Table 1 Quality of acid mine drainage mg/L

1.2 工艺流程
根据实验步骤,其工艺流程图如图1所示。
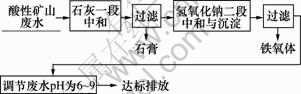
图1 二段中和法处理酸性矿山废水工艺流程图
Fig.1 Process of acid mine drainage treated by two-step neutralization method
1.3 分析与检测
用火焰型原子吸收光谱仪(TAS-990)测定总铁、锰、锌浓度,用重铬酸钾法测定亚铁浓度,用X线荧光分析(XRF)仪分析中和渣成分,用X线衍射(XRD)仪(日本理学,Cu Kα,50 kV,300 mA)分析中和渣 物相。
2 实验结果与讨论
2.1 石灰一段中和时pH对废水中铁锰锌去除率的 影响
实验将500 mL模拟废水加入烧杯,启动搅拌,加入石灰乳液。pH对废水中铁锰锌去除率的影响如图2所示。由图2可知:Fe,Mn和Zn去除率随pH增加而增加;pH为5时,Fe,Mn和Zn去除率分别为14.14%,5.94%和13.91%。pH≥5时,Fe,Mn和Zn去除率随pH增加而增加。石灰中和酸性矿山废水时发生如下反应:
H++OH-=H2O;
Ca2++SO42-=CaSO4↓;
Fe2++2OH-=Fe(OH)2↓;
Fe3++3OH-=Fe(OH)3↓;
Mn2++2OH-=Mn(OH)2↓;
Zn2++2OH-=Zn(OH)2↓。
根据上述反应,显然废水中金属离子去除率随废水pH增加而增加。根据KspFe(OH)2=8×10-16,KspFe(OH)3= 4×10-38,KspMn(OH)2=1.9×10-13和KspZn(OH)2=1.2×10-17 (Ksp为溶度积),当废水中Fe2+,Fe3+,Mn2+和Zn2+质量浓度分别为2 742,158,315和150 mg/L时,Fe2+,Fe3+,Mn2+和Zn2+开始沉淀的pH分别为7.11,2.38,8.76和6.86,完全沉淀即金属离子浓度c(Mn+)≤10-5 mol/L(n=2,3)时,pH分别为8.95,3.2,9.86和8.04。因此,当废水pH为6.9时,Zn去除率最高达到83.87%。有研究表明[15],Fe2+氧化速率为:
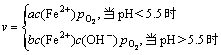
式中:a为1×10-30 Pa-1?min-1;b为8×108 L2?Pa-1?min-1?mol-2;c(Fe2+)为Fe2+的浓度;![]() 为氧气压;
为氧气压;![]() 为OH-的浓度。
为OH-的浓度。
可见:当pH<5.5时,Fe2+被氧化速率极小;当pH>5.5时,Fe2+被氧化速率非常快。因此,当废水pH<5时,铁、锰、锌去除率均较低;当pH>5时,由于Fe2+被快速氧化而形成Fe(OH)3,铁的去除率迅速增加,同时,Mn2+和Zn2+去除率也随之迅速增加。石灰中和时,为了减少石灰渣中铁、锰、锌含量,废水适宜的pH为5。
pH为5时,石灰一段中和渣成分如表2所示,X线衍射图谱如图3所示。
表2 石灰一段中和渣成分(质量分数)
Table 2 Composition of fist-step slag neutralized by lime %

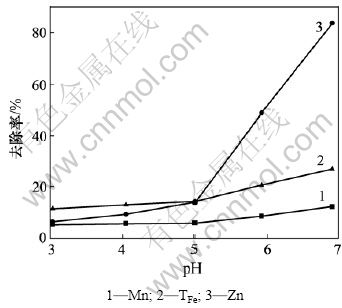
图2 石灰一段中和时pH对铁锰锌去除率的影响
Fig.2 Influence of pH on removal rates of TFe, Mn and Zn using lime as neutralizer
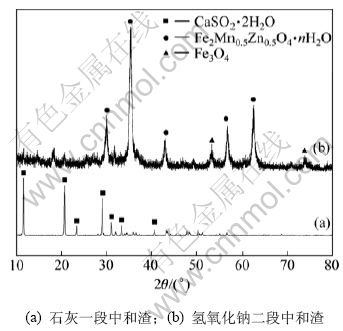
图3 二段中和渣XRD图谱
Fig.3 XRD patterns of slags produced by two-step neutralization
由表2可知:石灰一段中和渣中Ca和S的质量分数分别达到29.560%和21.370%。而根据图3可知:石灰一段中和渣为石膏(CaSO4·2H2O)。
2.2 氢氧化钠二段中和时pH对废水中铁、锰、锌去除率的影响
使用石灰乳液调废水pH约为5后过滤,然后,加氢氧化钠溶液将废水pH调到一定值后,开始曝气,并继续加氢氧化钠溶液控制终点pH。氢氧化钠二段中和终点pH对废水中铁、锰、锌去除率的影响如图4所示。
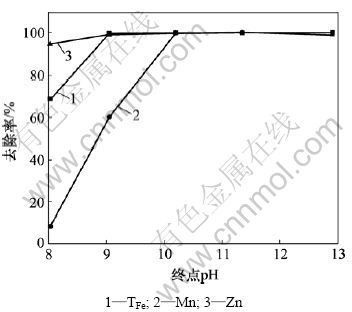
图4 氢氧化钠二段中和时pH对铁、锰、锌去除率的影响
