文章编号:1004-0609(2011)12-3082-05
废锂离子电池负极材料的机械分离与回收
周 旭,朱曙光,次西拉姆,贺文智,李光明,黄菊文
(同济大学 环境科学与工程学院,上海 200092)
摘 要:
基于锂电池负极结构及其组成材料铜与碳粉的物料特性,采用锤振破碎、振动筛分与气流分选组合工艺对废锂电池负极组成材料进行分离与回收。实验采用ICP-AES分析实验样品与分离富集产品的金属品位。结果表明:该负极材料经破碎筛分后,粒径大于0.250 mm的破碎料中铜的品位为92.4%,而粒径小于0.125 mm的破碎料中碳粉的品位为96.6%,均可直接回收;粒度为0.125~0.250 mm的破碎料中,铜的品位较低,可通过气流分选实现铜与碳粉的有效分离回收;气流分选过程中,操作气流速度为1.00 m/s时,铜的回收率达92.3%,品位达84.4%。
关键词:
中图分类号:X705 文献标志码:A
Mechanical separation and recovery process of anode materials from spent lithium-ion batteries
ZHOU Xu, ZHU Shu-guang, CIXI La-mu, HE Wen-zhi, LI Guang-ming, HUANG Ju-wen
(College of Environmental Science and Engineering, Tongji University, Shanghai 200092, China)
Abstract: Based on the analysis of the structure and properties of anodes, the pulverization, vibratory screening and pneumatic separation were utilized to separate and recover the copper and carbon powder in the anodes from spent lithium-ion batteries. The metal grade in the samples and separation products were determined by ICP-AES. The results indicate that anode materials are pulverized first and then sieved, the grades of copper (>0.250 mm) and carbon powder (<0.125 mm) in the crushed particles reach 92.4% and 96.6%, respectively, which can be recycled directly. As for the specific particles (0.125-0.250 mm) with low copper grade, the pneumatic separation was applied to achieve an effective separation of copper and carbon particle. When the flow velocity is 1.00 m/s, the recovery rate and grade of copper reach 92.3% and 84.4%, respectively.
Key words: lithium-ion battery; anode; pulverization; pneumatic separation
锂离子电池(以下简称锂电池)因具有电压高、比容量大、寿命长和无记忆效应等显著优点,自其商业化以来便快速占领了便携式电子电器设备的动力源市场,且产量逐年增大[1]。锂电池是电子消耗品,使用寿命约3 a。报废后的锂电池,如处理处置不当,其所含的六氟磷酸锂、碳酸酯类有机物以及钴、铜等重金属必然会对环境构成潜在的污染威胁。而另一方面,废锂电池中的钴、锂、铜及塑料等均是宝贵资源,具有极高的回收价值[2]。因此,对废锂电池进行科学有效的处理处置,不仅具有显著的环境效益,而且具有良好的经济效益。
