Trans. Nonferrous Met. Soc. China 27(2017) 2663-2672
Optimization of Cr/Mo molar ratio in FeCoCrMoCBY alloys for high corrosion resistance
Cheng-jie WANG, Qing-jun CHEN, Huai-xiao XIA
School of Materials Science and Engineering, Nanchang Hangkong University, Nanchang 330063, China
Received 6 July 2016; accepted 23 February 2017
Abstract:
The corrosion behavior of bulk metallic glasses (BMGs) (Fe41Co7Cr15Mo14C15B6Y2)100-xCrx (x=0, 4, 8, 12, molar fraction, %) was investigated in 1 mol/L HCl aqueous solution with electrochemical tests. The electrochemical measurements demonstrate that the passive current density of Fe-based amorphous alloy is reduced by about one order of magnitude, and meanwhile, the stability of passive film can be guaranteed by the Cr/Mo molar ratio. The Mott–Schottky (M–S) curves show that the passive film is the densest when the molar ratio of Cr/Mo is between 1.37 and 1.69. X-ray photoelectron spectroscopy (XPS) analysis was performed to clarify chemical states of elements in the passive films. The results show that the corrosion resistance of the alloy is related to the molar ratio of Cr/Mo. The stability of passive film is determined by the synergistic action of Cr and Mo elements. The main component of the passive film is Cr3+ oxide. When the potential is greater than 0.5 V (vs SCE), Mo6+ ions play an important role in keeping the stability of the passive film. The appropriate molar ratio of Cr/Mo can reduce the dissolution rate of the passive film.
Key words:
bulk metallic glasses; corrosion resistance; passive film; electrochemical measurement; Cr/Mo molar ratio;
1 Introduction
In the last few decades, many different amorphous alloys have been studied. However, the Fe-based amorphous alloys stand out, which can be attributed to their outstanding corrosion and wear resistance [1-3]. Now minor alloying elements addition has already been proven to be an effective method in enhancing the corrosion resistance [4-6], which can facilitate the passivation and result in a denser passive film. Fe-based bulk amorphous alloys have been investigated by variations of the metalloid content [7], rare earth additions [8,9] and transition metal additions [10]. At the same time, the addition of transition metals has been proven a useful method in developing new Fe-based bulk amorphous alloys with high corrosion resistance. According to the previous studies, Cr and Mo elements play the key role in keeping corrosion resistance in Fe-Cr-based alloy [11,12]. It has been reported that the corrosion resistance and mechanical properties of Fe-based amorphous alloys could be improved simultaneously by the appropriate addition of Cr element [13-17]. MADINEHEI et al [18] studied the effect of the addition of slight amount of Cr on the corrosion resistance of Fe(65-x)CrxMo14C15B6 (x=0, 2, 4, 6, 8, 10, molar fraction, %) ribbons, and found that corrosion rates in H2SO4 decreased with an increase of Cr content and the alloys containing more than 4% Cr (molar fraction) can spontaneously be passivated with low current density in 0.1 mol/L H2SO4, whereas the alloys with Cr content <4% (molar fraction) showed transpassive Mo dissolution. WANG et al [19] studied the effect of Cr content on the corrosion resistance and found that the corrosion resistance increases with larger Cr content. A homogeneous passive layer on the amorphous sample with 12.3% Cr (molar fraction) can be formed leading to a superior corrosion resistance of the amorphous sample to an austenitic stainless steel (SUS304) in 0.5 mol/L H2SO4 and 1 mol/L HCl solutions at 298 K. The addition of Mo in the alloy can promote the enrichment of Cr in the passive film and consequently enhances the corrosion resistance of the alloy [20,21]. The presence of Mo cations in the passive film indicates the possibility of the formation of passive film on the alloy surface. The formation of a Mo-enriched passive film is possible in Fe-based amorphous alloys when the enrichment of Cr ions in the surface film becomes insignificant [22]. The excessive Mo can lower the corrosion resistance of the alloy, and when the content of Mo is 8% (molar fraction), the corrosion resistance of the alloy is the best under acidic condition [23].
So far, the roles of Cr and Mo elements in the corrosion resistance have been studied, and little investigation has been carried out on the influence of the ratio of Cr to Mo elements on the corrosion resistance of the Fe-based bulk amorphous alloys. In the previous work [18,23], we found that the Cr and Mo elements with higher or lower content would be detrimental to the corrosion resistance in amorphous alloys. So, in this work, the corrosion resistance of the alloy with different Cr/Mo molar ratios is measured and the aim of the present work is to investigate the effect of Cr/Mo molar ratio on the corrosion resistance by doping Cr in Fe41Co7Cr15Mo14C15B6Y2. The main function of Fe, Co, C, B and Y elements is to ensure the glass forming ability (GFA) of the alloy [24-27].
2 Experimental
Multicomponent alloys with nominal compositions of (Fe41Co7Cr15Mo14C15B6Y2)100-xCrx (x=0, 4, 8, 12, molar fraction, %) were produced by arc-melting a mixture of Fe (99.9%), Cr (99.99%), Mo (99.99%), C (99.9%), Co (99.9%), Y (99.99%) elements and FeB (20.57%) alloy in a Ti-gettered argon atmosphere. In order to ensure chemical homogeneity, each ingot was re-melted four times, and then suck-cast into copper molds and bulk alloy samples with the size of 10 mm × 10 mm × 2 mm were prepared by cutting. The amorphous nature of the as-cast samples was checked by X-ray diffractometer (XRD) of Bruker-axs D8 type with Cu Kα radiation (λ=0.1541 nm) over a 2θ range from 20° to 80° and a scanning step of 0.02°. For convenience, the samples were numbered as BMG1 (Fe41Co7Cr15Mo14C15-B6Y2), BMG2 ((Fe41Co7Cr15Mo14C15B6Y2)96Cr4), BMG3 ((Fe41Co7Cr15Mo14C15B6Y2)92Cr8), BMG4 ((Fe41Co7Cr15- Mo14C15B6Y2)88Cr12).
