J. Cent. South Univ. Technol. (2008) 15: 808-813
DOI: 10.1007/s11771-008-0149-x![]()
Thermal decomposition behaviour of polyacrylamidomethyltrimethyl ammonium chloride in red mud separation process
HU Hui-ping(胡慧萍), ZHANG Kun-yu(张琨瑜), ZHANG Li-juan(张丽娟), CHEN Qi-yuan(陈启元)
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract:
In order to provide references for selecting highly efficient red mud flocculants, the behaviour of polyacryl- amidomethyltrimethyl ammonium chloride (PATAC) in red mud separation process was investigated. PATAC was employed as a flocculant for red mud separation from the caustic aluminate liquor at 95 ℃. The used red mud was generated from Chinese diaspore bauxite in Bayer process of alumina production. And the changes of PATAC before or after being treated in caustic solution at 95 ℃ were studied by thermogravimetry (TG) and Fourier transform infrared (FTIR) spectral analysis. The results show that PATAC fails in effectively flocculating red mud and PATAC is readily converted to a quaternary ammonium hydroxide (PATAH) in caustic solution. PATAH can be decomposed to a new polymer (HPATAH) even at 95 ℃. Furthermore, there is an intramolecular hydrogen bond formed in the HPATAH polymer chain with two functional groups of —CH2—OH and —CONH2. Therefore, the poor flocculation property of PATAC for red mud separation can be attributed to the thermal decomposition of PATAC in the caustic red mud slurry at 95 ℃ and the formation of intramolecular hydrogen bond in the polymer chain of HPATAH during the thermal decomposition, which causes the absorbable functional groups of PATAC to decrease greatly.
Key words:
1 Introduction
Red mud is an alkaline leaching waste of bauxite. Red mud separation from aluminate liquor is a critical step in the process of alumina production, and is often enhanced by the addition of various flocculants to promote the growth of red mud aggregation. The aluminate liquor overflowing the settler often contains an unacceptable concentration of suspended substance even after red mud separation, which results in a high supernatant turbidity. Poly quaternary ammonium salts, a new type of cationic polymers with good solubility, high cationic density, high effectiveness, no poison and low price, are used extensively in paper manufacturing, textile finishing, petroleum industry, mining, daily chemical industry and water treatment[1]. Because there are some organic impurities such as humate in aluminate liquor, ROE and MALITO[2] and KOSESTER[3] disposed aluminate liquor containing red mud generated from a gibbsite bauxite by employing polydiallyl dimethyl ammonium chloride, a cationic quaternary ammonium salt, to remove a portion of organic impurities present in aluminate liquor. Their studies demonstrated that polydiallyl dimethyl ammonium chloride was helpful for the removal of organic impurities in aluminate liquor. LU et al[4] showed that the application of cationic flocculants in red mud separation could reform the clarification degree of supernatant aluminate liquor rather than improve red mud settling rate, especially in the strong caustic soda liquor of Bayer process.
Cationic poly quaternary ammonium salts could flocculate negatively charged solid particles effectively by not only polymer bridging but also charge neutralization during the flocculation process[5]. However, SHUBIN[6] found a decrease in the adsorbed amount of cationic polyacrylamide onto negatively charged colloidal silica from basic electrolyte solutions with the increase of pH value. Red mud particles in alumina production process also brought negative surface charges on their surface[7]. In this work, in order to analyze the behaviour of polyacrylamidomethyltrimethyl ammonium chloride (PATAC) in red mud separation process and provide references for selecting highly efficient red mud flocculants, PATAC, a cationically modified polyacrylamide, was employed as a red mud flocculant to improve the clarity of supernatant aluminate liquor. And then the changes of PATAC before or after being treated in caustic solution at 95 ℃ were investigated by thermogravimetry (TG) and Fourier transform infrared (FTIR) spectral analysis.
