文章编号:1004-0609(2016)-04-0807-14
石墨烯在锂离子电池材料性能优化中的应用
曹 亮1,王安安2,艾立华2,贾 明1,刘业翔1
(1. 中南大学 冶金与环境学院,长沙 410000;
2. 湖南艾华集团股份有限公司,益阳 413000)
摘 要:
结合当前利用石墨烯材料特殊二维结构、优良物理化学特性来改善锂离子电池较低能量密度、较差循环性能等缺陷的研究热点,综述石墨烯材料及石墨烯复合材料在锂离子电池正极、负极材料中的研究进展,指出现有电极材料的缺陷和不足,讨论作为锂离子电池电极的石墨烯复合材料结构与功能调控的重要性,并简要评述石墨烯在相关领域中所面临的挑战和发展前景。
关键词:
中图分类号:TM912.9;O646.54 文献标志码:A
全球环境污染与能源危机的日趋严重,迫使各国努力寻找可持续发展的新型能源及能量储运系统以替代传统的化石燃料,以二次电池为代表的高效、实用、“绿色”(低污染或零污染)的能量储运体系已成为当前的关注焦点与研究热点。在新体系的研究过程中,学术界及产业界初步建立和完善了储能材料与器件的评测体系,普遍达成以高功率密度、高能量密度作为衡量新型的储能体系,特别是二次电池性能的重要指标的共识。然而,与评测系统的飞速发展形成鲜明对比的是储能材料体系的缓慢发展。从WHITTINGHAM[1]首次提出锂电池的概念以来,经过长达近40年的发展,虽然一系列新的、具有重要应用前景的电池材料体系层出不穷,但必须正视的是这些新型电极材料还远没有发挥出其理论水平。因此当前储能体系性能的一大核心问题在于发现性能优异的储能材料或者修饰材料以保证材料性能的最大发挥。当一种新型的先进储能材料被发现时,科技工作者苦恼的往往不是将其制备出来,而是怎样将该材料的性能尽量发挥出来。石墨烯材料的问世给解决这类问题带来了曙光。
2004 年, Manchester 大学的 GEIM等[2]首次用机械剥离法获得了单层或薄层的新型二维原子晶体——石墨烯。石墨烯是一种结构独特并且性能优异的新型材料,它是由碳原子以sp2杂化连接的单原子层二维蜂窝状结构,被认为是富勒烯、碳纳米管和石墨的基本结构单元[3]。零维富勒烯是由石墨烯弯曲成足球状得到的,一维的碳纳米管是由石墨烯卷曲而成,三维结构的石墨则被认为是石墨烯片层的紧密堆叠[4],如图1所示。石墨烯的发现在充实了碳材料家族的同时,也充实了储能材料优化及改性的方法体系。由于石墨烯具有高导电性、高导热性、高比表面积、高强度和刚度等诸多优良特性,在储能、光电器件、化学催化等诸多领域获得了广泛的应用,特别是在未来实现基于石墨烯材料的高能量密度、高功率密度应用有着非常重要的理论和工程价值。本文作者就石墨烯材料应用于锂离子电池领域的研究进展情况进行了系统的分析和综述。

图1 石墨烯的结构及由石墨烯为基本单元构筑的sp2杂化碳质材料[3]
Fig. 1 Structure of grapheme (a) and sp2 hybrid carbon materials constructed by grapheme as basic unit (b)[3]
理想的石墨烯是真正的表面性固体,其所有碳原子均暴露在表面,具有用作锂离子电池正负极材料的独特优势[5-6]:
1) 石墨烯具有超大的比表面积(2630 m2/g),比表面积的增大可以降低电池极化,减少电池因极化造成的能量损失。
2) 石墨烯具有优良的导电和导热特性,即本身已具有了良好的电子传输通道,而良好的导热性确保了其在使用中的稳定性。
3) 在聚集形成的宏观电极材料中,石墨烯片层的尺度在微纳米量级,远小于体相石墨的,这使得Li+在石墨烯片层之间的扩散路径较短;而且片层间距也大于结晶性良好石墨的,更有利于Li+的扩散传输。因此,石墨烯基电极材料同时具有良好的电子传输通道和离子传输通道,非常有利于锂离子电池功率性能的提高。
1 石墨烯在锂离子电池负极材料中的应用
1.1 石墨烯直接作为锂离子电池负极材料
商业化锂离子电池石墨负极的理论容量为372 mA·h/g[7]。为实现锂离子电池的高功率密度和高能量密度,提高锂离子电池负极材料的容量是一个关键性问题。无序或比表面积高的热还原石墨烯材料具有大量的微孔缺陷,能够提高可逆储锂容量。因此,相对石墨材料,石墨烯的储锂优点有:1) 高比容量:锂离子在石墨烯中具有非化学计量比的嵌入-脱嵌,比容量可达到 700~2000 mA·h/g,远超过石墨材料的理论比容量372 mA·h/g(LiC6);2) 高充放电速率:多层石墨烯材料的面内结构与石墨的相同,但其层间距离要明显大于石墨的层间距,因而更有利于锂离子的快速嵌入和脱嵌。
YOO等[8]把石墨烯作为锂电池负极材料,当采用50 mA/g的电流密度充放电时,该石墨烯电极材料的比容量为540 mA·h/g;GUO等[9]采用热膨胀氧化石墨法制备石墨烯,并将其应用于锂离子电池负极材料中,在 1 mA/g的电流密度下充放电时,其比容量可达 554 mA·h/g。并且他们发现,如果在其中掺入 C60和碳纳米管后,其比容量高达 784 mA·h/g,远高于传统石墨负极材料的。使用肼的氢氧化物还原事先预构建的石墨烯氧化物片,将退火后的石墨烯片直接作为锂离子电池的负极,不仅避免聚合物粘接剂的使用,而且化学法制备的石墨烯片含有部分残余的含氧基团,展现了相比石墨负极更优异的电化学性能[10-11]。但石墨烯直接作为锂离子电池负极材料所制得的电池器件性能并不稳定。如YOO等[8]所制备的的电池,经20次循环后,容量从初始的540 mA·h/g降低为340 mA·h/g,发生了一定程度的衰减。同样的情况也发生在GUO等[9]的实验中,首次循环之后,比容量就下降到 100 mA·h/g以下 (充放电电流密度 50 mA/g)。即便采用化学法制得的石墨烯片负极材料在较低的充放电倍率下(50~100 mA/g)的可逆容量能达到1264 mA·h/g(是运用于商业化锂离子电池石墨负极的3倍多),但在较高的充放电倍率(500 mA/g以上)下,石墨烯负极表面生成的SEI膜(固体电化学界面膜)使得石墨烯负极材料的充放电容量大幅度衰减。不仅如此,部分含氧基团在脱锂过程中会分解或与Li+发生不可逆副反应,导致充放电过程中容量损失以及负极材料循环过程的电化学性能不稳定[12-13]。有研究表明,通过退火处理可降低石墨烯片层的含氧量,进而减少不可逆副反应的发生。倍率方面的缺陷是制约石墨烯直接作为锂离子电池负极材料的瓶颈之一。为了解决这一问题,很多研究工作者尝试使用N掺杂以及B掺杂来提高石墨烯负极材料的性能[14-20]。WANG等[17]制备了高倍率性能良好的的N、B元素掺杂石墨烯负极材料,通过在液态前驱体中使用CVD(气相沉积法)得到了生长可控制的N-掺杂石墨烯(见图2)。由图2中可以看到,掺杂的N、B原子使石墨烯的蜂窝状结构产生了变形。在50 mA/g的充放电倍率下,N-掺杂石墨烯材料的容量为1043 mA·h/g,B-掺杂石墨烯材料的容量为1540 mA·h/g,是未掺杂N元素的石墨烯材料容量的两倍。不仅如此,掺杂N、B后的石墨烯材料可以在较短的时间内进行快速充放电,在快速充放电倍率为25 A/g下,电池充满时间为30 s。这种性能的改善可能是由于杂原子以及杂原子带来的缺陷改变了石墨烯负极材料的表面形貌,进而改善电极/电解液之间的润湿性,缩短电极内部电子传递的距离,提高Li+在电极材料中的扩散传递速度,从而提高电极材料的导电性和热稳定性。
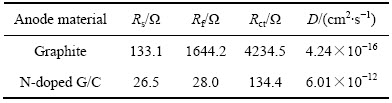
WU等[20]在石墨负极中添加了掺杂N的石墨烯材料,通过电导率的测试,复合电极材料的电导率为5912 S/m,要远高于石墨电极的4018 S/m。并且通过EIS(交流阻抗)测试可知,N掺杂石墨烯结构复合电极材料的电阻要远小于纯石墨电极材料的电阻,仅仅只有石墨材料电阻的3.14%。锂离子扩散速率的对比也证明了复合材料中Li+传输速率得到了提升,如表1所列。