The corrosion resistance of the BMGs was investigated by electrochemical measurements in 1 mol/L HCl solution. HCl solutions were prepared from reagent grade chemical and distilled water. Prior to electrochemical tests, the specimens were mechanically polished with silicon carbide paper up to 2000# grit, degreased in acetone, washed in alcohol and dried in air. Electrochemical measurements were conducted in a three-electrode-cell using a platinum counter electrode and a standard saturated calomel reference electrode (SCE). The potentiodynamic polarization experiments were carried out in electrochemical workstation (PARSTAT 2273, USA) at a potential sweep rate of 0.5 mV/s after immersing the specimens for about 30 min when the open-circuit potentials became almost steady. Mott-Schottky (M-S) plots were determined for each film by performing a potential scan toward the anodic direction (0.3 and 0.75 V (vs SCE) for 104 s) to evaluate the semiconductor properties of the passive film. The passive films for M-S analysis were obtained by potentiostatic polarisation testing in 1 mol/L HCl solution under the given applied potentials. The measurement frequency in the M-S experiments was 1 kHz. All measurements were repeated at least twice to ensure good reproducibility. The surface film was analysed by XPS using an AXIS Ultra DLD spectrometer (Kratos Analytical) with Al Kα X-ray source after potentiostatic polarization measurements.
3 Results and discussion
3.1 Characterisation of alloys
Figure 1 shows the XRD patterns of the as-cast (Fe41Co7Cr15Mo14C15B6Y2)100-xCrx (x=0, 4, 8, 12) alloys. All samples show almost identical broad peak around 43°, which is the typical characteristic of the amorphous structure.
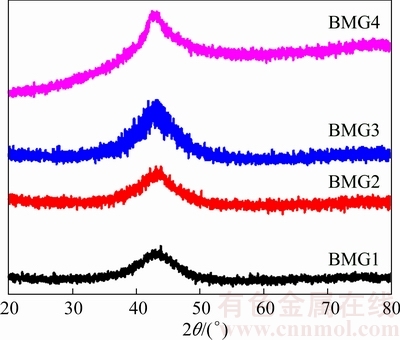
Fig. 1 XRD patterns of Fe-based BMGs
3.2 Electrochemical measurements
Figure 2(a) shows the polarization curves of Fe-based BMGs obtained in 1 mol/L HCl aqueous solution at room temperature. It is obvious that all samples are spontaneously passivated with a significant passivation region. It can be seen that the passive current density of the other alloys is reduced by about one order of magnitude compared with that of BMG1 from Table 1. However, when the potential reaches 0.5 V (vs SCE), the growth rate of the current density is different, in the order of BMG3 < BMG2 < BMG4. BMG3 shows the best corrosion resistance. Compared with the polarization curve of BMG4, the passive region of BMG2 and BMG3 is straighter, which means that the passive film is more stable. As seen from Table 1 and Fig. 2(a), there is no obvious difference in the breakdown potential. The passive region of BMG1 is large, but its corrosion resistance is the worst, so the passive region size cannot be used to estimate the corrosion resistance.
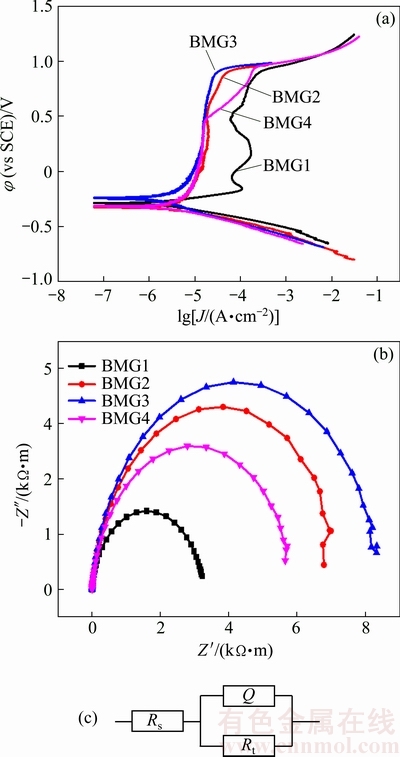
Fig. 2 Potentiodynamic polarisation curves (a), Nyquist plots (b) and equivalent circuit (c) of Fe-based BMGs in 1 mol/L HCl solution
Table 1 Results of potentiodynamic polarisation curves of Fe-based BMGs in 1 mol/L HCl solution
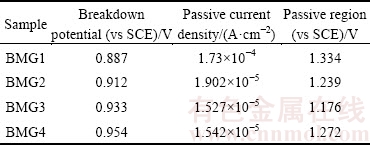
Figure 2(b) shows the Nyquist plots of Fe-based BMGs obtained in 1 mol/L HCl aqueous solution. There is only one capacitive loop, implying one single time constant in the electrochemical measurement. The radius of capacitive loop is in the order of BMG1 < BMG4 < BMG2 < BMG3. The radius of capacitive loop can roughly reflect the corrosion resistance of alloy: the greater the radius is, the smaller the possibility of ion erosion is.