2 Experimental
2.1 Materials
The red mud slurry used in this study was prepared from Bayer digestion of diaspore bauxite by a bauxite refinery at Zhengzhou Institute of China Aluminium Co. Ltd (CHALCO). The average grain size of red mud particles was measured by a Malvern laser granulometer (Mastersize 2000, U.K.) and found to be about 10 μm. The red mud slurry contains 70 g/L red mud solids, 154 g/L Al2O3 and 160 g/L Na2O.
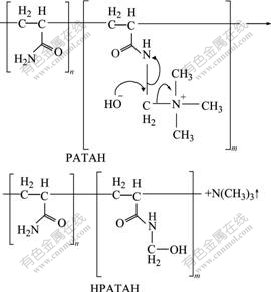
PATAC used as a red mud flocculant in this study was provided by a chemical company in China. PATAC was obtained by Mannich reaction and then quaternization of polyacrylamide. Its chemical structure is shown in Fig.1. The relative molecular mass and positive charge density of PATAC are 3×10-6-8×10-6 and 40%, respectively. As another flocculant, sodium polyacrylate (SPA) with an average relative molecular mass of about 3×10-6 was purchased from Tianjin Kermel Development Centre of Chemical Reagents in China. Both PATAC and SPA were dissolved in distilled water to form a 0.05% (mass fraction) flocculant solution.
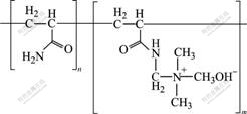
Fig.1 Chemical structure of PATAC
2.2 Settling tests of red mud
Red mud settling tests were carried out in a clear-sided glass water bath maintained at 95 ℃. A well-mixed sample of the boiled red mud slurry was portioned out into six 150-mL graduated cylinders with a diameter of 30 mm and a length of 220 mm, and then these cylinders were immediately placed into the water bath. Following the addition of PATAC or SPA, red mud slurry in the cylinder was mixed by several plunges. The added PATAC or SPA was calculated in the g/t unit based on the mass of dry red mud solids in the cylinder. Subsequently, the height of the interface was recorded with settling time. Settling rates were calculated according to Eqn.(1):
v=60(h1-h2)/5 (1)
where v is the red mud settling rate, m/h; h1 is the initial interface height, m; h2 is the interface height at the fifth minute of settling test, m.
The red mud slurry was allowed to settle for 30 min. After that, about 15 mL supernatant liquid was pipetted off and transferred to a glass cuvette to determine the turbidity, which was measured in nephelometric turbidity units (NTU) using a turbidity meter (WGZ-3, China).
2.3 Alkali treatment of PATAC
A caustic solution containing 0.3% PATAC was prepared by dissolving 0.6 g PATAC in 200 mL 85 g/L NaOH solution. In the caustic solution, PATAC contacted sufficiently with NaOH at 30 ℃ and 150 r/min for 24 h to obtain a solution containing the derivative of PATAC, which is abbreviated as PATAH. After that, PATAH solution was precipitated with acetone. Subsequently, the precipitated PATAH was extracted with acetone for 48 h by a Soxhlet’s apparatus to remove the residual water in PATAH, and then dried under vacuum at 50 ℃ for 48 h. The dried sample was kept in a desiccator for analysis later.
2.4 Thermalgravimetric analysis
The thermal behaviours of PATAC and PATAH were investigated by thermalgravimetry (TG). TG was performed on a METTLER TOLEDO thermoanalyzer (TGA/SDTA851e, Switzerland) using highly pure N2 (99.999%) as purge gas. And the flow rate of N2 was 70 mL/min. 10-15 mg PATAC and PATAH samples were heated from 25 to 300 ℃ at a heating rate of 5 ℃/min. The changes in the mass or the mass differential difference with temperature were recorded to obtain TG curves and differential thermalgravimetric (DTG) analysis curves of PATAC or PATAH.