此外,锂离子电池作为大规模推广的储能体系,对安全性能也有较高的要求。锂离子电池在短路、大电流充放电的情况下,会产生大量的热量,从而导致电池性能下降,并且存在爆炸等危险。如何有效地将电池体系的热量及时的扩散出去将是一个重要的问题。单层石墨烯不仅具备优良的电子导电性,同时还具备优良的导热性。在室温下,单层石墨烯的热导率可达5000 W/(m·K),锂离子电池的散热性问题可以利用这一优良的特性得到解决。BALANDIN等[21-22]和SHAHIL等[23]将石墨烯掺杂到相变材料中得到的复合材料导热率比不添加石墨烯的单一相变材料高出两个数量级,大幅度提高了材料的传热效率。

图2 N、B掺杂在石墨烯晶格中的成键结构示意图[20]
Fig. 2 Schematic diagram of binding structure of N and B in grapheme lattice[20]
表1 石墨和掺氮石墨烯在25 ℃、0.2C下循环50次得到的阻抗数据[17]
Table 1 Impedance parameters of graphite and N-doped G/C electrodes after 50 cycles at 0.2C and 25 ℃[17]
虽然将石墨烯作为锂电池负极材料可以提高电导率并改善锂电池的散热性能,但石墨烯材料直接作为电池负极存在如下缺点:1) 制备的单层石墨烯片层极易堆积。丧失了因其高比表面积而具有的高储锂空间的优势;2) 首次库伦效率低。由于大比表面积和丰富的官能团及空位等因素,循环过程中电解质会在石墨烯表面发生分解,形成SEI膜,造成部分容量损失,因此首次库伦效率与石墨负极相比明显偏低,一般低于 70%;同时,碳材料表面残余的含氧基团与锂离子发生不可逆副反应,填充碳材料结构中的储锂空穴,造成可逆容量的进一步下降;3) 初期容量衰减快。一般经过十几次循环后,容量才逐渐稳定;4) 存在电压平台及电压滞后等缺陷。因此,将石墨烯和其他材料进行复合制作成石墨烯基复合负极材料是现在锂电池研究的热点,也是今后发展的趋势。
1.2 石墨烯与过渡金属氧化物复合作锂离子电池负极材料
过渡金属氧化物是具有广泛应用前景的锂电池负极材料。在动力学方面,过渡金属氧化物有很大的比表面积,同时还可以提供额外的活性位点来提高储锂容量。因此,其具有较高的理论储锂容量(大于600 mA·h/g)、较长的循环性能以及较好的倍率性能。
然而,过渡金属氧化物的低电导率以及Li+在嵌入和脱嵌过程中引起的体积效应导致其作为锂离子电池负极性能的不稳定,往往需要通过复合改性处理。添加石墨烯材料一方面可以提高过渡金属氧化物材料的电导率,缓解Li+嵌入脱嵌过程中的体积效应;另一方面,过渡金属氧化物粒子的加入,有效地避免了石墨烯片层间的团聚,保持了石墨烯材料的高比表面积。因此,一系列对于石墨烯过渡金属氧化物的合成方法及性能提升的研究不断被提出[24-30]。
RAI等[31]通过尿素辅助、自蔓延燃烧的方法合成了含有10%石墨烯的石墨烯/Co3O4复合材料 ,复合材料中的石墨烯呈现出的柔软片层状将Co3O4粒子紧紧包裹住并向各个方向延生,构成伸展的空间网络结构。这种稳定的空间网络结构,可以有效缓冲Co3O4电极材料在充放电过程中体积的膨胀收缩,提高材料的循环寿命性能。首次,放电容量为890.44 mA·h/g,充放电循环30次之后,放电容量为801.31 mA·h/g,容量的保持率为90%。而单一的Co3O4电极材料在首次放电容量为877.98 mA·h/g的情况下,充放电循环30次后的容量只有523.94 mA·h/g,其容量保持率仅有59.7%。ZHOU等[26]使用工艺简单、可操作性强的微波辐射法制备了Co3O4量子点石墨烯负极材料。在0.1C下充放电90次后的容量为1785 mA·h/g,在5C下放电容量为485 mA·h/g,得到的容量要高于Co3O4的理论容量。这很可能是嵌锂脱嵌过程中电极界面的Co3O4量子点具有较高的电化学活性所致。
缩小Co3O4的分子尺寸有助于材料性能的提高,SUN等[21]使用介孔尺寸的Co3O4纳米片与石墨烯复合构建了整体有序的3D电极结构。微观显微镜检测结果显示,复合材料的比表面积为34.5 m2/g,介孔的尺寸为3.8 nm。高倍扫描电镜下,复合材料中介孔纳米片均匀的分散在基底上。石墨烯较大的比表面积以及构建的多孔结构不仅有利于电解液的扩散和渗透,而且提高了Li+在活性材料中的传输速率。相比于使用石墨烯和Co3O4进行复合,LAI等[28]尝试将N-掺杂的石墨烯与Co3O4进行复合,Co3O4/NMEG(N-掺杂石墨烯)复合材料的合成示意图如图3所示。N-掺杂石墨烯材料中的含N功能基团,尤其是吡啶氮和叽咯氮有利于Co3O4的生长,且含氮基团的掺杂有利于金属氧化物纳米分子的分散,降低石墨烯中氧的含量。这使得不可逆副反应造成的首次充放电容量损失减少,首次充放电库伦效率得到提高。
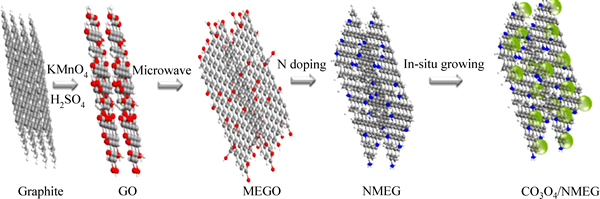
图3 Co3O4/NMEG复合材料制备示意图[28]
Fig. 3 Schematic diagram of preparation of Co3O4/NMEG composite[28]
Fe3O4是一种很有潜力的锂离子负极材料。单位Fe3O4可以储存8个Li+(Fe3O4+8Li++8e=4Li2O+3Fe), 拥有较高的理论容量(924 mA·h/g),且由于价格低廉、易于合成、环境友好等诸多优点使其成为大规模商业化应用的潜在材料。但电导率低、体积效应大以及电压滞后等缺陷制约了Fe3O4的应用。
CHEN等[24]在高温高压条件下用原位碳化法制备了掺杂54.6%碳的Fe3O4复合材料,在50 mA/g下循环100次后容量为610 mA·h/g。相比于掺杂碳材料,DONG等[32]使用水热法将石墨烯与Fe3O4复合,得到一种Fe3O4结构可调的介孔Fe3O4纳米片/石墨烯复合材料。当铁的乙酰丙酮化物和柠檬酸的初始摩尔比调整为3:2时,Fe3+原位还原成Fe2+,进而在石墨烯片层上形成8~20 nm大小的Fe3O4纳米分子。然而,当初始摩尔比改变为3:3时,Fe3O4纳米分子(约15 nm)自组装成内部中空的介孔尺寸(约100 nm)的Fe3O4纳米片附着在石墨烯片上。这种介孔Fe3O4纳米片/石墨烯复合材料展现优良的电化学性能。声波降解检测表明,Fe3O4纳米分子和石墨烯片层间具有很强的相互作用力,并非简单的掺杂、复合。在200 mA/g的电流密度下充放电循环160次后,复合材料的容量达到1070 mA·h/g。在水热体系中,石墨烯表面的含氧官能团以及空位缺陷有利于Fe3O4纳米分子的形核长大,有效地避免了Fe3O4纳米分子的团聚,使得Fe3O4纳米分子、纳米片层能够更好的分散到石墨烯片层上。而且,石墨烯表面的活化核点能控制在其表面生长的金属氧化物颗粒保持在纳米尺寸,使锂离子和电子的扩散距离变小,从而改善材料的倍率性能。 XIAO等[33]通过简易的聚乙烯吡咯烷酮辅助水热法制备Fe2O3/石墨烯复合材料,并通过试验找到了最佳的石墨烯掺杂量。当石墨烯掺杂量为30%(质量分数),复合材料在50 mA/g的电流密度下充放电循环后50次后,可逆放电容量为1069 mA·h/g;当电流密度提高到1000 mA/g,可逆容量仍达到534 mA·h/g。通过SEM和TEM检测发现,Fe2O3分子被石墨烯分子片层包裹住,形成了分散性良好的电极微观结构。WU等[34]制备了Fe@Fe2O3核壳状石墨烯复合材料。通过XRD和SEM检测,可以观测到尺寸为100 nm左右的核壳状Fe@Fe2O3纳米分子均匀地分散在石墨烯片层上形成多孔的三维结构。首次充电容量为1110.3 mA·h/g,在100 mA/g电流密度下充放电循环90次后,充电容量为959.3 mA·h/g,容量保持率为86.4%。而在1000 mA/g下的容量为472.3 mA/g,比XIAO[33]试验中相同电流密度下得到的容量低了11.67%。可能是石墨烯成分的比例不同所致。此外,在实验中还发现过渡金属氧化物电极经循环后容量有所上升的现象,这很可能是放电过程中电极物质逐步激活引起的[35]。PARK等[36]通过水热法合成了Mn3O4/掺氮石墨烯复合负极材料。在HR-TEM下Mn3O4纳米分子镶嵌到石墨烯纳米片层中,扩大了石墨烯片层的间距,增加了石墨烯的比表面积,从而增加了石墨烯材料的储锂容量。而且这种镶嵌结构提高了电极材料的稳定性,加速了Li+的脱嵌。经过循环伏安测试,在200 mA/g的电流密度下循环40次后得到的可逆容量为471 mA·h/g,高出单一Mn3O4材料容量的118 mA·h/g。