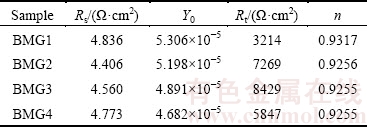
Figure 2(c) shows the equivalent circuit of Fe-based BMGs and Table 2 gives the fitted results for EIS of Fe-based BMGs in 1 mol/L HCl solution, in which n represents dispersion coefficient, Rs the solution resistance, Rt the charge transfer resistance, and Y0 constant phase element. The Y0 represents the non-ideal capacitive response of the interface. The n value of all the samples is close to 1, which indicates that the surface oxide layers act almost as a pure capacitor. The charge transfer resistance, Rt, characterizes the charge transfer across the electrolyte/electrode interface: the greater the value of Rt is, the stronger the ability of the passive film barrier solution ions into the matrix is and the more difficult the electrode reaction occurs [28]. Thus, the EIS data agree well with the results of the polarization curves.
Table 2 Fitted results for EIS of Fe-based BMGs in 1 mol/L HCl solution
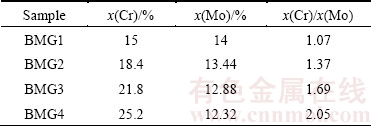
In Figs. 2 (a) and (b), the corrosion resistance of the alloy is not increased with the increase of the content of Cr element or decreased with the decrease of the content of Mo element. In previous work, we found that the stability of passive film is determined by the synergistic action of Cr and Mo elements. So, the Cr/Mo molar ratio is used to measure the corrosion resistance of the alloy. Table 3 gives the contents of Cr and Mo elements. From Fig. 2(a), when the Cr/Mo molar ratio is between 1.37 and 1.69, the passivation region is very stable and the passive current density does not increase with the increase of the potential. From Table 2, Rt increases significantly with the increase of Cr/Mo molar ratio and reaches the highest value at x(Cr)/x(Mo)=1.69. However, with the increase of the molar ratio of Cr/Mo from 1.69 to 2.05, the value of Rt decreases from 8429 to 5847 Ω·cm2. The corrosion resistance of the alloy is the best when the molar ratio of Cr/Mo reaches 1.69. Therefore, only when Cr and Mo reach an optimum proportion, the alloy can exhibit the best corrosion resistance. It is known that the excessive Mo can reduce the corrosion resistance of the alloy [23]. In BMG2, Mo element content is too much; however, Cr element content is too much and Mo element is in short supply in the BMG4. The Cr and Mo contents in the BMG3 achieve the best ratio. The molar ratio of Cr/Mo only changes the passive current density, and there is no effect on the breakdown potential. At the same time, it can ensure the stability of the passive film.
Table 3 Cr and Mo element contents of Fe-based BMGs
In order to explore the effect of Cr/Mo molar ratio on the corrosion resistance of the alloy, XPS analysis was performed to characterize the chemical composition of the passive film formed on the surfaces of the amorphous alloys after potentiostatic polarisation testing in 1 mol/L HCl solution.
3.3 Mott–Schottky (M-S) curves and XPS spectra
The current density–time curves of the Fe-based BMGs obtained in 1 mol/L HCl solution are displayed in Fig. 3(a). The values of the current density yield the total current due to the film formation and dissolution of the alloy. In Fig. 3(a), the current density initially decreases rapidly with time, because the growth rate of the passive film is much larger than the dissolution rate [29]. With the prolongation of time, the current density is relatively stable due to the formation of the passive film on the surface. Compared with the current density under the potential of 0.75 V, the current density under the potential of 0.3 V (vs SCE) is larger, and the order of the magnitude of the current density in the above two potentials is BMG4 > BMG2 > BMG3.
The semiconducting behavior of the films is described by M-S plot analysis. When the space charge capacitance (CSC) in passive films is minimal, the CSC of an n-type semiconductor can be given by the following equation [30]:
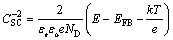
where εr is the dielectric constant of the film (for the Fe-Cr alloy, εr=15.6), εo is the vacuum permittivity (8.85 ×10-14 F/cm), e is the charge of an electron, ND is the donor density of the n-type passive film, E is the applied potential, EFB is the flat band potential, k is the Boltzmann constant (1.38×10-23 J/K), and T is the temperature (K).