2.5 Thermal treatment of PATAC and PATAH
The prepared PATAC and PATAH samples were heated to 95 ℃, and then kept at that temperature for 5 h in a tubular furnace (China) purged by highly pure N2 (99.999%) with a flow rate of 70 mL/min. After being cooled to ambient temperature, the treated PATAC and PATAH, which were abbreviated as corresponding HPATAC and HPATAH, were taken out and kept in a desiccator for analysis later.
2.6 Fourier transform infrared spectroscopy
PATAC, PATAH, HPATAC and HPATAH were analyzed by Fourier transform infrared (FTIR) spectroscopy. The FTIR spectra (KBr pellets) were recorded on a Nicolet FTIR spectrophotometer (AVATAR-360, America).
3 Results and discussion3.1 Red mud settling performance of PATAC and SPA
The settling rate and supernatant turbidity of red mud slurry treated by different types and different dosages of flocculants are listed in Table 1.
Table 1 Settling rate and supernatant turbidity contrast for PATAC and SPA
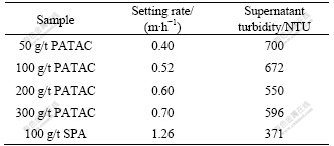
It can be seen from Table 1 that with 100 g/t flocculants, the settling rate (0.52 m/h) of red mud slurry treated by PATAC is much lower than that by SPA (1.26 m/h) and the supernatant turbidity (672 NTU) of red mud slurry treated by PATAC is higher than that by SPA (371 NTU). When the dosage of PATAC is 300 g/t, red mud settling rate and supernatant turbidity are 0.70 m/h and 596 NTU, respectively. It is concluded that red mud settling performance of PATAC cannot be improved obviously, even if its dosage is increased to 300 g/t.
The flocculation of fine particles in suspension may occur by polymer bridging and charge neutralization[8]. The minerals commonly existing in red mud include sodium aluminosilicate hydrate, hydrogarnet, katoite and other complex oxysalt, which makes red mud surface bear lots of hydroxyl groups[9]. Due to the deprotonation of hydroxyl groups on red mud particles in caustic solution, red mud has a negative surface charge[7]. However, the anionic polymers (e.g. SPA) can adsorb on these negatively charged red mud particles by polymer bridging. This effect has also been observed in flocculating negatively charged kaolinite dispersion[10]. In this case, polymer bridging mechanism is of primary importance[11], and bridging absorption is formed mostly by hydrogen bonding between red mud surface hydroxyl groups and such anionic functional groups as —COO- groups in polymer chain or by an ion bridge such as Ca2+ and Mg2+, which can result in local coulombic force between anionic particle surface and negatively charged carboxylate groups in anionic polymers[12]. Indeed, SPA is a highly efficient flocculant for red mud settlement. When red mud particles are flocculated by cationic polymers (e.g. PATAC) with a high relative molecular mass, charge neutralization can also play an important role as well as polymer bridging[8]. In other words, electrostatic attraction between oppositely charged polymers and fine particles can enhance the absorption action of cationic flocculants onto negatively charged red mud surface. Therefore, PATAC should produce a better settling property for red mud than SPA. In fact, red mud settling performance of PATAC is much worse than that of SPA in this study. Maybe, a series of chemical changes appear in PATAC when it is employed in caustic solution at 95 ℃.
3.2 Thermal behaviour of PATAC and PATAH
To investigate the changes of PATAC in caustic red mud slurry at 95 ℃, PATAC was treated by alkali solution. Then both PATAC and its derivative after alkali treatment (i.e. PATAH) were analyzed by TG. Fig.2 shows the TG and DTG curves of PATAC and PATAH.