锂电池主要由外壳、正极、负极、电解液与隔膜组成。正极是通过起粘结作用的PVDF将钴酸锂粉末涂布于铝箔集流体两侧构成;负极结构与正极类似,由碳粉粘结于铜箔集流体两侧构成[3]。
目前,废锂电池资源化研究主要集中于价值高的正极贵重金属钴和锂的回收[4],对负极材料的分离回收鲜见报道。为缓解经济快速发展而引发的日趋严重的资源短缺与环境污染问题,对废旧物资实现全组分回收利用已成为全球共识。废锂电池负极中的铜(含量达35%左右)是一种广泛使用的重要生产原料,粘附于其上的碳粉,可作为塑料、橡胶等添加剂使用。因此,对废锂电池负极组成材料进行有效分离,对于最大限度地实现废锂电池资源化,消除其相应的环境影响具有推动作用。
常用的废锂电池资源化方法包括湿法冶金[5-12]、火法冶金[13-15]及机械物理法[16-17]。相比于湿法及火法,机械物理法无需使用化学试剂,且能耗更低,是一种环境友好且高效的方法。本文作者基于锂电池负极结构特点,采用破碎筛分与气流分选组合工艺,对其进行分离富集研究,以实现废锂电池负极铜与碳粉的高效分离回收。
1 实验
1.1 实验原料
手工拆解手机废锂电池,分离得到电池正极、负极及隔膜,收集负极材料并将其剪成2 cm×2 cm的小片待用。实验过程中使用的试剂包括硝酸(分析纯)、盐酸(分析纯)及去离子水。
1.2 实验过程
1.2.1 负极材料的破碎解离
将负极样品放入锤式破碎机中粉碎,排料口设孔径1 mm的筛网,确保粒径小于1 mm的破碎料排出设备,而大于1 mm的破碎料继续粉碎,直至粒径小于1 mm;收集破碎料,采用标准筛进行分级并确定其粒径分布。
1.2.2 破碎料的气流分选
称取一定质量的筛分物料加入图1所示的实验装置进行气流分选,破碎料置于流化床分布板上形成固定床层;开启风机调节气体流速,依次使颗粒床层经固定床、床层松动、初始流态化直至充分流化而使金属与非金属颗粒相互分离,其中轻组分被气流带出流化床,经旋风分离器进行收集,重组分则停留在流化床底部。
1.3 评价方法
使用金属品位、金属回收率及分选效率对分选效果进行评价。实验样品及分离产品经消解处理,使用ICP-AES (电感耦合等离子体原子发射光谱)测定其金属品位(即金属含量)。
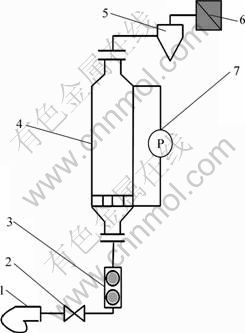
图1 气流分选装置示意图
Fig.1 Illustration of pneumatic separation devices: 1—Blower; 2—Valve; 3—Rotameter; 4—Fluidized bed; 5—Cyclone; 6—Bag filter; 7—Pressure drop manometer
选择应用广泛的汉考克分选效率(EH)定量表征本研究的分选情况[18]:
![]() (1)
(1)
式中:![]() 、
、![]() 、
、![]() 分别表示分选物料、重组分和纯组分的品位;
分别表示分选物料、重组分和纯组分的品位;![]() 表示产率,即产物质量与分选物料的质量比。
表示产率,即产物质量与分选物料的质量比。
2 结果与分析
2.1 负极材料的破碎筛分
构成废锂电池负极的碳粉和铜箔通过PVDF相互连接,实验通过锤式破碎以使其相互解离,筛分结果见表1与图2。
由表1及图2可见,废锂电池负极破碎料主要集中在大于0.590 mm和小于0.074 mm的粒径范围内,其质量分数分别为 20.6%和40.4%;粉碎料中的铜含量随颗粒粒径的减小而降低,相应地碳粉含量随颗粒粒径减小而增大。由表1可见,粒径大于0.250 mm和小于0.125 mm的粉碎料分别为高度富集的金属铜(平均品位达92.4%)与碳粉(平均品位达96.6%),可分
别将其送于下游企业回收并利用;而粒径为0.125~ 0.250 mm的粉碎料中,金属铜的品位较低,可通过气流分选提高其纯度。