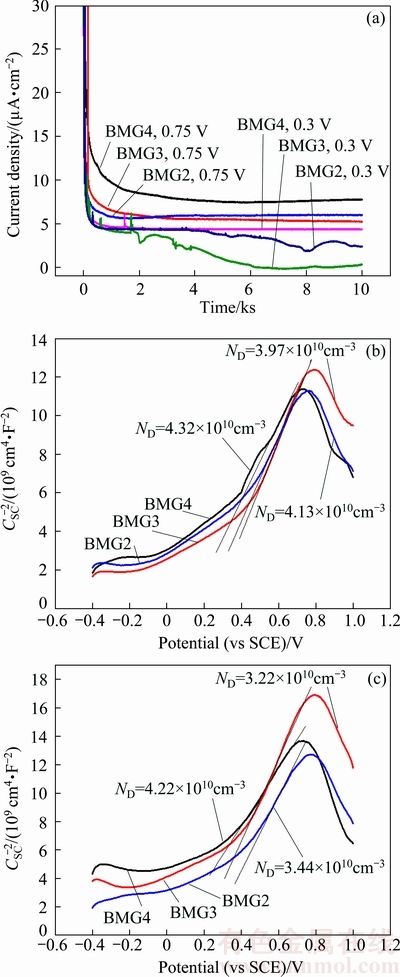
Fig. 3 Current density-time curves of Fe-based BMGs (a), and Mott-Schottky plots of passive films formed at 0.3 V (b) and 0.75 V (c) (vs SCE) in 1 mol/L HCl solution
The M–S curves of passive films formed on Fe- based BMGs at 0.3 V (vs SCE) and 0.75 V (vs SCE) in HCl solution are shown in Figs. 3(b) and (c), respectively. At the potential between 0.4 and 0.7 V (vs SCE), the type of semiconductor is n-type; however, when the potential is between 0.7 and 1 V (vs SCE), the semiconductor type changes to p-type. The type of semiconductor is determined by the structure and composition of the passive film. Different oxide layers exhibit different semiconductor properties. Oxides such as Cr2O3 and MoO2 are usually considered as p-type semiconductor, while oxides like MoO3 are regarded as n-type semiconductor [31]. Taking the results into consideration, it is reasonable to draw an inference that the corrosion resistance of the alloy mainly depends on the oxides of Cr and Mo. The ND value of the passive film formed under the potential of 0.3 V (vs SCE) is similar. However, the ND value of the passive film formed under the potential of 0.75 V (vs SCE) is similar to that of BMG2 and BMG3, and is much smaller than that of BMG4, which shows that when the Cr/Mo molar ratio is between 1.37 and 1.69, the passive film is the most stable, and the protection of the substrate is the best. This is in good agreement with the results of electrochemical experiments.
The XPS spectra show the presence of Fe, Cr, Mo, C, Y, O and B, but the peak intensity of Y and B is very low, which indicates that the elements of Fe, Cr, and Mo are the dominant components of the passive film. The spectra of Fe 2p, Cr 2p, and Mo 3d of the passive films formed in 1 mol/L HCl solution at 0.3 and 0.75 V (vs SCE) are depicted in Figs. 4 and 5, respectively. Figure 6 shows the raw area of the oxide species in the passive film.
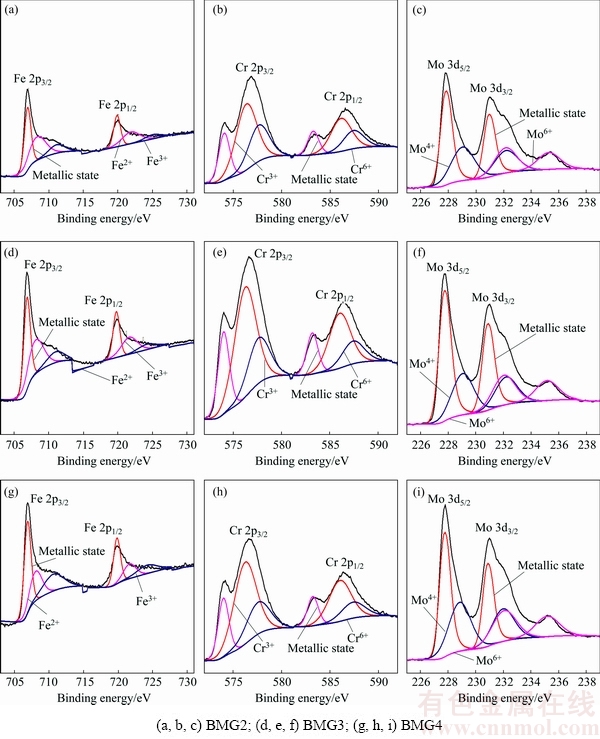
Fig. 4 XPS spectra of Fe 2p, Cr 2p, and Mo 3d recorded from surface of Fe-based BMGs after potentiostatic polarisation testing in 1 mol/L HCl solution at 0.3 V (vs SCE)
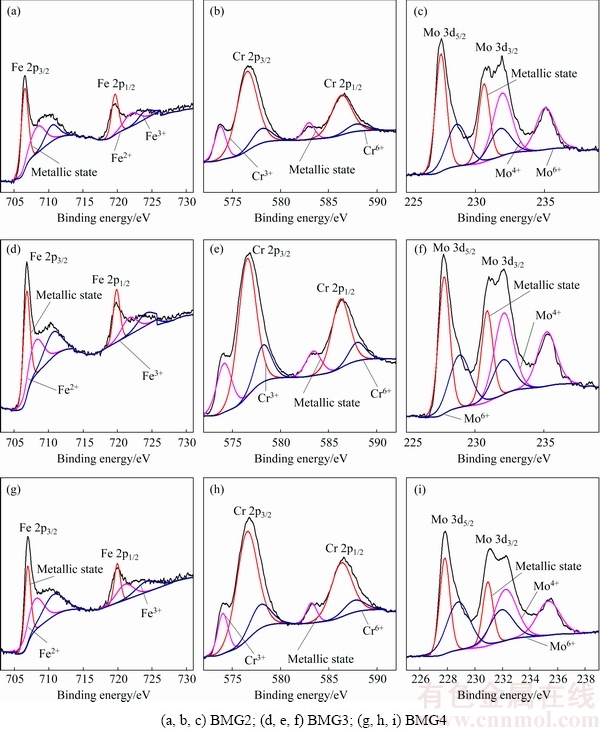
Fig. 5 XPS spectra of Fe 2p, Cr 2p, and Mo 3d recorded from surface of Fe-based BMGs after potentiostatic polarisation testing in 1 mol/L HCl solution at 0.75 V (vs SCE)
The contents of Cr element in BMG2, BMG3 and BMG4 alloys gradually increase, and the contents of Fe and Mo elements decrease in turn. From Fig. 3, Cr0, Cr3+ and Cr6+ states still increase in the passive film formed under the potential of 0.3 V (vs SCE); however, Fe0, Fe2+, Fe3+, Mo0, Mo4+ and Mo6+ states do not decrease. Among them, the contents of Cr3+, Fe0 and Mo0 states are relatively large. It is proven that Fe and Mo are enriched on the surface of the alloy, and the main component of the passive film is Cr3+ oxide, which indicates that Cr3+ is the main ion to determine the corrosion resistance of the alloy under 0.5 V (vs SCE). The contents of high valence cations gradually increase, which indicates that the oxidation reaction on the alloy surface is enhanced in turn. From Fig. 5, compared with the passive film formed under the potential of 0.3 V (vs SCE), the contents of most ions in the passive film formed under the potential of 0.75 V (vs SCE) are significantly reduced, and only the content of Mo6+ state in BMG2 and BMG3 increases, which indicates that the increase of potential accelerates the oxidation of Mo. When the potential increases from 0.3 to 0.75 V (vs SCE), the content of Cr3+ and Mo4+ states in the passive film of BMG4 is significantly larger than that of BMG2, and the corrosion resistance of BMG2 is better than that of BMG4. It is a remarkable fact that the content of Mo6+ state in the passive film changes from BMG2 < BMG3 < BMG4 to BMG4 < BMG2 < BMG3, which indicates