
Effect of added cobalt ion on copper electrowinning from sulfate bath using doped polyaniline and Pb-Ag anodes
HUANG Hui(黄 惠)1, ZHOU Ji-yu(周继禹)2, GUO Zhong-cheng(郭忠诚)1
1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology,Kunming 650093, China;
2. The No. 705 Research Institute, Kunming 650118, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract:
Effect of added Co2+(aq) on copper electrowinning was studied using doped polyaniline (Pani) and Pb-Ag(1%) anodes and a stainless steel cathode. The presence of added Co2+(aq) in the electrolyte solution was found to decrease the anode potentials. The optimum level of Co2+(aq) concentration in the electrolyte, with respect to the maximum saving of power consumption was established. Linear sweep voltammetry (LSV) was used to study the influence of added Co2+(aq) on the anodic processes in a copper sulfate-sulfuric acid electrolyte. The oxygen-evolution potential for Pani anode is depolarised at lower current densities (≤0.01 A/cm2) and attains saturation at ρ(Co2+)o≈0.789 g/L; whilst the oxygen-evolution potential for Pb-Ag(1%) anode is depolarised at higher current densities (≤0.02 A/cm2) and attains saturation at ρ(Co2+)o≈1.315 g/L. The preferred orientations of the copper deposits change from (220) to (111) with the addition of 0.394-0.789 g/L Co2+ but higher concentrations favor (220) orientation again.
Key words:
copper electrowinning; cobalt additive; electrode potentials; polarisation;
____________________________________________________________________________________________
1 Introduction
It is well-known that any insoluble anode material used for electrowinning from sulphate solution should possess at least three characteristics: high electrical conductivity[1-2], good electrocatalytical capabilities (for oxygen evolution)[3] and good stability (corrosion resistance)[4-5]. Evidently, pure Pb and its alloying with other metals do not satisfy these requirements. Despite the large number of studies, the most useful material appears to be Pb-Ag alloy in which the Ag content ranges from 0.5% to 1.0% (mass fraction)[6]. This alloy has gained a major position in the production of anodes for hydrometallurgical winning of zinc. Because Ag is an expensive metal, extensive research into its replacement with no resulting changes in the properties of the standard anodes used in industry has been carried out. In search of a suitable alternative to Pb-Ag alloy anode, polyaniline (Pani) anode has been found to be very potential in the anodes. It notably reduces the oxygen overpotential and the corrosion rate of anode.
The electrolytic recovery of copper from an aqueous acidic sulfate solution is an attractive method of producing copper[7]. Copper is also electrolytically recovered from effluents of many industrial units involving metal plating and finishing[8]. However, the high power consumption associated with this process has been the subject of many investigations[9-10].
The addition of Co2+ ion to acidic copper sulfate bath electrolyte is likely to be used for the following reasons[11]:
1) There is a considerable decrease in the over-potential for oxygen evolution, in which ![]() is oxide electrode potential:
is oxide electrode potential:
2H2O→O2↑+4H++4e, ![]() (1)
(1)
2) There is a significant control of the lead corrosion of the lead-antimony anode, and subsequent improvement in the cathode copper quality by reducing lead contamination.
3) The electrolytic process can be used in a conventional copper-electrowinning cell without any modification to the existing plant cell.
Although the presence of added Co2+(aq) in the copper electrolyte with lead alloy anodes[12-13] was shown to decrease the anode potential during the electrowinning process, it has been not shown in the addition of Co2+(aq) to the copper electrolyte using doped polyaniline (Pani) anode during the electrowinning. Furthermore, the effect of cobalt is very sensitive to the composition of the anode substrates[14].
Pani, as one of the most important conducting polymers, has been intensively studied recently due to its high conductivity, ease of preparation, good environment stability, and a large variety of applications especially in light-emitting and electronic devices and chemical sensors[15-17]. In this work, an attempt was made to compare the effect of added Co2+on anode polarization characteristics during the electrowinning of copper with Pani and Pb-Ag(1%) anodes. A comparison of cell voltage, anode potential, current efficiency, power consumption and the crystal orientation for the Pani and Pb-Ag(1%) anode systems were reported. The optimum level of Co2+concentration in the electrolyte to give the maximum current efficiency and the minimum power consumption was established.
2 Experimental
2.1 Materials
Aniline (An) (analytical grade) was distilled twice under vacuum and stored under nitrogen in a refrigerator. Ammonium peroxydisulfate (APS) (analytical grade), sulfuric acid (analytical grade, H2SO4), 5-sulfosalicylic acid (SSA) (analytical grade) and ammonium hydroxide (technical grade, NH3·H2O) were used without purification.