在负极材料制备过程中,往往会使用粘接剂、导电添加剂以及集流体。这不仅降低了活性材料的比率,而且在一定程度上影响了电极材料的纯度。于是DENG等[37]在水热法的基础上结合了CVD法将MnO2纳米薄片直接生长在泡沫状石墨烯纳米模板上制成电极,避免使用导电添加剂、粘接剂、集流体。图4所示为MnO2NFs@GF(石墨烯纳米薄层核壳包覆MnO2纳米片)电极微观结构。由图4可看出,生长在石墨烯薄片上的MnO2骨架呈花瓣状,在充放电过程中,锂离子可以更快的迁移,在500 mA/g的电流密度下循环300次后,容量为1200 mA·h/g。通过CVD法直接生长的金属氧化物纳米片和石墨烯片之间接触紧密,有效提升电子的传输速率、缓解体积效应。而且金属氧化物的纳米颗粒又能覆盖住石墨烯表层,最大程度防止电解质插入石墨烯片层导致的电极材料剥落,从而改善材料的循环稳定性能。得到的三维复合材料拥有较单一材料更大的比表面积,增大了电极与电解液间有效的接触面积,提供了更多的电化学反应活性位点。因此,复合材料的容量性能、倍率性能以及循环性能都有了大幅度的提升。
掺杂石墨烯后,电极材料的电化学性能得到了改善,可归纳为如下几点:1) 石墨烯分子可以有效地避免过渡金属氧化物在充放电循环中的团聚;2) 石墨烯柔韧卷曲的片层结构可以有效地缓解充放电过程中的体积膨胀,从而维持电极材料的稳定;3) 石墨烯表面的活性位点可提供额外的储锂空间。但是,目前的实验对于石墨烯复合材料微观结构的研究还不够深入,尤其是充放电循环后,造成电化学性能差异的机理还未明了,尚未得出本证性的结论。所以,可以针对复合材料微观组织形貌充放电循环前后的变化进行对比研究,深入分析和归纳,找出影响电极电化学性能的重要因素。
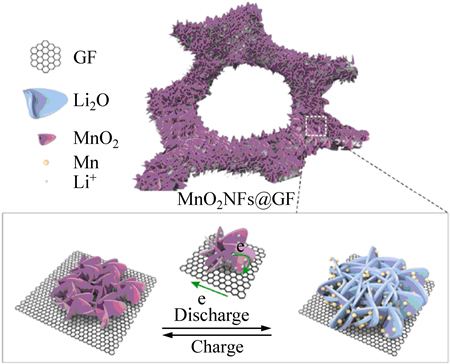
图4 互相连接的MnO2NFs@GF结构充放电行为示意图[37]
Fig. 4 Scheme diagram of discharge and charge behavior for interconnected MnO2NFs@GF[37]
1.3 石墨烯与硅基、锡基材料复合作锂离子电池负极材料
硅基、锡基材料拥有很高的理论比容量,但Li+在其中嵌入、脱出时,电极材料体积的膨胀收缩变化明显,材料内应力大,造成反复充放电后电极材料粉化脱落,导致活性物质减少,从而降低电池容量。很多研究表明,采用纳米尺寸的SnO2材料[38-39]或使用碳包覆的方法可以在缓解充放电过程中体积膨胀的同时,进一步提高电导率。类似的研究,例如:碳包覆SnO2复合材料[40-41]、SnO2-多孔碳材料[42-43]、SnO2@碳核壳纳米结构[44-45]以及SnO2-碳纳米管结构[46-48]。相比于使用其他碳材料的改性方法,石墨烯的引入不仅能更好地阻止锡纳米颗粒的团聚,缓冲材料的体积变化,同时能有效地提高硅材料的锂离子和电子的传输能力。
原位合成法能有效地提高纳米颗粒在石墨烯片层表面的分散程度。Sn2+在与含氧基团间静电作用力的作用下被固定在氧化石墨烯片层表面,随后还原得到 Sn 纳米颗粒,再由高温氧化形成 SnO2/石墨烯材料。虞帧君等[49]采用原位还原法制备了SnO2/ 石墨烯材料,将其与单一SnO2材料和单一石墨烯材料充放电的结果进行对比发现,SnO2/石墨烯复合材料首次充放电容量分别为1682.4 mA·h/g 和1008.2 mA·h/g, 均高于单一石墨烯和单一SnO2 材料的充放电容量,三者放电容量保持率依次为32.8%、23.9%、和7.4%。这表明石墨烯包覆的SnO2材料性能较单一组分的SnO2材料更加优越。
石墨烯和SnO2复合后不仅可以有效降低石墨烯的堆积程度,还可以进一步提高储锂比容量。TAN等[50]使用水热溶剂法一步合成了SnO2掺氮石墨烯复合材料。在SEM下,复合材料呈现出独立、分散的海绵状结构,其中存在的空位缺陷和电子缺陷更便于Li+在电极材料中脱嵌。电化学测试结果显示:在充放电循环100次后,容量为1100 mA·h/g。在电流密度为0.2、0.5、1、2、5 A/g下,复合材料的平均比容量分别为1326、1151、915、709和460 mA·h/g。相比未添加石墨烯的SnO2材料均有了明显的提升。而SHAHID等[51]用石墨烯包覆SnO2纳米球体颗粒,构建了三明治状夹层结构的SnO2/石墨烯复合材料,如图5所示。这种“三明治”状夹层结构一方面提高了电极材料的稳定性;另一方面,最大化利用了SnO2分子的比表面积,有利于SnO2分子均匀的分散在石墨烯片层上,避免了SnO2分子的团聚,缓解了体积膨胀,加强了纳米分子间的相互联系,从而避免了导电添加剂和粘结剂的使用。石墨烯/SnO2球状颗粒复合材料的首次充放电容
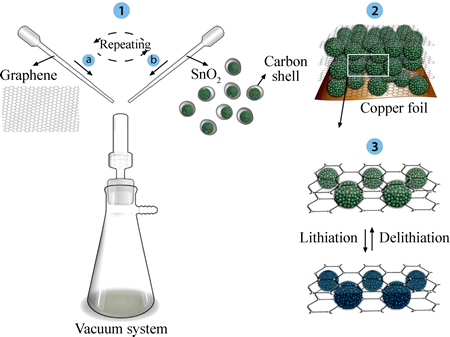
图5 夹层状石墨烯包覆SnO2球体合成流程示意图[51]
Fig. 5 Schematic diagram of preparation of assembled graphene/C-coated mesoporous SnO2 spheres[51]
量为1783 mA·h/g和1247 mA·h/g,较石墨烯/SnO2纳米片层材料的充放电容量提升了24.08%和41.06%。同时研究发现,经过石墨烯的包覆,SnO2材料的不可逆容量降低。这可能是石墨烯包覆在SnO2表面,降低了部分吸附在材料细小孔道中不能可逆脱除Li+的量,从而减少了容量的损失。
就如何减小吸附在细小微孔中不能可逆脱出的Li+造成的容量损失这一难题。LIU等[52]利用柯肯特尔效应制备了克量级石墨烯-介孔SnO2复合电极材料。氮气吸附-解吸等温测试结果表明:复合材料内部的孔径尺寸为中孔和微孔,减少了不能可逆脱出的Li+的量,在100 mA/g的电流密度下充放电循环50次后,可逆容量为1354 mA·h/g,在2 A/g倍率下放电测试容量为664 mA·h/g。图6所示为利用柯肯特尔效应制备复合电极材料流程图。经过高温处理后,SnO2纳米分子镶嵌在石墨烯材料的表面。此类利用柯肯特尔效应构建中孔复合材料的方法亦可借鉴到燃料电池以及超级电容器领域。
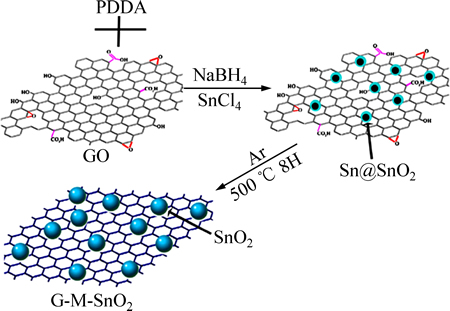
图6 G-M-SnO2合成示意图[52]
Fig. 6 Schematic diagram of synthesis process for G-M-SnO2[52]
为了进一步改善SnO2材料的导电性,提高电子在一维材料中的传递效率,尤其是提高远离集流体的那部分材料在嵌锂和脱嵌过程中的电子传递效率,XU等[53]的团队提出一种添加导电聚合物来构建多相分支状核壳结构的SnO2-聚苯胺纳米棒阵列复合电极材料的方法。复合材料的充放电循环示意图如图7所示。
通过电沉积法将聚苯胺(PANI)直接生长在SnO2纳米棒的表面,这样既省去添加粘接剂,又提高电极材料的纯度。由此构建的PANI核壳结构,一方面可以缓解Li+在SnO2纳米棒上嵌入、脱出时产生的体积膨胀,避免电极材料的崩塌与粉化,另一方面,高导电性的PANI材料可以提高电子在SnO2纳米棒上的传输效率,在100、500、1000、2000、3000 mA/g倍率下的容量分别为1080、866、649、407 和 312 mA·h/g。