that Mo6+ is the main ion that determines the stability of the passive film, not low-valence Cr(III) or Mo(IV) species. At the same time, most of the cations are dissolved into the solution, but the content of Cr3+ state is still the highest, indicating that Cr3+ state is the main component of passive film in the whole electrochemical process, and it is also the main ion to determine the densification degree of passive film. However, when the potential reaches a certain value, the effect of Cr ions on corrosion resistance becomes insignificant [22]. Therefore, combined with the results of electrochemical experimental, it is reasonable to draw an inference that when the potential is below 0.5 V (vs SCE), Cr is the main element to determine the corrosion resistance. When the potential is higher than 0.5 V (vs SCE), the protective film resulted from Cr ions is damaged. At the same time, Mo can promote repair of protective film, and Mo ion itself can also form a layer of protective film, so Mo ion also plays a key role in determining the corrosion resistance. The reduction of the cations content in the passive film of BMG4 is the most serious, which results in a larger passive current density at a higher potential. This is consistent with the change in the polarization curve when the potential is higher than 0.5 V (vs SCE). The previous studies have shown that the formation of insoluble Mo(IV) species is responsible for the maintenance of the passivity of Fe-Cr amorphous alloys [21]. Mo can form a protective insoluble Mo(IV) film (such as MoO2 [32] and MoO(OH)2 [33]) that acts as an effective barrier against the diffusion of the species, therefore decreasing the dissolution rate in the H2SO4 solutions. However, the corrosion behavior of the Fe-based BMGs in HCl solution is different from that in H2SO4 solution, which may be attributed to the structures of the passive films and the presence of Cl-. The MoO2 is alkaline and MoO3 is acidic. When pH is between 1.5 and 2.9, Mo element usually exists in the form of Mo8O24. So, the formation of insoluble Mo(VI) species is responsible for the maintenance of the passivity of Fe-Cr amorphous alloys in 1 mol/L HCl solution. In addition, the Mo element can promote the enrichment of Cr elements, repairing and ensuring the stability of the passive film [25,26].
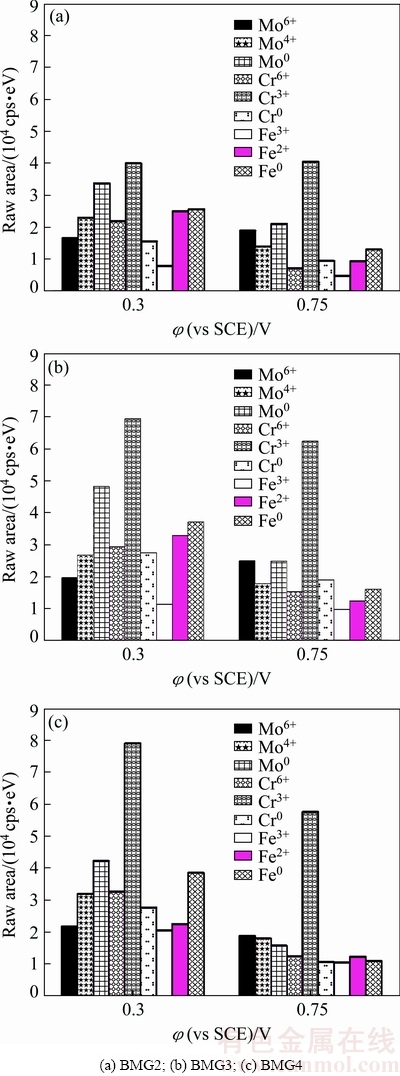
Fig. 6 Raw area of oxide species detected by XPS from surface
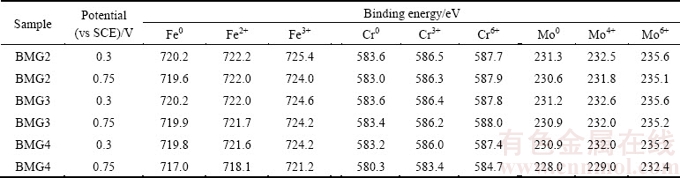
The change of binding energy is also an important index to measure the corrosion resistance. From Table 4, compared with the changes of the binding energy of all cations in the passive film of BMG1, BMG2 and BMG4, the corresponding binding energy of all cations in the passive film of BMG3 does not change and is within the error range. The binding energy of the BMG2 changes relatively little, and that of BMG4 has a significant shift. It can be seen that when the Cr/Mo molar ratio is between 1.37 and 1.69, the passive film formed on the alloy surface is relatively stable, and the dissolution rate is slow. For BMG4, it is known that the binding energies of Fe0, Cr0 and Mo0 states decrease from about 719.8, 583.2 and 230.9 to 717.0, 580.3 and 228.0 eV, respectively. The previous studies [34-36] have shown that the change in chemical environment can cause binding energy to shift. In this work, the binding forces between nuclei and electrons can be affected by the change of chemical environment around atoms to some extent, and the local chemical environment around metallic Cr atoms can be influenced by electrochemical process. Therefore, it is considered that the decrease in binding energy of about 2.9 eV is a binding energy shift, which results from a slowing down of the oxidation rate of the alloy surfaces. Fe2+, Fe3+, Cr3+, Cr6+, Mo4+ and Mo6+ ions have a binding energy shift about 