上述破碎解离效果因铜与碳粉的物料特性及负极结构所致。研究发现,因铜具有良好的延展性与优良的强度与韧性,在锤振冲击和挤压等作用下不易破碎而弯曲团绕,因此,在破碎过程中趋于富集在较粗粒级范围内;而铜箔表面的碳粉依靠 PVDF与其粘结,随着锤振时间的延长,PVDF的粘合作用逐渐减弱直至消失,石墨碳粉随即脱落而富集于较细的粒级中。
表1 负极材料的筛分结果
Table 1 Sieving results of anode materials
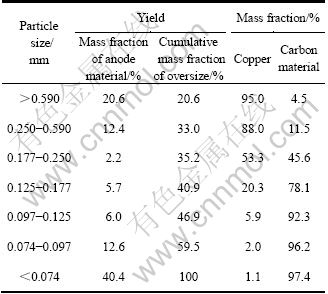
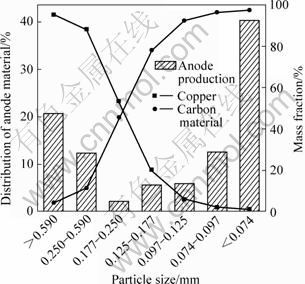
图2 破碎产物粒径分布与铜、碳含量
Fig.2 Size distribution of anode production and contents of copper, carbon purities in each size range
2.2 破碎料中金属与非金属的气流分选
气流分选是借助组分间的密度差,以气体为介质对混合物中组分进行分离富集的技术,具有设备简单、生产能力大、生产成本低和环境污染少等特点。物料气选的难易程度取决于轻重组分与流体介质间的密度差,可以用两者的比值C来衡量,即
![]() (2)
(2)
式中:![]() 、
、![]() 分别为轻物料和重物料的密度;
分别为轻物料和重物料的密度;![]() 为流体介质的密度。
为流体介质的密度。
表2所列密度差异与分选难易程度间的相互 关系。废锂电池负极的主要组成材料铜与石墨碳的密度分别为8.9×103 kg/m3和(1.9~2.3)×103 kg/m3,空气介质的密度为1.2 kg/m3。经计算,C为3.9~4.7,表明利用气流分选能有效实现铜与碳粉的分离富集。表3所列为0.125~0.250 mm粒径范围内粉碎料在不同气流速度下的气流分选实验结果。
由表3可见,相比于原料中铜的品位,经气流分选后,负极材料中的铜得到有效分离与富集,且流态化表观气流速度越大,铜的富集程度越高。比较不同气流速度下铜品位与铜回收率的关系(见图3)可见:随着气流速度的增加,铜品位呈增加趋势,而铜回收率呈下降趋势。在气流速度由0.71 m/s增至1.00 m/s时,铜品位的提高幅度较大;当气流速度继续增大,铜品位提高趋缓,铜回收率却显著下降。铜的品位与回收率随气流速度的这一变化规律,与流态化分离本质及破碎料的特性密切相关。
实验过程中,分选物料在气流速度作用下经历松散—分层与输送—分离过程时,由于颗粒尺寸不同,故随着气流速度增大,随同气流被带出的非金属颗粒总量逐渐增多,驻留于床层内重组分中铜的品位提高。因该破碎料中金属铜与非金属碳的密度差较大,因此在气流速度增大初期,大部分非金属会随同气流被带出;而对于金属铜,只有较微小的粒子,才会因颗粒间的相互干扰而被少量带出,因此,表现为分离所得重组分中铜的品位随气流速度大幅增加。当气流速度继续增大,分选物料样品中的大部分非金属已被气流带出,而粒径较小的铜颗粒会因其沉降速度小于气流上升速度而被气流带出,气流速度越大,带出的数量增多,表现为铜品位的提高随之趋缓,回收率下降。因此,铜的回收率与其品位间存在着一个较优组合。
表2 基于密度差的分选难易程度
Table 2 Grades of pneumatic separation according to material density

表3 负极粉碎料气流分选结果
Table 3 Pneumatic separation results of anode materials
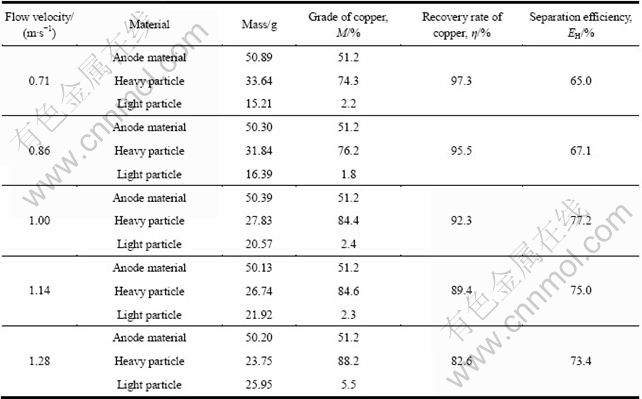
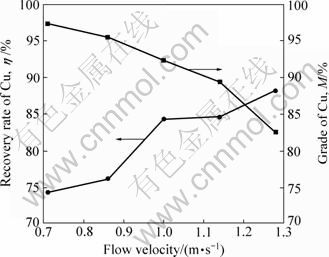
图3 气流速度对铜品位(M)与回收率(η)的影响
Fig.3 Effect of flow velocity on recovery rate (M) and grade of Cu (η)
图4所示为气流速度与分选效率的关系。由图4可见,随着表观气流速度的增大,分选效率EH也随之增大,且在气流速度增大初期,EH提高幅度较大;当气流速度达到1.00 m/s时,分选效率最大,达77.2%。气流速度继续增大时,由于回收率的大幅下降,导致了分选效率的降低。综上所述,最佳操作气流速度为1.00 m/s,此时铜的回收率达92.3%,品位达84.4%。