硅基类材料的理论比容量高达4200 mA·h/g,同时,其放电电压低,自然储量丰富,使其成为具有极好应用前景的负极材料。但其在充放电过程中体积效应严重(形成 Li3.75Si 体积变化能高达270%),造成材料的循环稳定性差。随着石墨烯研究的兴起,利用石墨烯材料优良的物理化学性质来改善硅基材料,发挥两者的协同作用,将使硅基材料的性能得到大幅度提高[54-56]。WANG等[57]通过CVD沉积法构建了多孔纳米硅/碳纳米管一维异质结构的电极,并利用事先预构建好的结构来实现理想的充放电环境。电极材料的合成结构如图8所示。
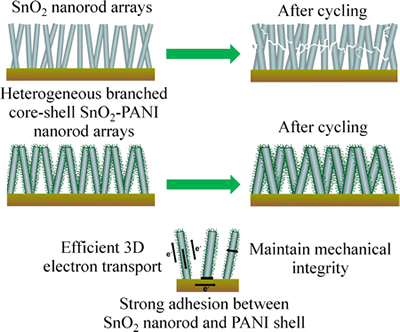
图7 SnO2和SnO2-PANI核壳结构纳米棒阵列循环过程示意图[53]
Fig. 7 Schematic diagram of SnO2 and heterogeneous branched core-shell SnO2-PANI nanorod arrays during cycling[53]
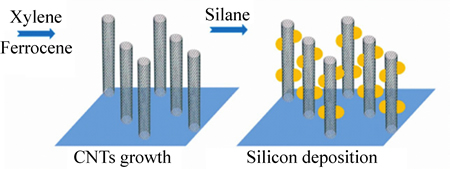
图8 硅/碳纳米管混合纳米结构示意图[57]
Fig. 8 Schematic diagram showing fabrication of silicon/carbon nanotube hybrid nanostructures[57]
HE等[58]使用喷雾干燥法制备出高性能硅-石墨烯复合材料,其扫描电镜和透射电镜图如图9所示,在石墨烯包覆纳米硅(GS-Si)复合材料扫描电镜及透射电镜图中可以看到,石墨烯构成具有内部空腔的三维立体导电网络,将硅粉包裹在其内部空腔内,形成浴花形的复合颗粒。该复合材料不仅容量高,而且具有较好的循环性能,在 200 mA/g电流密度下进行恒流充放电测试,30 次循环后,可逆容量仍能保持在1502 mA·h/g,容量保持率高达98%。该方法简单易行,无需加入表面活性剂,适合大规模工业化生产,但复合材料的倍率性能仍有待提高。
CHEN等[59]在高能热球磨Si纳米颗粒和氧化石墨烯材料混合材料后添加黄原胶作为粘结剂制备Si/石墨烯复合材料。该材料的倍率性能在2000 mA/g和4000 mA/g下容量为766 mA·h/g和458 mA·h/g,相比初始的Si负极材料倍率性能得到了一定的改善。Si基/石墨烯材料在充放电循环后性能下降的原因可能是石墨烯材料的化学惰性使得其与Si基材料之间的作用力很弱,没有形成类似于化学键的稳定联系,在经过充放电循环后,结构出现了粉化和崩塌。有报道提到使用芳香族添加剂可以使石墨烯和Si基以共价键的形式结合[60]。LI等[55]在对Si基/有缺陷的石墨烯间的微观结构进行深入研究后发现,石墨烯中那些由于晶体生长、高能粒子轰击或化学处理所产生的单空位缺陷、双空位缺陷以及Stone-Wales缺陷可以大幅度提高石墨烯/Si分子间的结合能,使复合材料的稳定性更好。而且空位缺陷可以提供额外的储锂活性位点,提高了电极材料的容量。为了进一步提高Si基/石墨烯复合材料的性能,FENG等[61]通过原位生长纳米碳的方法制备了C/Si/石墨烯复合网络结构的电极材料。由于原先的石墨烯纳米片不能很好地包覆在Si纳米分子表面,所以复合材料的循环稳定性不佳。通过在Si分子、石墨烯片层间生长纳米碳的方式在石墨烯纳米片和Si基间搭建了稳定的导电桥梁,并在宏观上构建了稳定的导电网络。这种稳定的导电网络结构既减少了Li+嵌入、脱出过程中产生的体积效应,避免电极材料的破碎,又保持了SEI膜的稳定性。在0.1C、1C下的容量分别为1139 mA·h/g和894 mA·h/g;在1C下循环300次后,容量保持率为94%,在2C下的容量为770 mA·h/g。
此外,不同类型的Si/C复合材料和复合方式也会影响电极材料的性能。高鹏飞[62]综合比较不同类型Si/C复合方式得到的材料性能,其中包括Si/石墨烯包覆型复合材料、Si/微孔碳包裹型复合材料、Si/碳纳米管扎钉型复合材料。研究表明:虽然包裹型、包覆型复合材料发挥了C和Si的协同效应,但在30次循环后,其比容量仍有大幅度下降,原因可能是包裹在石墨烯导电网络内的Si纳米颗粒与石墨烯间仍是物理接触,强度和稳定性不高,在反复充放电过程中会发生电接触丧失。而在扎钉型Si/C复合材料中,纳米碳原位生长在Si基的表面,形成了稳定的导电网络结构,进一步提高Si的利用率,降低电池的内阻,同时,扎钉型结构能够有效缓解Si基材料充放电过程中的体积膨胀,大幅提高复合材料的循环性能。因此,对Si基材料进行石墨烯改性时要优化复合方式。
通过上述可以看出,过渡金属氧化物或者有机分子掺杂石墨烯材料相比单一的过渡金属氧化物负极材料展现了更优异的电化学性能(更高的容量、更好的倍率性能),掺入的杂原子引入了更多的表面缺陷,提高石墨烯材料的电导率,因此,使得复合材料拥有更优良的性能。后阶段石墨烯提高锂离子电池负极材料能量密度的着重点将在于找寻性能更为稳定、优良的杂原子掺杂系统,以及构建更为合适、更为有效的电极微观结构。
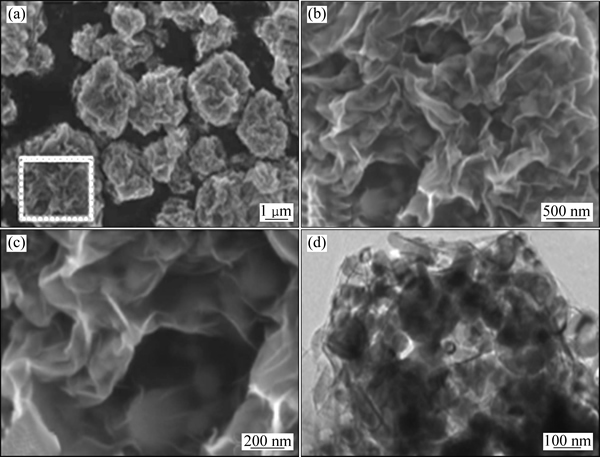
图9 浴花形石墨烯包覆纳米硅(GS-Si)复合材料扫描电镜图及透射电镜图[58]
Fig. 9 SEM ((a)~(c)) and TEM (d) images of graphene nano silicon coated composite materials[58]
2 石墨烯复合正极材料
现阶段,正极材料比容量低,循环性能不佳等缺陷成为制约锂离子电池实现高功率、高能量密度指标的瓶颈。为了突破这一瓶颈,过去的十年里,广大科研工作者投入了大量的精力去寻找新的材料体系或对现有的材料体系进行掺杂和包覆杂原子来提高锂离子电池正极材料的性能。石墨烯及其衍生物已经被广泛的引入到锂离子电池正极材料以弥补现有锂离子电池正极材料电导率低、电子和Li+传输速率低、纳米分子易团聚等缺陷。
2.1 石墨烯与聚阴离子型正极材料的复合
尖晶石型的LiMn2O4以及橄榄石型的LiFePO4[63]是目前应用较好的锂电池正极材料。但是应用过程中存在Li+迁移过慢、电极的电子传导性差、大倍率充放电下电极与电解液间的电阻率大等问题。引入石墨烯材料,为解决这些问题带来了可行的途径。
SU等[64]通过原位溶剂热法制得了LiFePO4/石墨烯/碳复合材料,通过微观形貌分析观察到石墨烯呈二维片层状包覆LiFePO4分子,并通过石墨烯包覆碳构成了三维的空间导电网络。这为离子和电子的传输提供了更好的接触条件,因此,提高复合材料的导电性能,容量达到141 mA·h/g。ZHANG等[65]在乙醇为介质的条件下用水热溶剂法,制得了包覆在橄榄石型LiFePO4分子表面的薄膜状片层石墨烯,包覆的石墨烯薄膜限制了LiFePO4分子的长大和团聚,并进而构建了三维导电的空间结构。这不仅有利于锂离子的传输,而且由于石墨烯的高导电率使制得的石墨 烯/LiFePO4复合材料的性能有了显著提高。在0.1C下,放电的容量为160 mA·h/g;在20C和40C下,放电容量为72.7 mA·h/g和42 mA·h/g。WANG等[66]通过微波加热的方法制得LiFePO4/石墨烯复合材料,控制复合材料的分子在200 nm以下,LiFePO4分子嵌入到石墨烯的片层中,形成了紧密的包覆状。电化学测试表明,在0.1C下,放电容量为158.7 mA·h/g,通过EIS得到的传导电阻以及锂离子在固相中的传输电阻较LiFePO4/C材料有了明显降低,表明石墨烯形成的三维结构能更好地提高LiFePO4的导电性能。MO等[67]在空位限制思路的基础上提出了在油包水的乳液体系中制备碳包覆LiFePO4/石墨烯纳米晶片的方法,如图10所示。这种制备方法有望成为制备更复杂石墨烯复合材料的一般性方法。通过此种方法可以得到具有高度分散纳米结构的晶体。电化学测试结果显示,在17 mA/g的电流密度下充放电循环100次后,可逆储锂容量为158 mA·h/g,库伦效率高于97%。在60C下充放电后的可逆容量为83 mA·h/g。结果表明了油包水体系下制备的碳包覆LiFePO4/石墨烯复合材料具有很好的倍率性能。