3.5, 3.0, 2.6, 2.7, 3.0 and 2.8 eV, respectively. According to the previous studies [37,38], the Cr3+ spectrum was composed of several peaks of Cr species in states of Cr2O3, CrOOH, etc. So, the decrease in binding energy of these cations can be mainly attributed to the increase in contents of the corresponding species with lower binding energies. The experimental results show that when the Cr/Mo molar ratio is 2.05, the passive film exhibits rapid dissolution under the potential of 0.75 V (vs SCE), which results from many cations entering into the solution. This clearly explains why the current density suddenly begins to increase when the potential is up to 0.5 V (vs SCE).
Table 4 Binding energy of oxidised species detected by XPS
Based on the electrochemical experiments and XPS analysis, the passive film of BMG4 is destroyed when the potential is up to 0.5 V (vs SCE). At the same time, the main components of the passive film are oxides. According to the principle of Pilling-Bedworth, it is well known that the stress existing in the oxide film is the main cause of the failure of the oxide film. When the passive film is subjected to erosion of Cl- ion and is unable to timely repair, pitting corrosion occurs and a concave is formed. When the cation diffusion is dominated, tensile stress exists in the film, which will result in the breakdown of passive film.
4 Conclusions
1) The passive current density of Fe-based amorphous alloy is reduced by about one order of magnitude with doping the Cr element in 1 mol/L HCl solution. The Cr/Mo molar ratio is the most important factor affecting the corrosion resistance, and the optimal Cr/Mo molar ratio range is 1.37-1.69 in FeCoCrMoCBY alloys. The electrochemical results and M-S curves show that the passive film is the densest when the molar ratio of Cr/Mo is between 1.37 and 1.69. The type of semiconductor is n-type at the potential between 0.4 and 0.7 V (vs SCE), and the semiconductor type is changed from n-type to p-type at the potential between 0.7 and 1 V (vs SCE).
2) Based on XPS analysis, it can be concluded that the stability of passive film is determined by the synergistic action of Cr and Mo elements. The main component of the passive film is Cr3+ oxide. When the potential is greater than 0.5 V (vs SCE), Mo6+ is the main component that determines the stability of the passive film. When the Cr/Mo molar ratio is between 1.37 and 1.69, the binding energy shift is relatively small and the passive film is more stable.
References
[1] LI Zhi, ZHANG Cheng, LIU Lin. Wear behavior and corrosion properties of Fe-based thin film metallic glasses [J]. Journal of Alloys and Compounds, 2015, 650: 127-135.
[2] WU Hong, LAN Xiao-dong, LIU Yong, LI Fei, ZHANG Wei-dong, CHEN Zi-jin, ZAI Xiong-fei, ZENG Han. Fabrication, tribological and corrosion behaviors of detonation gun sprayed Fe-based metallic glass coating [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 1629-1637.
[3] GUALCO A, MARINI C, SVOBODA H, SURIAN E. Wear resistance of Fe-based nanostructured hardfacing [J]. Procedia Materials Science, 2015, 8: 934-943.
[4] SHI B, XU Y L, MA W L, LI C, ESTEFANIA C V, LI J G. Effect of Ti addition on the glass forming ability, crystallization, and plasticity of (Zr64.13Cu15.75Ni10.12Al10)100-xTix bulk metallic glasses [J]. Materials Science and Engineering A, 2015, 639: 345-349.
[5] ZHUANG Yan-xin, WANG Shen-ci, WANG Chang-jiu, WANG Nai-peng, HE Ji-cheng. Effect of Ti on microstructure, mechanical and corrosion properties of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix bulk metallic glasses [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 138-143.
[6] WANG T, WU Y D, SI J J, CAI Y H, CHEN X H, HUI X D. Novel Ti-based bulk metallic glasses with superior plastic yielding strength and corrosion resistance [J]. Materials Science and Engineering A, 2015, 642: 297-303.