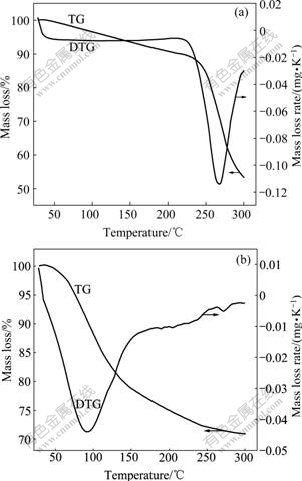
Fig.2 TG and DTG curves of PATAC (a) and PATAH (b)
Fig.2 shows that PATAC has a higher decomposition temperature (267 ℃) at the maximum pyrolysis rate, while PATAH has a lower decomposition temperature (92 ℃) at the maximum pyrolysis rate. LU et al[13] reported that decomposition temperature of organic quaternary ammonium salts was above 190 ℃ in the air. GAO et al[14] also found that phenolic resin containing quaternary ammonium hydrochloride groups was transformed into phenolic resin containing quaternary ammonium hydroxide groups by KOH solution, and thermal decomposition temperature (120 ℃) of quaternary ammonium hydroxide groups was much lower than that (224 ℃) of quaternary ammonium hydrochloride groups. From ambient temperature to 95 ℃, the total mass losses of PATAC and PATAH are 3.2% and 10.2%, respectively. These results show that PATAC is thermostable at 95 ℃, but its derivative in NaOH solution, i.e. PATAH, has a much lower thermal stability. PATAH can be decomposed to a great extent at 95 ℃ due to its nature. As a quaternary ammonium salt, PATAC is readily converted to a quaternary ammonium hydroxide in caustic solution by Eqn.(2)[15]. Therefore, PATAH is a quaternary ammonium hydroxide. And quaternary ammonium hydroxide is easily decomposed at a higher temperature. The decomposition reaction is summarized in Eqn.(3)[15]. As a result, PATAH is unstable under a heating condition.
(2)
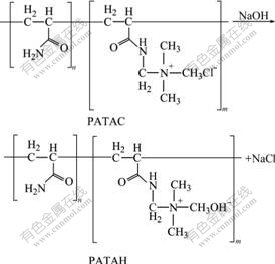
(3)
3.3 FTIR spectral analysis
For the further study on the decomposition reaction of PATAC and PATAH, FTIR analysis was carried out. FTIR spectra of PATAC, HPATAC, PATAH and HPATAH are shown in Fig.3.
In Fig.3, PATAC, HPATAC and PATAH exhibit the similar characteristic absorption peaks. The spectra clearly mark the presence of amide groups and quaternary ammonium groups. The band at 3 427 cm-1 arises from the asymmetric stretching vibration of the NH2 groups, the band at 1 668 cm-1 from stretching vibration of the C=O bond[16], the band at 1 169 cm-1 from in-plane bending vibration of the NH2 groups[17], and the band at 620 cm-1 from out-of-plane rocking vibration of the NH2 groups[18]. The CH2—N+ bending vibration occurs at 953 cm-1[19] and the band at 1 455 cm-1 is the —CH2— bending vibration[20].
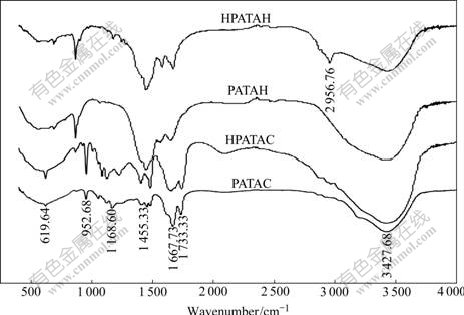
Fig.3 FTIR spectra of PATAC, HPATAC, PATAH and HPATAH
In addition to the similar absorption peaks of amide and quaternary ammonium groups, a new absorption peak appears at 2 957 cm-1 in the spectrum of HPATAH, which maybe results from the stretching vibration of an associated hydroxyl group in —CH2—OH[18]. It is concluded that an intramolecular hydrogen bond (as described in Fig.4) composed of —CH2—OH and —CONH2 from HPATAH can be constructed when HPATAH exists in alkali solution.