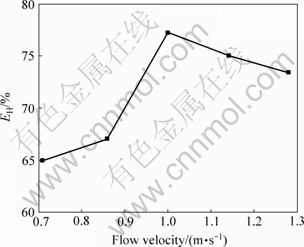
图4 气流速度对铜分选效率(EH)的影响
Fig.4 Effect of flow velocity on separation efficiency of copper (EH)
3 结论
1) 通过锤振破碎、振动筛分与气流分选组合工艺可实现对废锂电池负极材料中金属铜与碳粉的资源化利用。
2) 负极材料经过锤振破碎可有效实现碳粉与铜箔间的相互剥离,后经基于颗粒间尺寸差和形状差的振动过筛可使铜箔与碳粉得以初步分离。锤振剥离与筛分分离结果显示,铜与碳粉分别富集于粒径大于0.250 mm和粒径小于0.125 mm的粒级范围内,品位分别高达92.4%和96.6%,可直接送下游企业回收 利用。
3) 对于粒径为0.125~0.250 mm且铜品位较低的破碎颗粒,可采用气流分选实现铜与碳粉间的有效分离,当气流速度为1.00 m/s时即可取得良好的回收效果,金属铜的回收率可达92.3%,品位达84.4%。
REFERENCES
[1] 戴永年, 杨 斌, 姚耀春, 马文会, 李伟宏. 锂离子电池的发展状况[J]. 电池, 2005, 35(3): 193-195.
DAI Yong-nian, YANG Bing, YAO Yao-chun, MA Wen-hui, LI Wei-hong. Development status of Li-ion batteries[J]. Battery Bimonthly, 2005, 35(3): 193-195.
[2] ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451: 652-657.
[3] GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries[J]. Chemistry of Materimals, 2010, 22(3): 587-603.
[4] XU Jin-qiu, THOMAS H R, FRANCIS R W, LUM K R, WANG Jing-wei, LIANG Bo. A review of processes and technologies for the recycling of lithium-ion secondary batteries[J]. Journal of Power Sources, 2008, 177: 512-527.
[5] FERREIRA D A, PRADOS L M Z, MAJUSTE D, MANSUR M B. Hydrometallurgical separation of aluminum, cobalt, copper and lithium from spent Li-ion batteries[J]. Journal of Power Sources, 2009, 187: 238-246.
[6] SWAIN B, JEONG J, LEE J C, LEE G H. Separation of cobalt and lithium from mixed sulphate solution using Na-cyanex 272[J]. Hydrometallurgy, 2006, 84: 130-138.
[7] SWAIN B, JEONG J, LEE J C, LEE G H, SOHN J S. Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries[J]. Journal of Power Sources, 2007, 167: 536-544.
[8] DORELLA G, MANSUR M B. A study of the separation of cobalt from spent Li-ion battery residues[J]. Journal of Power Sources, 2007, 170: 210-215.