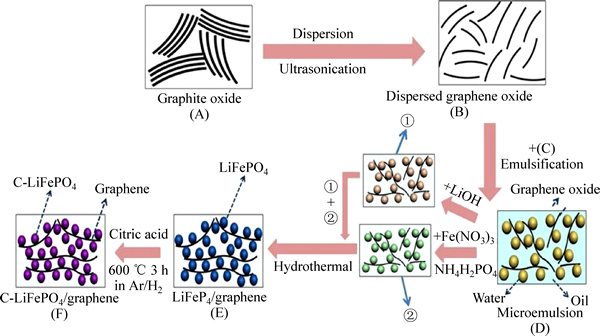
图10 C-LFP/GNs复合材料合成机理示竟图[67]
Fig. 10 Schematic diagram of synthesis steps for C-LFP/GNs composite[67]
此外,利用石墨烯对尖晶石型LiMn2O4进行改性,也展现出了优越的性能。CUI等[68]采用溶胶-凝胶法制备纳米LiMn2O4活性材料,使用改进了的Hummer法制得多层石墨烯,并用冷冻干燥法制备了石墨烯/LiMn2O4纳米复合材料。在扫描电镜检测下,复合材料中的二维石墨烯纳米片层呈现出褶皱状并将LiMn2O4纳米分子牢牢包裹住,构成稳定的三维空间网状结构。经测试,最大充放电容量分别为130.24 mA·h/g和 124.30 mA·h/g,循环50次后,充放电容量衰减到 122.49 mA·h/g和87.22 mA·h/g,容量的保持率分别为 81.40%和 98.54%;循环100次后,容量保持率为 74.31%和 96.66%。LIN等[69]采用水热法合成LiMn2O4/石墨烯纳米片复合材料,避免了复杂的MnO2/石墨烯前驱体合成过程。首次充放电容量为151 mA·h/g和139 mA·h/g,首次库伦效率92%,而单一的LiMn2O4电极材料的首次库伦效率为86%。充放电循环200次后,复合材料的可逆容量121 mA·h/g,而单一电极材料的可逆容量只有71 mA·h/g。实验结果表明:具有完整尖晶石结构的LiMn2O4纳米分子能很好地固定在石墨烯纳米片层的表面。石墨烯片层的存在,有效地降低了LiMn2O4分子之间的团聚,使得活性比表面积未减少。而且导电性能良好的石墨烯纳米片层构成的导电框架可以有效地减少尖晶石型LiMn2O4的Jahn-Teller形变并避免Mn离子与电解液的接触,因而获得了电化学性能优越的电极材料。SAAD等[70]使用溶胶-凝胶法制得LiMn2O4,并通过掺杂石墨烯材料达到增加比容量、提高倍率性能的目的。实验中运用原子层沉淀技术(ALD)可以精确控制覆盖层的厚度。在制得的LiMn2O4/石墨烯复合材料表面包覆了一层6个原子层厚度的ZnO薄膜,ZnO层一方面可以消耗充放电过程中电解液产生的HF,抑制Mn的溶解,另一方面可以提高LiMn2O4的循环稳定性,提高电极材料的寿命。在SEM和TEM扫描下,合成的纳米复合材料呈现出类似“三明治”状的结构,此种结构能有效提升电极材料的容量和电导率。电化学测试显示,在1C的放电倍率下,初始放电容量为135 mA·h/g,要比未添加石墨烯的LiMn2O4电极材料容量高出26 mA·h/g。
通过以上可以看出,在使用石墨烯改性LiFePO4和LiMn2O4材料后,电子的传导率和倍率性能有了明显提升。这是由于石墨烯材料的使用大大缩短了锂离子在正极材料中的扩散路径,加深了锂离子的嵌入和脱出,同时改性材料内部的高空隙率也为锂离子提供了大量的可嵌入空间,提高储锂容量和能量密度。
2.2 石墨烯与钒系材料复合
近些年,钒系材料在锂电池正极材料研究方面取得了巨大的进展[71-73]。钒系材料成本低廉、电化学活性较高、较宽的价态(从+3~+5价)[74]以及相对较高的能量密度的特点,使其受到了广泛的关注。然而,钒系材料较差的循环倍率、较高的电荷转移电阻以及晶体结构容易粉化等缺陷制约了钒系材料的发展。其中VO5作为一种拥有很大潜力的锂离子正极材料,其理论比容量(440 mA·h/g)远高于现在商业化的锂离子电池的正极材料的比容量(<170 mA·h/g)[75-76]。目前,将VO5纳米颗粒与纳米尺度的碳类材料复合来解决钒系材料电导率低、锂离子传输速率慢的的研究已经取得了巨大的进展[75, 77-78]。然而钒系材料的纳米颗粒易于团聚以及长循环下性能变差等仍然是亟待解决的问题。近期,LEE等[79]通过将超薄的VO5纳米线与石墨烯纳米片相缠绕的方法制备循环寿命较长的复合材料。复合材料中的石墨烯纳米片可以有效地遏止VO5纳米线的团聚,在10000 mA/g电流密度下循环10000次后,15%(质量分数)的VO5/石墨烯复合材料仍展现出94.4 mA·h/g的容量。
V2O5是另一种备受关注的钒系材料[80-83],大色散度的量子点可以为活性物质的体积膨胀提供更多的缓冲空间,并且缩短Li+的扩散距离,这将大大提升锂离子电池的循环寿命以及容量上限。HAN等[81]通过控制形核和晶核长大过程得到了V2O5量子点/石墨烯纳米复合材料(VQDG),如图11所示。在电流密度为50、100、200、500 mA/g充放电检测,容量保持率分别为100%、96.92%、89.16%以及65.72%。不仅如此,高倍率充放电后,恢复到50 mA/g下充放电循环,容量保持率恢复到先前50 mA/g下的94.73%。结果表明V2O5量子点/石墨烯材料拥有可靠地稳定性和可逆性。在500和1000 mA/g的电流密度下,初始容量为128 mA·h/g和118 mA·h/g,循环300次后,容量下降到102和100 mA·h/g,单次循环衰减为0.074%和0.055%,这种构建复合电极材料的思路可以拓宽到其他的材料体系中。
SHI等[85]通过原位水热法合成的石墨烯/VO2编织状的纳米带展现出了较低的电荷转移电阻,未添加石墨烯的VO2材料的电荷传递电阻为281 Ω,而石墨烯/VO2复合材料的电荷转移电阻为191 Ω,只有单一VO2材料的67%。将VO2和石墨烯纳米带复合得到的材料取得了较高的可逆容量以及快速充放电的性能,结果证明晶型完整的VO2-石墨烯纳米带可提供快速充放电、较高的可逆容量以及在高温下的长循环性能。这也为制备高能量密度、高功率密度的锂电池石墨烯掺杂复合正极材料提供了突破口。
到目前为止,引入石墨烯材料到锂离子电池正极材料系统可以提高正极材料的电导率,保护正极材料避免粉化、崩塌,抑制正极材料的溶解。在今后的工作中,一方面,应该加强开发具备高容量特性的新材料体系;另一方面,通过构建合理的正极材料结构,如材料的尺寸、形貌、表面缺陷等来提高电池的性能,也将是今后研究工作的重点。
3 结论
1) 石墨烯和石墨烯复合电极在比容量、电压特性、内阻、充放电性能、循环性能、倍率性能等电化学性能方面已经表现出了优异的特性。但是,碳质材料微观结构的复杂性,材料结构和电极的电化学性能间的关系制约着高性能、高效能锂离子电池的发展。
2) 锂盐电解质在碳负极表面形成钝化薄膜(SEI 膜),致使石墨烯电极在首次充放电过程中的库伦效率只有50%~70%,不可逆比容量损失高达 30%~50%。
3) 为了进一步解决目前存在的循环寿命差、快速大电流充放电性能不佳、能量密度和功率密度较低等缺陷,应该深入研究大规模工业化生产单层或几层石墨烯材料的方法、加大对石墨烯材料充放电机理的研究,提出构建合理有效微观形貌石墨烯材料的可行性方法,并深入研究石墨烯的尺寸、结构、缺陷及孔径等因素对石墨烯材料电化学性能的影响。
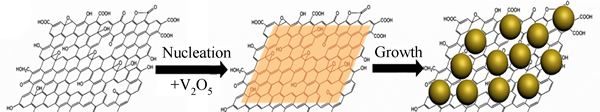
图11 VQDG制备流程示意图[81]
Fig. 11 Schematic diagram of construction processes of VQDG[81]
REFERENCES
[1] WHITTINGHAM M S. Electrical energy storage and intercalation chemistry[J]. Science, 1976, 192(4244): 1126-1127.