3.4 Discussion on red mud settling performance of PATAC
From the results as discussed above, PATAC is readily converted to PATAH in caustic solution and PATAH at 95 ℃ can be decomposed to HPATAH, in which a —CH2—OH~—CONH2 intramolecular hydrogen bond will be formed when HPATAH exists in alkali solution. Red mud separation process during alumina production is conducted usually in strong caustic aluminate liquor at a high temperature from 95 to 110 ℃. Red mud settling tests in this study are done at 95 ℃ and the alkali content in red mud slurry is also quite high. In such a red mud system, PATAC is probably converted to HPATAH. Furthermore, PATAC used in this study bears 40% of positive charge density, so the content of amide groups in HPATAH is almost equal to that of alcoholic hydroxyl groups. Once the intramolecular hydrogen bond, where one amide group is bonded with one alcoholic hydroxyl group, is formed in the polymer chain of HPATAH, HPATAH will have few free absorbable functional groups and then have a much worse flocculation property for red mud. As a result, red mud settling performance of PATAC is rather poor.
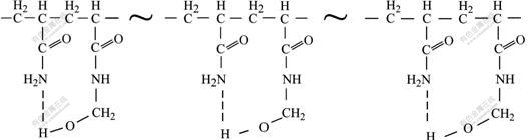
Fig.4 Intramolecular hydrogen bond formed in polymer chain of HPATAH
4 Conclusions1) Although cationic PATAC can absorb on negatively charged red mud surface by not only polymer bridging but also charge neutralization, anionic SPA is more effective for caustic red mud separation at 95 ℃ than cationic PATAC instead.
2) At the same flocculant dosage, settling rate of red mud slurry treated by SPA is much higher than that by PATAC and the supernatant liquor of red mud slurry treated by SPA is also clearer than that by PATAC.
3) PATAC is unstable in caustic solution at a higher temperature and can be decomposed to HPATAH with alcoholic hydroxyl groups.
4) HPATAH has two functional groups of —CH2— OH and —CONH2 to form an intramolecular hydrogen bond in caustic solution, which can greatly decrease the effective absorbable functional groups in HPATAH and result in a poor red mud settling performance of PATAC.
References[1] LIU Li-hua, GONG Zhu-qing, ZHENG Ya-jie. Synthesis and structure characterization of diethyldiallylammonium chloride [J]. J Cent South Univ Technol, 2003, 10(4): 347-351.
[2] ROE W J, MALITO J T. Purification of Bayer process liquors: US 4578255 [P]. 1986-08-27.
[3] KOESTER G E. Process for making alumina: EP 0211338A2 [P]. 1987-12-29.
[4] LU Hong-mei, ZHONG Hong, ZHANG Lei, YU Jia-geng, ZENG Xiang-hui. The present situation and prospect of flocculants in red mud settling [J]. Light Met, 2000(9): 23-26. (in Chinese)
[5] BIGGS S, HABGOOD M, JAMESON G J, YAN Y D. Aggregate structures formed via a bridging flocculation mechanism [J]. Chem Eng J, 2000, 80(1): 13-22.
[6] SHUBIN V. Adsorption of cationic polyacrylamide onto monodisperse colloidal silica from aqueous electrolyte solutions [J]. J Colloid Interf Sci, 1997, 191(2): 372-377.
[7] CHVEDOV D, OSTAP S, LE T. Surface properties of red mud particles from potentiometric titration [J]. Colloid Surface A, 2001, 182(2): 131-141.
[8] NASSER M S, JAMES A E. The effect of polyacrylamide charge density and molecular weight on the flocculation and sedimentation behaviour of kaolinite suspensions [J]. Sep Purif Technol, 2006, 52(2): 241-252.
[9] APAK R, T?TEM E, H?G?L M, HIZAL J. Heavy metal cation retention by unconventional sorbents (red muds and fly ashes) [J]. Wat Res, 1998, 32(2): 430-440.