Fig.4 Influence of pH on removal rates of TFe, Mn and Zn using sodium hydroxide as neutralizer
由图4可知,TFe,Mn和Zn去除率随着终点pH升高而增加,当终点pH达到10.20时,TFe,Mn和Zn去除率均达到99.7%以上,其废水中残留质量浓度分别为80,810和30 μg/L。
终点pH为10.20的氢氧化钠二段中和渣的XRD分析结果如图3所示。由图3可知:氢氧化钠二段中和生成了铁氧体(Fe2Mn0.5Zn0.5O4·nH2O)和四氧化三铁(Fe3O4)。其反应原理为[16-17]:
Fe2++2OH-=Fe(OH)2↓;
2Fe(OH)2+0.5O2+H2O→2Fe(OH)3↓;
Fe(OH)2+2Fe(OH)3→Fe(OH)2·2Fe(OH)3→
Fe3O4+4H2O;
Zn2++4Fe3++Mn2++16OH-→
2Fe2Mn0.5Zn0.5O4·nH2O+(8-2n)H2O。
2.3 石灰中和法与二段中和法处理酸性矿山废水比较
实验各取5 L模拟酸性矿山废水,在曝气条件下,采用石灰中和法处理酸性矿山废水,终点pH为10.46,完全沉淀后过滤;采用石灰-氢氧化钠二段中和法,石灰中和废水pH至5后过滤,氢氧化钠调节滤液至终点pH为10.46,完全沉淀后过滤。石灰中和处理和石灰-氢氧化钠二段中和处理后废水水质如表3所示,
表3 石灰中和法和二段中和法处理后水质
Table 3 Component of wastewater treated by lime neutralization process and two-step neutralization process
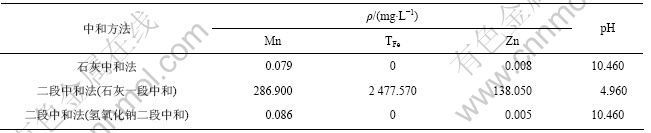
表4 石灰中和法和二段中和法干渣成分及干渣量
Table 4 Composition and mass of dried slag produced by lime neutralization process and two-step neutralization process
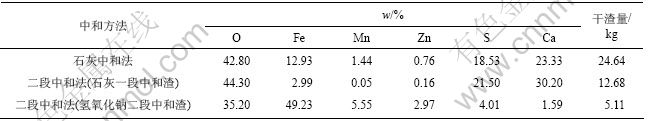
石灰中和渣和石灰-氢氧化钠中和渣干渣成分及干渣量如表4所示,石灰中和渣XRD分析结果如图5 所示。
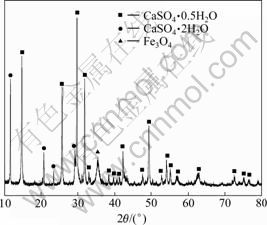
图5 石灰中和渣XRD图谱
Fig.5 XRD pattern of slag produced by lime neutralization
由表3可知,石灰中和法和石灰-氢氧化钠二段中和法处理酸性矿山废水,Mn2+和Zn2+均可达到国家污水综合排放标准。
由表4可知,每处理1 m3酸性矿山废水石灰中和法比二段中和法产生的渣量多6.85 kg,而且石灰中和法产生的渣Fe,Mn和Zn含量(质量分数)低,分别仅为12.93%,1.44%和0.76%;石灰-氢氧化钠二段中和法渣量少,产生的石膏纯度高,氢氧化钠二段中和渣Fe,Mn和Zn含量(质量分数)分别达到49.23%,5.55%和2.97%,具有综合利用价值。
由图5可以看出:石灰中和产生的渣为石膏(CaSO4·0.5H2O)和Fe3O4,没有产生铁氧体(Fe2Mn0.5Zn0.5O4·nH2O)。
3 结论
(1) 石灰一段中和及氢氧化钠二段中和时,Fe,Mn和Zn去除率随pH增加而增加。二段中和且当终点pH为10.20,曝气流量为50 mL/min,反应时间为20 min时,TFe,Mn,Zn去除率均达到99.7%以上,其废水中TFe,Mn2+和Zn2+残留质量浓度分别为80,810和30 μg/L。
(2) 采用二段中和法,石灰一段中和废水pH为5时产生的渣为石膏,氢氧化钠二段中和pH为10.20时产生的渣含Fe3O4和铁氧体(Fe2Mn0.5Zn0.5O4·nH2O)。
(3) 石灰中和法和石灰-氢氧化钠二段中和法处理酸性矿山废水,当终点pH为10.46时,重金属Mn2+和Zn2+均可达到国家污水综合排放标准(GB 8978—1996)。石灰中和法渣量大,Fe,Mn和Zn质量分数分别为12.93%,1.44%和0.76%。二段中和法石灰一段中和渣为石膏,Ca和S含量分别达到30.2%和21.5%。氢氧化钠二段中和渣中Fe,Mn和Zn含量分别达到49.23%,5.55%和2.97%,二段中和渣渣量小,具有综合利用价值。
参考文献:
[1] 蔡美芳, 党志. 磁黄铁矿氧化机理及酸性矿山废水防治研究进展[J]. 环境污染与防治, 2006, 28(1): 58-61.