[2] GEIM A K ,NOVOSELOV K S, MOROZOV S V, JIANG D, ZHANG Y, DUBONOS S V, GRIGORIEVA I V, FIRSOV A A. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306(5696): 666-669.
[3] GEIM A K. Science, graphene: Status and prospects[J]. Science, 2009, 324(5934): 1530-1534.
[4] LU Xue-kun, YU Min-feng, HUANG Hui, RUOFF R S. Tailoring graphite with the goal of achieving single sheets[J]. Nature Nanotechnology, 1999, 1099(3): 269-272.
[5] LIANG Ming-hui, ZHI Lin-jie. Graphene-based electrode materials for rechargeable lithium batteries[J]. Journal of Materials Chemistry, 2009, 19(33): 5871-5878.
[6] LIANG Ming-hui, LUO Bin, ZHI Lin-jie. Application of graphene and graphene-based materials in clean energy-related devices[J]. International Journal of Energy Research, 2009, 33(13): 1161-1170.
[7] TARASCON, AMP J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414(6861): 359-367.
[8] YOO E J, KIM J, HOSONO E, ZHOU Hao-shen, KUDO T, HONMA I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries[J]. Nano Letters, 2008, 8(8): 2277-2282.
[9] GUO Peng, SONG Huai-he, CHEN Xiao-hong. Electrochemical performance of graphene nanosheets as anode material for lithium-ion batteries[J]. Electrochemistry Communications, 2009, 11(6): 1320-1324.
[10] WANG Cai-yun, LI Dan, TOO C O, WALLACE G G. Electrochemical properties of graphene paper electrodes used in lithium batteries[J]. Chemistry of Materials, 2009, 21(13): 2604-2606.
[11] CHEN Hai-qun, UUML, LLER M B, GILMORE K J, WALLACE G G, LI Dan. Mechanically strong, electrically conductive, and biocompatible graphene paper[J]. Journal of Advanced Materials, 2013, 20(18): 3557-3561.
[12] ZHUO Lin-hai, WU Ying-qiang, ZHOU Wei, WANG Ling-yan, YU Yan-cun, ZHANG Xin-bo, ZHAO Feng-yu. Trace amounts of water-induced distinct growth behaviors of NiO nanostructures on graphene in CO2-expanded ethanol and their applications in lithium-ion batteries[J]. Acs Applied Materials and Interfaces, 2013, 5(15): 7065-7071.
[13] ZHENG Liu-qun, LI Shu-jun, LIN Hai-jun, MIAO Yan-yue, ZHU Ling, ZHANG Zhong-jun. Effects of water contamination on the electrical properties of 18650 lithium-ion batteries[J]. Russian Journal of Electrochemistry, 2014, 50(9): 904-907.
[14] BAE S, KIM H, LEE Y, XU Xiang-fan, PARK J S, ZHENG Yi, BALAKRISHNAN J, LEI Tian, KIM H R, SONG Y I. Roll-to-roll production of 30-inch graphene films for transparent electrodes[J].Nature Nanotechnology, 2010, 5(8): 574-578.
[15] WANG Ying, SHAO Yu-yan, MATSON D W, LI Jing-hong, LIN Yue-he. Nitrogen-doped graphene and its application in electrochemical biosensing[J]. Acs Nano, 2010, 4(4): 1790-1798.
[16] JEONG H M, LEE J W, SHIN W H, CHOI Y J, SHIN H J, KANG J K, CHOI J W. Nitrogen-doped graphene for high-performance ultracapacitors and the importance of nitrogen-doped sites at basal planes[J]. Nano Letters, 2011, 11(6): 2472-2477.
[17] WANG Hai-bo, ZHANG Chuan-jian, LIU Zhi-hong, WANG Li, HAN Peng-xian, XU Hong-xia, ZHANG Ke-jun, DONG Shan-mu, YAO Jian-hua, CUI Guang-lei. Nitrogen-doped graphene nanosheets with excellent lithium storage properties[J]. Journal of Materials Chemistry, 2011, 21(14): 5430-5434.
[18] HUMMERS W S, OFFEMAN R E. Preparation of graphitic oxide[J]. Journal of the American Chemical Society, 1958, 80(6): 1339.
[19] REDDY A L M, SRIVASTAVA A, GOWDA S R, GULLAPALLI H, DUBEY M, AJAYAN P M. Synthesis of nitrogen-doped graphene films for lithium battery application[J]. ACS Nano, 2010, 4(11): 6337-6342.
[20] WU Zhong-shuai, REN Wen-cai, XU Li, LI Feng, CHENG Hui-ming. Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries[J]. ACS Nano, 2011, 5(7): 5463-5471.
[21] GOYAL V, BALANDIN A A. Thermal properties of the hybrid graphene-metal nano-micro-composites: Applications in thermal interface materials[J]. Applied Physics Letters, 2012, 100(7): 073113-073114.
[22] GOLI P, LEGEDZA S, DHAR A, SALGADO R, RENTERIA J, BALANDIN A A. Graphene-enhanced hybrid phase change materials for thermal management of Li-ion batteries[J]. Journal of Power Sources, 2014, 248(7): 37-43.
[23] SHAHIL K M, BALANDIN A A. Graphene-multilayer graphene nanocomposites as highly efficient thermal interface materials[J]. Nano Letters, 2012, 12(2): 861-867.
[24] CHEN Gen, ZHOU Meng, CATANACH J, LIAW T, FEI Ling, DENG Shu-guang, LUO Hong-mei. Solvothermal route based in situ carbonization to Fe3O4@C as anode material for lithium ion battery[J]. Nano Energy, 2014, 8(6): 126-132.
[25] CHEN Shuang-qiang, BAO Pei-te, WANG Guo-xiu. Synthesis of Fe2O3-CNT-graphene hybrid materials with an open three-dimensional nanostructure for high capacity lithium storage[J]. Nano Energy, 2013, 2(3): 425-434.
[26] ZHOU Xiao-yan, SHI Jing-jing, LIU Ya, SU Qing-mei, ZHANG Jun, DU Gao-hui. Microwave irradiation synthesis of Co3O4 quantum dots/graphene composite as anode materials for Li-ion battery[J]. Electrochimica Acta, 2014, 143: 175-179.
[27] SUN Hong-yu, LIU Yan-guo, YU Yan-long, AHMAD M, Nan Ding, ZHU Jing. Mesoporous Co3O4 nanosheets-3D graphene networks hybrid materials for high-performance lithium ion batteries[J]. Electrochimica Acta, 2014, 118(2): 1-9.
[28] LAI Lin-fei, ZHU Ji-xin, LI Zheng-gang, YU D Y W, JIANG Shu-ran, CAI Xiao-yi, YAN Qing-yu, LAM Y M, SHEN Ze-xiang, LIN Jian-yi. Co3O4/nitrogen modified graphene electrode as Li-ion battery anode with high reversible capacity and improved initial cycle performance[J]. Nano Energy, 2014, 3: 134-143.
[29] LIU Yan-guo, CHENG Zhi-ying, SUN Hong-yu, ARANDIYAN H, LI Jin-peng, AHMAD M. Mesoporous Co3O4 sheets/3D graphene networks nanohybrids for high-performance sodium-ion battery anode[J]. Journal of Power Sources, 2015, 273: 878-884.
[30] CHEN Bing-di, PENG Cheng-xin, CUI Zheng. Ultrasonic synthesis of CoO/Graphene nanohybrids as high performance anode materials for lithium-ion batteries[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(10): 2517-2522.
[31] RAI A K, GIM J, ANH L T, KIM J. Partially reduced Co3O4/graphene nanocomposite as an anode material for secondary lithium ion battery[J]. Electrochimica Acta, 2013, 100: 63-71.
[32] DONG Yu-cheng, YUNG K C, MA Ru-guang, YANG Xia, CHUI Ying-san, LEE J M, ZAPIEN J A. Graphene/acid assisted facile synthesis of structure-tuned Fe3O4 and graphene composites as anode materials for lithium ion batteries[J]. Carbon, 2015, 86: 310-317.
[33] XIAO Wei, WANG Zhi-xing, GUO Hua-jun, LI Xin-hai, WANG Jie-xi, HUANG Si-lin, GAN Lei. Fe2O3 particles enwrapped by graphene with excellent cyclability and rate capability as anode materials for lithium ion batteries[J]. Applied Surface Science, 2013, 266(2): 148-154.
[34] WU Chao, ZHANG Hong, WU Yong-xin, ZHUANG Quan-chao, TIAN Lei-lei, ZHANG Xin-xi. Synthesis and characterization of Fe@Fe2O3 core-shell nanoparticles/graphene anode material for lithium-ion batteries[J]. Electrochimica Acta, 2014, 134(21): 18-27.