[10] NASSER M S, JAMES A E. Effect of polyacrylamide polymers on floc size and rheological behaviour of kaolinite suspensions [J]. Colloid Surface A, 2007, 301(2): 311-322.
[11] PATIENCE M, ADDAI-MENASH J, RALSTON J. Investigation of the effect of polymer type on flocculation, rheology and dewatering behaviour of kaolinite dispersions [J]. Int J Miner Process, 2003, 71(4): 247-268.
[12] ERSOY B. Effect of pH and polymer charge density on settling rate and turbidity of natural stone suspensions [J]. Int J Miner Process, 2005, 75(3): 207-216.
[13] LU Gui-qian, WU Ding-cai, FU Ruo-wen. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate [J]. React Funct Polym, 2007, 67(4): 355-366.
[14] GAO Yu, ZHAN Xue-gui, XIE Hong-quan, GAN Zhi-wei. Synthesis and properties of water soluble photosensitive quaternary ammonium hydroxide groups containing phenolic resin [J]. Polym Mater Sci Eng, 2004, 20(1): 65-67. (inChinese)
[15] WEI Jun-jie. Organic chemistry [M]. Beijing: Higher Education Press, 2003: 290-298. (inChinese)
[16] DENG Y J, DIXON J B, WHITE G N, LOEPPERT R H, JUO A S R. Bonding between polyacrylamide and smectite [J]. Colloid Surface A, 2006, 281(1): 82-91.
[17] HASINE K, SAADET ?, MURAT O. Modified polyacrylamide hydrogels and their application in removal of heavy metal ions [J]. Polymer, 2003, 44(6): 1785-1793.
[18] LU Yong-quan, DENG Zhen-hua. Practical infrared spectrum analysis [M]. Beijing: Electronic Industry Press, 1989. (inChinese)
[19] VASSILEV K, STAMENOVA R, TSVETANOV C. Epoxidation of styrene with hydrogen peroxide in the presence of polymer-supported quaternary ammonium salts and peroxo complexes of W(VI) [J]. React Funct Polym, 2000, 46(2): 165-173.
[20] HU Hui-ping, HUANG Ke-long, PAN Chun-yue. Study on syntheses and characterizations of polymethylphenethylsilane, polymethyl- cyclohexylsilane and their copolymers [J]. J Cent South Univ Technol, 2000, 7(2): 92-96.
Foundation item: Project(2005CB623702) supported by the Major State Basic Research and Development Program of China
Received date: 2008-01-25; Accepted date: 2008-04-15
Corresponding author: HU Hui-ping, Professor; Tel: +86-731-8877364; E-mail: phhuiping@hotmail.com
(Edited by ZHAO Jun)
Abstract: In order to provide references for selecting highly efficient red mud flocculants, the behaviour of polyacryl- amidomethyltrimethyl ammonium chloride (PATAC) in red mud separation process was investigated. PATAC was employed as a flocculant for red mud separation from the caustic aluminate liquor at 95 ℃. The used red mud was generated from Chinese diaspore bauxite in Bayer process of alumina production. And the changes of PATAC before or after being treated in caustic solution at 95 ℃ were studied by thermogravimetry (TG) and Fourier transform infrared (FTIR) spectral analysis. The results show that PATAC fails in effectively flocculating red mud and PATAC is readily converted to a quaternary ammonium hydroxide (PATAH) in caustic solution. PATAH can be decomposed to a new polymer (HPATAH) even at 95 ℃. Furthermore, there is an intramolecular hydrogen bond formed in the HPATAH polymer chain with two functional groups of —CH2—OH and —CONH2. Therefore, the poor flocculation property of PATAC for red mud separation can be attributed to the thermal decomposition of PATAC in the caustic red mud slurry at 95 ℃ and the formation of intramolecular hydrogen bond in the polymer chain of HPATAH during the thermal decomposition, which causes the absorbable functional groups of PATAC to decrease greatly.