[9] KIM D S, SOHN J S, LEE C K, LEE J H, HAN K S, LEE Y I. Simultaneous separation and renovation of lithium cobalt oxide from the cathode of spent lithium ion rechargeable batteries[J]. Journal of Power Sources, 2004, 132: 145-149.
[10] PAULINO J F, BUSNARDO N G, AFONSO J C. Recovery of valuable elements from spent Li-batteries[J]. Journal of Hazardous Materials, 2008, 150: 843-849.
[11] LI Jin-hui, SHI Pi-xing, WANG Ze-feng, CHEN Yao, CHANG Chein-chi. A combined recovery process of metals in spent lithium-ion batteries[J]. Chemosphere, 2009, 77: 1132-1136.
[12] WANG Rong-chi, LIN Yu-chuan, WU She-huang. A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries[J]. Hydrometallurgy, 2009, 99: 194-201.
[13] 胡传跃, 郭 军, 易秋艳, 汪形艳, 易 涛. 从废旧锂离子电池中回收制备LiAlO2材料[J]. 湖南人文科技学院学报, 2007, 6: 25-28.
HU Chuan-yue, GUO Jun, YI Qiu-yan, WANG Xing-yan, YI Tao. The recover and preparation of LiAlO2 from the waste Li-ion batteries[J]. Journal of Hunan Institute of Humanities, Science and Technology, 2007, 6: 25-28.
[14] 刘云建, 胡启阳, 李新海, 王志兴, 郭华军, 彭文杰. 锂离子电池边角料中直接回收合成LiCoO2的性能[J]. 中国有色金属学报, 2008, 18(2): 398-402.
LIU Yun-jian, HU Qi-yang, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, PENG Wen-jie. Synthesis and electrochemical performances of LiCoO2 recycled from incisors bound of Li-ion batteries[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(2): 398-402.
[15] FOUAD O A, FARGHALY F I, BAHGAT M. A novel approach for synthesis of nanocrystalline γ-LiAlO2 from spent lithium-ion batteries[J]. J Anal Appl Pyrolysis, 2007, 78: 65-69.
[16] SAYILGAN E, KUKRER T, CIVELEKOGLU G, FERELLA F, AKCIL A, VEGLIO F, KITIS M. A review of technologies for the recovery of metals from spent alkaline and zinc-carbon batteries[J]. Hydrometallurgy, 2009, 97: 158-166.
[17] HUANG Kui, LI Jia, XU Zhen-ming. A novel process for recovering valuable metals from waste nickel-cadmium batteries[J]. Environ Sci Technol, 2009, 43(23): 8974-8978.
[18] 王柏成. 对典型分选效率公式的分析和评价[J]. 西安冶金建筑学院学报, 1994, 26(2): 202-207.
WANG Bai-cheng. Analysis and evaluation on typical formulas of selection efficiency[J]. Journal of Xi’an Institute of Metallurgy and Construction Engineering, 1994, 26(2): 202-207.
(编辑 龙怀中)
基金项目:上海市科学技术委员会科研项目(07DZ12029);国家自然科学基金资助项目(51078286)
收稿日期:2010-12-06;修订日期:2011-02-20
通信作者:贺文智,教授;电话:021-65989215;E-mail: hithwz@163.com
摘 要:基于锂电池负极结构及其组成材料铜与碳粉的物料特性,采用锤振破碎、振动筛分与气流分选组合工艺对废锂电池负极组成材料进行分离与回收。实验采用ICP-AES分析实验样品与分离富集产品的金属品位。结果表明:该负极材料经破碎筛分后,粒径大于0.250 mm的破碎料中铜的品位为92.4%,而粒径小于0.125 mm的破碎料中碳粉的品位为96.6%,均可直接回收;粒度为0.125~0.250 mm的破碎料中,铜的品位较低,可通过气流分选实现铜与碳粉的有效分离回收;气流分选过程中,操作气流速度为1.00 m/s时,铜的回收率达92.3%,品位达84.4%。