[35] HU Ai-ping, CHEN Xiao-hua, TANG Yuan-hong, TANG Qun-li, YANG Lei, ZHANG Shao-peng. Self-assembly of Fe3O4 nanorods on graphene for lithium ion batteries with high rate capacity and cycle stability[J]. Electrochemistry Communications, 2013, 28(1): 139-142.
[36] PARK S K, JIN A, YU S H, HA J, JANG B, BONG S, WOO S, SUNG Y E, PIAO Y. In situ hydrothermal synthesis of Mn3O4 nanoparticles on nitrogen-doped graphene as high-performance anode materials for lithium ion batteries[J]. Electrochimica Acta, 2013, 120(7): 452-459.
[37] DENG Jing-wen, CHEN Lin-feng, SUN Yang-yong, MA Min-hao, FU Lei. Interconnected MnO2 nanoflakes assembled on graphene foam as a binder-free and long-cycle life lithium battery anode[J]. Carbon, 2015, 92: 177-184.
[38] JAHEL A, GHIMBEU C M, MONCONDUIT L. Confined ultrasmall SnO2 particles in micro/mesoporous carbon as an extremely long cycle life anode material for li-ion batteries[J]. Advanced Energy Materials, 2014, 4(11): 7963-7975.
[39] TANG Y, WU D, CHEN S, ZHANG F, JIA J, FENG X. Highly reversible and ultra-fast lithium storage in mesoporous graphene-based TiO2/SnO2 hybrid nanosheets[J]. Energy and Environmental Science, 2013, 6(8): 2447-2451.
[40] LOU Xiong-wen, CHEN Jun-song, CHEN Peng, ARCHER L A. One-pot synthesis of carbon-coated SnO2 nanocolloids with improved reversible lithium storage properties[J]. Chemistry of Materials, 2009(13): 2868-2874.
[41] YUAN Ling, KONSTANTINOV K, WANG G X, LIU H K, DOU S X. Nano-structured SnO2 -carbon composites obtained by in situ spray pyrolysis method as anodes in lithium batteries[J]. Journal of Power Sources, 2005, 146(1/2): 180-184.
[42] ZHOU Guang-ming, WANG Da-wei, LI Lu, LI Na, LI Feng, CHENG Hui-ming. Nanosize SnO2 confined in the porous shells of carbon cages for kinetically efficient and long-term lithium storage[J]. Nanoscale, 2013, 5(4): 1576-1582.
[43] WANG Xue-kun, LI Zhao-qiang, YIN Long-wei. Nanocomposites of SnO2@ordered mesoporous carbon (OMC) as anode materials for lithium-ion batteries with improved electrochemical performance[J]. Crystengcomm, 2013, 15(37): 7589-7597.
[44] CHEN Y F, HUANG Q Z, WANG J K, WANG Q, XUE J M. Synthesis of monodispersed SnO2@C composite hollow spheres for lithium ion battery anode applications[J]. Journal of Materials Chemistry, 2011, 21(43): 17448-17453.
[45] ZHANG Bao-hua, YU Xiao-yuan, GE Chun-yun, DONG Xian-ming, FANG Yue-ping, LI Ze-sheng, WANG Hong-qiang. Novel 3-D superstructures made up of SnO2@C core-shell nanochains for energy storage applications[J]. Chemical Communications, 2010, 46(48): 9188-9190.
[46] WU Guang-hui, LI Rui-yi, LI Zai-jun, LIU Jun-kang, GU Zhi-guo, WANG Gang-li. N-doped graphene/graphite composite as a conductive agent-free anode material for lithium ion batteries with greatly enhanced electrochemical performance[J]. Electrochimica Acta, 2015, 171: 156-164.
[47] WEN Zhen-hai, WANG Qiang, ZHANG Qian, LI Jing-hong. In situ growth of mesoporous SnO2 on multiwalled carbon nanotubes: A novel composite with porous-tube structure as anode for lithium batteries[J]. Advanced Functional Materials, 2007, 17(15): 2772-2778.
[48] WU Ping, DU Mi-yu, ZHANG Hui, YU Jing-xue, YANG De-ren. CNTs@SnO2@C coaxial nanocables with highly reversible lithium storage[J]. The Journal of Chemical Physics C, 2010, 114(51): 22535-22538.
[49] 虞祯君. 石墨烯及二氧化锡/石墨烯用作锂离子电池负极材料的电化学性能研究[D]. 上海: 华东理工大学, 2013.
YU Zhen-jun, The electrochemical research of graphene and graphene/SnO2 as lithium-ion anode materials[D]. Shanghai: East China University of Science and Technology, 2013.
[50] TAN Chun-hui, CAO Jing, KHATTAK A M, CAI Fei-peng, JIANG Bo, YANG Gai, HU Su-qin. High-performance tin oxide-nitrogen doped graphene aerogel hybrids as anode materials for lithium-ion batteries[J]. Journal of Power Sources, 2014, 270(3): 28-33.
[51] SHAHID M, YESIBOLATI N, REUTER M C, ROSS F M, ALSHAREEF H N. Layer-by-layer assembled graphene-coated mesoporous SnO2 spheres as anodes for advanced li-ion batteries[J]. Journal of Power Sources, 2014, 263(5): 239-245.
[52] LIU Xiao-wu, ZHONG Xiong-wu, YANG Zhen-zhong, PAN Fu-sen, LIN Gu, YAN Yu. Gram-scale synthesis of graphene-mesoporous SnO2 composite as anode for lithium-ion batteries[J]. Electrochimica Acta, 2015, 152: 178-186.
[53] XU Wang-wang, ZHAO Kang-ning, NIU Chang-jiang, ZHANG Lei, CAI Zheng-yang, HAN Chun-hua, HE Liang, SHEN Teng, YAN Meng-yu, QU Long-bing,MAI Li-qiang. Heterogeneous branched core-shell SnO2-PANI nanorod arrays with mechanical integrity and three dimentional electron transport for lithium batteries[J]. Nano Energy, 2014, 8(9): 196-204.
[54] PARK S H, KIM H K, AHN D J, LEE S I, ROH K C, KIM K B. Self-assembly of Si entrapped graphene architecture for high-performance Li-ion batteries[J]. Electrochemistry Communications, 2013, 34(5): 117-120.
[55] LI Meng, LIU Yue-jie, ZHAO Jing-xiang, WANG Xiao-guang. Si clusters/defective graphene composites as li-ion batteries anode materials: A density functional study[J]. Applied Surface Science, 2015, 345: 337-343.
[56] YONG S K, SHOORIDEH G, ZHMAYEV Y, LEE J, LI Zhong, PATEL B, CHAKRAPANI S, PARK J H, LEE S, YONG L J. The critical contribution of unzipped graphene nanoribbons to scalable silicon-carbon fiber anodes in rechargeable Li-ion batteries[J]. Nano Energy, 2015, 16: 446-457.
[57] WANG Wei, KUMTA P N. Nanostructured hybrid silicon/carbon nanotube heterostructures: Reversible high-capacity lithium-ion anodes[J]. Acs Nano, 2010, 4(4): 2233-2241.
[58] HE Yu-shi, GAO Peng-fei, CHEN Jun, YANG Xiao-wei, LIAO Xiao-zhen, YANG Jun, MA Zi-feng. A novel bath lily-like graphene sheet-wrapped nano-Si composite as a high performance anode material for li-ion batteries[J]. RSC Advances, 2011, 1(6): 958-960.
[59] CHEN Da, YI Ran, CHEN Shu-ru, GORDIN M L, WANG Dong-hai. Facile synthesis of graphene–silicon nanocomposites with an advanced binder for high-performance lithium-ion battery anodes[J]. Solid State Ionics, 2014, 254(9): 65-71.
[60] YANG Sheng-nan, LI Guo-rui, ZHU Qing, PAN Qin-min. Covalent binding of Si nanoparticles to graphene sheets and its influence on lithium storage properties of Si negative electrode[J]. Journal of Materials Chemistry, 2012, 22(8): 3420-3425.
[61] FENG Kun, AHN W, LUI G, PARK H W, KASHKOOLI A G, JIANG Gao-peng, WANG Xiao-lei, XIAO Xing-cheng, CHEN Zhong-wei. Implementing an in-situ carbon network in Si/reduced graphene oxide for high performance lithium-ion battery anodes[J]. Nano Energy, 2016, 19: 187-197.
[62] 高鹏飞. 锂离子电池硅基复合负极材料的制备及电化学研究[D]. 上海: 上海交通大学, 2013.
GAO Peng-fei. Preparation and electrochemical investigation of silicon-based composite anode materials for lithium-ion batteries[D]. Shanghai: Shanghai Jiao Tong University, 2013.
[63] ZHONG Sheng-kui, WANG You, LIU Jie-qun, WANG Jian. Synthesis of LiMnPO4/C composite material for lithium ion batteries by sol-gel method[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(10): 2535-2540.
[64] SU Chang, BU Xi-dan, XU Li-huan, LIU Jun-lei, ZHANG Cheng. A novel LiFePO4/graphene/carbon composite as a performance-improved cathode material for lithium-ion batteries[J]. Electrochimica Acta, 2012, 64(1): 190-195.