[7] DAN Z H, TAKENAKA K, ZHANG Y, UNAMI S, TAKEUCHIA, HARA N, MAKINO A. Effect of Si addition on the corrosion properties of amorphous Fe-based soft magnetic alloys [J]. Journal of Non-Crysttalline Solids, 2014, 402: 36-43.
[8] ZHANG Chun-zhi, WANG Jin-hua, QIU Nan-nan, XIE Kun, LI Hui-ping. Cerium addition on pitting corrosion of (Cu50Zr50)100-2xCe2x (x=0, 1, 2 and 3) metallic glasses in seawater [J]. Journal of Rare Earths, 2015, 33: 102-106.
[9] XING Q, ZHANG K, WANG Y Z, LENG J F, JIA H L, LIAW P K, WANG Y. Effects of pre-compression on the microstructure, mechanical properties and corrosion resistance of RE65Co25Al10 (RE=Ce, La, Pr, Sm and Gd) bulk metallic glasses [J]. Intermetallics, 2015, 67: 94-101.
[10] LIU Bin, LIU Lin, SUN Min, QIU Chun-lei, CHEN Qi. Influence of Cr micro-addition on the glass forming ability and corrosion resistance of Cu-based bulk metallic glasses [J]. Acta Metallurgica Sinica, 2005, 41: 738-742.
[11] LI Jia-wei, YANG Li-jing, MA Hao-ran, JIANG Ke-min, CHANG Cun-tao, WANG Jun-qiang, SONG Zhen-lun, WANG Xin-min, LI Run-wei. Improved corrosion resistance of novel Fe-based amorphous alloys [J]. Materials Design, 2016, 95: 225-230.
[12] SOUZA C A C, RIBEIRO D V, KIMINAMI C S. Corrosion resistance of Fe-Cr-based amorphous alloys: An overview [J]. Journal of Non-Crysttalline Solids, 2016, 442: 56-66.
[13] WANG L S, LI H X, ZHANG X F, YI S. Effects of Cr contents in Fe-based bulk metallic glasses on the glass forming ability and the corrosion resistance [J]. Materials Chemistry and Physics, 2009, 113: 878-883.
[14] PANG S J, ZHANG T, ASAMI K, INOUE A. Effects of Chromium on the glass formation and corrosion behavior of bulk glassy Fe-Cr-Mo-C-B alloys [J]. Materials Transactions, 2002, 43: 2137-2142.
[15] LONG L Z, SHAO Y, DENG X H, ZHANG Z C, JIANG Y, ZHANG P, SHEN B L, INOUE A. Cr effects on magnetic and corrosion properties of Fe-Co-Si-B-Nb-Cr bulk glassy alloys with high glass-forming ability [J]. Intermetallics, 2007, 15: 1453-1458.
[16] SHEN B L, AKIBA M, INOUE A. Effect of Cr addition on the glass-forming ability, magnetic properties, and corrosion resistance in FeMoGaPCBSi bulk glassy alloys [J]. Journal of Applied Physics, 2006, 100(4): L2248.
[17] JIAO Z B, LI H X, GAO J E, WU Y, LU Z P. Effects of alloying elements on glass formation, mechanical and soft-magnetic properties of Fe-based metallic glasses [J]. Intermetallics, 2011, 19: 1502-1508.
[18] MADINEHEI M, BRUNA P, DUARTE M J, PINEDA E, KLEMM J, RENNER F U. Glass-formation and corrosion properties of Fe-Cr-Mo-C-B glassy ribbons with low Cr content [J]. Journal of Alloys and Compounds, 2014, 615: 128-131.
[19] WANG S L, LI H X, ZHANG X F. Effects of Cr contents in Fe-based bulk metallic glasses on the glass forming ability and the corrosion resistance [J]. Materials Chemistry and Physics, 2009, 113: 878-883.
[20] TAN M W, AKIYAMA E, HABAZAKI H, KAWASHIMA A, ASAMI K, HASHIMOTO K. The role of chromium and molybdenum in passivation of amorphous Fe-Cr-Mo-P-C alloys in deaerated 1 M HCl [J]. Corrosion Science, 1996, 38: 2137-2151.
[21] TAN M W, AKIYAMA E, HABAZAKI H, KAWASHIMA A, ASAMI K, HASHIMOTO K. The effect of air exposure on the corrosion behavior of amorphous Fe-8Cr-Mo-13P-7C alloys in 1 M HCl [J]. Corrosion Science, 1995, 37: 1289-1301.
[22] GOSTIN P F, GEBERT A, SCHULTZ L. Comparison of the corrosion of bulk amorphous steel with conventional steel [J]. Corrosion Science, 2010, 52: 273-281.
[23] JAYARIJ J, KIMA Y C, KIM K B, SEOK H K, FLEURY E. Corrosion studies on Fe-based amorphous alloys in simulated PEM fuel cell environment [J]. Science and Technology of Advanced Materials, 2005, 6: 282-289.
[24] LI Duan-xian, YUAN Zi-zhou, KANG Jian, ZHANG Xiang-yun. Effects of yttrium addition on structure, mechanical properties and corrosion resistance of bulk glassy Zr-Co-Al-(Y) alloys [J]. Foundry, 2015, 35: 525-528.
[25] BOTTA W J, BERGER J E, KIMINAMI C S, ROCHE V, NOGUEIRA R P, BOLFARINI C. Corrosion resistance of Fe-based amorphous alloys [J]. Journal of Alloys and Compounds, 2014, 586: 105-110.