CAI Mei-fang, DANG Zhi. A review on pyrrhotite oxidation mechanism and acid mine drainage prevention[J]. Environmental Pollution and Control, 2006, 28(1): 58-61.
[2] 罗凯, 张建国. 矿山酸性废水治理研究现状[J]. 资源环境与工程, 2005, 19(1): 45-48.
LUO Kai,ZHANG Jian-guo. Status quo of the disposal of acidic mining wastewater[J]. Resources Environment & Engineering, 2005, 19(1): 45-48.
[3] Akcil A, Koldas S. Acid mine drainage (AMD): Causes, treatment and case studies[J]. Journal of Cleaner Production, 2006, 14(12/13): 1139-1145.
[4] 饶俊, 张锦瑞, 徐晖. 酸性矿山废水处理技术及其发展前景[J]. 矿业工程, 2005, 3(3): 47-49.
RAO Jun, ZHANG Jin-rui, XU Hui. The technology of treating acid mine drainage and its developmental prospects[J]. Mining Engineering, 2005, 3(3): 47-49.
[5] 黄万抚, 王淑君. 硫化沉淀法处理矿山酸性废水研究[J]. 环境污染治理技术与设备, 2004, 5(8): 60-62.
HUANG Wan-hu, WANG Shu-jun. Research on treatment of mine wastewater using sulfide precipitation[J]. Techniques and Equipment for Environmental Pollution Control, 2004, 5(8): 60-62.
[6] Aziz H A, Adlan M N, Ariffin K S. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(Ⅲ)) removal from water in Malaysia: Post treatment by high quality limestone[J]. Bioresource Technology, 2008, 99(6): 1578-1583.
[7] Feng D, van Deventer J S J, Aldrich C. Removal of pollutants from acid mine wastewater using metallurgical by-product slags[J]. Separation and Purification Technology, 2004, 40(1): 61-67.
[8] Mohanan D, Chander S. Removal and recovery of metal ions from acid mine drainage using lignite: A low cost sorbent[J]. Journal of Hazardous Materials, 2006, 137(3): 1545-1553.
[9] Tabak H H, Scharp R, Burckle J, et al. Advances in biotreatment of acid mine drainage and biorecovery of metals: 1. Metal precipitation for recovery and recycle[J]. Biodegradation, 2003, 14(6): 423-436.
[10] 丁希楼, 丁春生. 石灰石—石灰乳二段中和法处理矿山酸性废水[J]. 能源环境保护, 2004, 18(2): 27-29.
DING Xi-lou, DING Chun-sheng. Two stage neutralization of limestone and lime treat mineral acidic wastewater[J]. Energy Environmental Protection, 2004, 18(2): 27-29.
[11] Kurniawan T A, Chan G Y S, Lo W H, et al. Physico-chemical treatment techniques for wastewater laden with heavy metals[J]. Chemical Engineering Journal, 2006, 118(1/2): 83-98.
[12] WEI Xin-chao, Viadero Jr R C. Synthesis of magnetite nanoparticles with ferric iron recovered from acid mine drainage: Implications for environmental engineering[J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2007, 294(1/3): 280-286.
[13] 汤兵, 张俊浩. 铁氧体法处理含Zn2+、Ni2+废水研究[J]. 环境保护科学, 2002, 28(1): 12-15.
TANG Bing, ZHANG Jun-hao. Treating wastewater containing Zn2+, Ni2+ by ferrite process[J]. Environmental Protection Science, 2002, 28(1): 12-15.
[14] Erdem M, Tumen F. Chromium removal from aqueous solution by the ferrite process[J]. Journal of Hazardous Materials, 2004, 109(1/3): 71-77.
[15] 王凯雄. 水化学[M]. 北京: 化学工业出版社, 2001: 230.
WANG Kai-xiong. Water chemistry[M]. Beijing: Chemical Industry Press, 2001: 230.
[16] YANG Xi-yun, GONG Zhu-qing, LIU Feng-liang. Kinetics of Fe3O4 formation by air oxidation[J]. Journal of Central South University of Technology, 2004, 11(2): 152-155.
[17] Lou J C, Chang C K. Completely treating heavy metal laboratory waste liquid by an improved ferrite process[J]. Separation and Purification Technology, 2007, 57(3): 513-518.
收稿日期:2010-04-07;修回日期:2010-06-17
基金项目:广东省教育部产学研重大项目(2008A090300016)
通信作者:郑雅杰(1959-),男,湖南常德人,教授,博士生导师,从事冶金资源综合利用及水污染控制研究;电话:0731-88836285;E-mail: zzyyjj01@yahoo.com.cn