[65] ZHANG Yin, WANG Wen-chao, LI Peng-hui, FU Yan-bao, MA Xiao-hua. A simple solvothermal route to synthesize graphene-modified LiFePO4 cathode for high power lithium ion batteries[J]. Journal of Power Sources, 2012, 210(4): 47-53.
[66] WANG Zhao-zhi, GUO Hai-fu, YAN Peng. A rapid microwave heating route to synthesize graphene modified LiFePO4/C nanocomposite for rechargeable lithium-ion batteries[J]. Ceramics International, 2014, 40(10): 15801-15806.
[67] MO Run-wei, LEI Zheng-yu, ROONEY D, SUN K. Facile synthesis of nanocrystalline LiFePO4/graphene composite as cathode material for high power lithium ion batteries[J]. Electrochimica Acta, 2014, 130(4): 594-599.
[68] CUI Yong-li, XU-Kun, YUAN Zhen, XIE Ren-jian, ZHU Guang-lin, ZHUANG Quan-chao, QIANG Ying-huai. Synthesis and electrochemical performance of graphene modified nano-spinel LiMn2O4 cathode materials[J]. Chinese Journal of Inorganic Chemistry, 2013, 29(1): 50-56.
[69] LIN Bing-hui, YIN Qing, HU Heng-run, LU Fu-jia, XIA Hui. Chem inform abstract: LiMn2O4 nanoparticles anchored on graphene nanosheets as high-performance cathode material for lithium-ion batteries[J]. Journal of Solid State Chemistry, 2014, 209(7): 23-28.
[70] AZIZ S, ZHAO Jian-qing, CAIN C, WANG Ying. Nanoarchitectured LiMn2O4 /Graphene/ZnO composites as electrodes for lithium ion batteries[J]. Journal of Materials Science Letters, 2014, 30(5): 427-433.
[71] RAHMAN M M, WANG Jia-zhao, IDRIS N H, CHEN Zhi-xin, LIU Hua-kun. Enhanced lithium storage in a VO2-multiwall carbon nanotube microsheet composite prepared via an in situ hydrothermal process[J]. Electrochimica Acta, 2010, 56(2): 693-699.
[72] REDDY C V S, WALKER E H, SR. S A W, WILLIAMS Q L, KALLURU R R. Synthesis of VO2 nanorods for Li battery application[J]. Current Applied Physics, 2009, 9(6): 1195-1198.
[73] 梁叔全, 潘安强, 刘 军, 钟 杰, 陈 涛, 周 江. 锂离子电池纳米钒基正极材料的研究进展[J]. 中国有色金属学报, 2011, 21(10): 2448-2464.
LIANG Shu-quan, PAN An-qiang, LIU Jun, ZHONG Jie, CHEN Tao, ZHOU Jiang. Research developments of V-based nanomaterials as cathodes for lithium batteries[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(10): 2448-2464.
[74] ZHAO Qian-qian, JIAO Li-fang, PENG Wen-xue, GAO Hai-yan, YANG Jia-qin, WANG Qing-hong, DU Hong-mei, LI Li, QI Zhan, SI Yu-chang, WANG Yi-jing, YUAN Hua-tang. Facile synthesis of VO2(B)/carbon nanobelts with high capacity and good cyclability[J]. Journal of Power Sources, 2012, 199(1): 350-354.
[75] LI Gui-chun, PANG Shu-ping, JIANG Li, GUO Zhi-yan, ZHANG Zhi-kun. Environmentally friendly chemical route to vanadium oxide single-crystalline nanobelts as a cathode material for lithium-ion batteries[J]. Journal of Physical Chemistry B, 2006, 110(19): 9383-9386.
[76] WANG Su-qing, LI Si-rong, SUN Yi, FENG Xu-yong, CHEN Chun-hua. Three-dimensional porous V2O5 cathode with ultra high rate capability[J]. Energy and Environmental Science, 2011, 4(8): 2854-2857.
[77] RUI Xian-hong, ZHU Ji-xin, DAOHAO S, XU Chen, ZENG Yi, HUEY HOON H, TUTI MARIANA L, YAN Qing-yu. Reduced graphene oxide supported highly porous V2O5 spheres as a high-power cathode material for lithium ion batteries[J]. Nanoscale, 2011, 3(11): 4752-4758.
[78] LIU Jun, XU Hui, XUE Dong-feng, LU Li. Double-shelled nanocapsules of V2O5-based composites as high-performance anode and cathode materials for Li ion batteries[J]. Journal of the American Chemical Society, 2009, 131(34): 12086-12087.
[79] LEE J W, LIM S Y, JEONG H M, HWANG T H, KANG J K, CHOI J W. Extremely stable cycling of ultra-thin V2O5 nanowire-graphene electrodes for lithium rechargeable battery cathodes[J]. Energy and Environmental Science, 2012, 5(12): 9889-9894.
[80] LIN Shan, SHAO Bin, TANIGUCHI I. One-step synthesis of dense and spherical nanostructured V2O5 particles for cathode of lithium batteries and their electrochemical properties[J]. Materials Research Bulletin, 2014, 49(1): 291-296.
[81] HAN Chun-hua, YAN Meng-yu, MAI Li-qiang, TIAN Xiao-cong, XU Lin, XU Xu, AN Qin-you, ZHAO Yun-long, MA Xin-yu, XIE Jun-lin. V2O5 quantum dots/graphene hybrid nanocomposite with stable cyclability for advanced lithium batteries[J]. Nano Energy, 2013, 2(5): 916-922.
[82] KUMAR N S, CHANDRASEKARAN J, MARIAPPAN R, SETHURAMAN M, CHAVALI M. V2O5 nano-rods using low temperature chemical pyrophoric reaction technique: The effect of post annealing treatments on the structural, morphological, optical and electrical properties[J]. Superlattices and Microstructures, 2014, 65(4): 353-364.
[83] KIM T, SHIN J, YOU T S, LEE H, KIM J. Thermally controlled V2O5 nanoparticles as cathode materials for lithium-ion batteries with enhanced rate capability[J]. Electrochimica Acta, 2015, 164: 227-234.
[84] PENG Cheng-xin, CHEN Bing-di, QIN Yao, YANG Shi-he, LI Chun-zhong, ZUO Yuan-hui, LIU Si-yang, YANG Jin-hu. Facile ultrasonic synthesis of CoO quantum dot/graphene nanosheet composites with high lithium storage capacity[J]. ACS Nano, 2012, 6(2): 1074-1081.
Application of graphene in performance optimization of lithium ion battery materials
CAO Liang1, WANG An-an2, AI Li-hua2, JIA Ming1, LIU Ye-xiang1
(1. School of Metallurgy and Environment, Central South University, Changsha410000, China; 2. Aihua Group Co., Ltd., Yiyang 413000, China)
Abstract: Combining with the current hotspot of using the special two-dimensional structure, excellent physical and chemical properties of graphene materials, the low energy density and poor cycle performance of lithium ion batteries were improved. Based on the recent research progress of graphene and its composite materials in lithium ion battery cathode, anode materials, the defects and deficiencies of the existing electrode material were pointed. The importance of the structure and function regulation of graphene composites as lithium ion batteries electrode was discussed, the current challenge and future perspectives in the applications of graphene materials were also reviewed.
Key words: graphene; lithium-ion battery; electrode material; graphene composite electrode; energy storage material
Foundation item: Project (51204211, 51222403) supported by the National Natural Science Foundation of China; Project (0714-EMTC02-5271/6) supported by the Special Foundation for Industrial Upgrading Transformation and Strengthen the Foundation of Ministry of Industry and Information Technology, China
Received date: 2015-06-29; Accepted date: 2015-12-22
Corresponding author: JIA Ming; Tel: +86-13975127722; E-mail: jiamingsunmoon@aliyun.com
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(51204211,51222403);工信部工业转型升级强基工程专项资助的课师(0714-EMTC02-5271/6)
收稿日期:2015-06-29;修订日期:2015-12-22
通信作者:贾明,讲师,博士;电话:13975127722;E-mail:jiamingsunmoon@aliyun.com
摘 要:结合当前利用石墨烯材料特殊二维结构、优良物理化学特性来改善锂离子电池较低能量密度、较差循环性能等缺陷的研究热点,综述石墨烯材料及石墨烯复合材料在锂离子电池正极、负极材料中的研究进展,指出现有电极材料的缺陷和不足,讨论作为锂离子电池电极的石墨烯复合材料结构与功能调控的重要性,并简要评述石墨烯在相关领域中所面临的挑战和发展前景。
[3] GEIM A K. Science, graphene: Status and prospects[J]. Science, 2009, 324(5934): 1530-1534.
[49] 虞祯君. 石墨烯及二氧化锡/石墨烯用作锂离子电池负极材料的电化学性能研究[D]. 上海: 华东理工大学, 2013.
[62] 高鹏飞. 锂离子电池硅基复合负极材料的制备及电化学研究[D]. 上海: 上海交通大学, 2013.
[73] 梁叔全, 潘安强, 刘 军, 钟 杰, 陈 涛, 周 江. 锂离子电池纳米钒基正极材料的研究进展[J]. 中国有色金属学报, 2011, 21(10): 2448-2464.