[26] TSAI P H, XIAO A C, LI J B, JANG J S C, CHU J P, HUANG J C. Prominent Fe-based bulk amorphous steel alloy with large supercooled liquid region and superior corrosion resistance [J]. Journal of Alloys and Compounds, 2014, 586: 94-98.
[27] CUI L Z, YUAN Z Z, ZHANG X Y, FENG X L. Effects of Fe on structure and mechanical properties of Cu-based amorphous alloys [J]. Rare Metal Materials and Engineering, 2015, 44: 2831-2835.
[28] KOCIJAN A, MERL D K, JENKO M. The corrosion behaviour of austenitic and duplex stainless steels in artificial saliva with the addition of fluoride [J]. Corrosion Science, 2011, 53: 776-783.
[29] QIAO X Y, ZHENG Y G, KE W, OKAFOR P C. Electrochemical behaviour of high nitrogen stainless steel in acidic solutions [J]. Corrosion Science, 2009, 51: 979-986.
[30] SIMMOES A M P, FERREIRA M G S, RONDOT B, BELO M D C. Study of passive films formed on AISI 304 stainless steel by impedance measurements and photoelectrochemistry [J]. Journal of The electrochemical Society, 1990, 137: 82-87.
[31] SUNSERI C, PIAZZA S, DIQUARTO F. Photocurrent spectroscopic investigations of passive films on chromium [J]. Journal of The Electrochemical Society, 1990, 137: 2411-2417.
[32] HASHIMOTO K. 2002 W.R. Whitney award lecture: In pursuit of new corrosion-resistant alloys [J]. Corrosion, 2002, 58: 715-722.
[33] LU Y C, CLAYTON C R. An XPS study of the passive and transpassive behavior of molybdenum in deaerated 0.1 M HCl [J]. Corrosion Science, 1989, 29: 927-937.
[34] RODRIGUEZ J A, GOODMAN D W. The nature of the metal-metal bond in bimetallic surfaces [J]. Science, 1992, 257: 897-903.
[35] SUN Chang-qing. Thermo-mechanical behavior of low-dimensional system: The local bond average approach [J]. Progress in Materials Science, 2009, 54: 179-307.
[36] JIAO W, WANG X L, LAN S, PAN S P, LU Z P. Propensity of bond exchange as a window into the mechanical properties of metallic glasses [J]. Applied Physics Letters, 2015, 106: 061910.
[37] HASHIMOTO K. What we have learned from studies on chemical properties of amorphous alloys? [J]. Applied Surface Science, 2011, 257: 8141-8150.
[38] BIESINGER M C, PAYNE B P, GROSVENOR A P, LAU L W M, GERSON A R, SMART R S C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni [J]. Applied Surface Science, 2011, 257: 2717-2730.
高耐腐蚀性能FeCoCrMoCBY合金中的最优Cr/Mo摩尔比
王诚杰,陈庆军,夏怀效
南昌航空大学 材料科学与工程学院,南昌 330063
摘 要:采用水冷铜模吸铸法制备(Fe41Co7Cr15Mo14C15B6Y2)100-xCrx (x=0,4,8,12,摩尔分数,%)块体金属玻璃,利用电化学方法研究其在1 mol/L HCl中的耐腐蚀性能。电化学测试发现钝化电流密度降低了大约一个数量级,同时发现适当的Cr/Mo摩尔比可以保证钝化膜的稳定性。Mott–Schottky曲线证实当Cr/Mo摩尔比为1.37~1.69 时,合金的钝化膜更致密。采用XPS分析钝化膜中元素的化学状态,发现合金耐腐蚀性能与Cr/Mo摩尔比有关,钝化膜的稳定性由Cr和Mo协同作用决定。钝化膜的主要成分为Cr3+的氧化物。当电位高于0.5 V (vs SCE)时,Mo6+是决定钝化膜稳定性的主要因素。适当的Cr/Mo摩尔比可以降低钝化膜的腐蚀速率。
关键词:块体金属玻璃;耐腐蚀性能;钝化膜;电化学测试;Cr/Mo摩尔比
(Edited by Bing YANG)
Foundation item: Project (51261021) supported by the National Natural Science Foundation of China; Project (KJLD13056) supported by the Science and Technology Landing Plan of Jiangxi Province, China
Corresponding author: Qing-jun CHEN; Tel: +86-791-83953322; E-mail: qjchen@nchu.edu.cn
DOI: 10.1016/S1003-6326(17)60295-4
Abstract: The corrosion behavior of bulk metallic glasses (BMGs) (Fe41Co7Cr15Mo14C15B6Y2)100-xCrx (x=0, 4, 8, 12, molar fraction, %) was investigated in 1 mol/L HCl aqueous solution with electrochemical tests. The electrochemical measurements demonstrate that the passive current density of Fe-based amorphous alloy is reduced by about one order of magnitude, and meanwhile, the stability of passive film can be guaranteed by the Cr/Mo molar ratio. The Mott–Schottky (M–S) curves show that the passive film is the densest when the molar ratio of Cr/Mo is between 1.37 and 1.69. X-ray photoelectron spectroscopy (XPS) analysis was performed to clarify chemical states of elements in the passive films. The results show that the corrosion resistance of the alloy is related to the molar ratio of Cr/Mo. The stability of passive film is determined by the synergistic action of Cr and Mo elements. The main component of the passive film is Cr3+ oxide. When the potential is greater than 0.5 V (vs SCE), Mo6+ ions play an important role in keeping the stability of the passive film. The appropriate molar ratio of Cr/Mo can reduce the dissolution rate of the passive film.