2.2 Preparation of Pani anode
The aqueous Pani dispersion was prepared by mixing 0.01 mol An in a solution of 0.01 mol APS in 100 mL deionized water. Then, 100 mL of an aqueous solution of 0.1 mol/L SSA was added into the stirred solution of An/APS. The polymerization was carried out at 15 ℃ for 6 h. It was then terminated by pouring the reaction mixture into methanol, acetone or water. The obtained dark green powder was filtrated, washed with acetone, methanol and water, respectively. And then the powder was dried in vacuum at 60 ℃ for 48 h.
Pani anode was prepared by mixing active materials with 5% (mass fraction) polyvinyl to form slurry and titanium net as framework. The slurry was pressed under 10 MPa pressure by oil press (8.0 cm×5.0 cm×0.2 cm). The prepared anode was dried at 40 ℃ for 6 h under vacuum.
2.3 Electro-deposition experiments
The electrolysis cell consisted of a lidded 200 cm3 double wall beaker. A stainless steel cathode (8.0 cm×5.0 cm×0.2 cm) and Pani or Pb-Ag(1%) anode of the same dimensions were used. The inter-electrode space was maintained at 3.0 cm for all the experiments. All electro-deposition experiments were carried out for 2 h at (45±1)℃ using an electrolyte solution containing 40 g/L Cu2+, 170 g/L H2SO4 and 0.789 g/L. After electrolysis, the cathode was washed thoroughly with water and acetone and dried. The current efficiency was calculated from the mass of copper gained by the cathode.
2.4 Polarisation measurements
Linear sweep voltammetry (LSV) was used to examine the anodic polarisation behaviour, during copper electro-deposition in the absence or presence of Co2+. A Pani (1 cm2) or Pb-Ag (1 cm2) electrode was used as the working anodic electrode for polarisation studies. A platinum wire and a saturated calomel electrode (SCE) were used as the counter electrode and the reference electrode, respectively. A scanning potentiostat (CHI660B) was used for carrying out polarisation experiments between 0.0 and 2.0 V for the Pani anode and 1.5 to 2.2 V for the Pb-Ag anode. The microcosmic structure of cathode copper deposits was tested by XRD (D/max-3BX) at a scan rate of 5 (?)/min. A scanning electron microscope (XL30ESEM-TMP) was utilized for determining the surface characteristics of the Pani anode.
3 Results and discussion
3.1 Anode potential
In the absence of added Co2+(aq), the anode potential for Pani and Pb-Ag anodes was 1.62 V and 1.67 V(Fig.1), respectively. However, the presence of added Co2+ in the copper electrolyte influenced the anode potentials significantly. Addition of 0.026 3-0.131 5 g/L Co2+ to the electrolyte decreased the Pani anode potential more significantly. An increase in added ρ(Co2+)o up to 0.789 g/L decreased the anode potential to a minimum value of 1.562 V for the Pani anode. But a further increase in ρ(Co2+)o to 5.260 g/L increased the anode potential until saturation was observed.
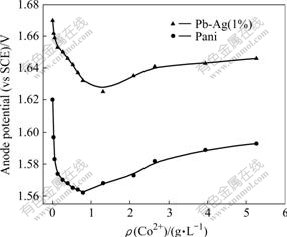
Fig.1 Effect of Co2+ on anode potential (ρ(Cu2+)=40 g/L; ρ(H2SO4)=170 g/L; JCD=300 A/m2; θ=(45±1)℃; td=2 h)
In the case of the Pb-Ag anode system (Fig.1), the decrease in the anode potential with increasing ρ(Co2+)o was less complex. The anode potential decreased to a minimum value of 1.629 V with ρ(Co2+)o≈1.315 g/L and marginally increased with higher Co2+ concentrations.
3.2 Cell voltage
Combining the anode potential and cathode potential, the overall change in the cell voltage with increasing concentrations of added Co2+ is shown in Fig.2. The trend in the cell voltage is nearly the same for both the anodes with an initial decrease as the added Co2+ increases from 0.026 3 to 0.789 g/L and a small increase in cell voltage at higher concentrations. However, the cell voltage obtained using the Pani anode was always lower than that observed on the Pb-Ag anode in accord with open circuit potentials.
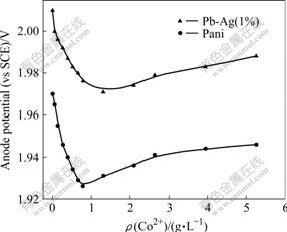
Fig.2 Effect of Co2+ on cell voltage (Condition same as in Fig.1)
3.3 Anode polarization
It was initially observed that the current density vs anode potential curves remained identical, both in the absence and presence of 0.263 g/L Co2+ in the scan range of 0-1.1 V. This indicated that Co2+ had no perceptible effect on the initial sulfation of the Pani surface. The results of anode polarisation studies above 1.1 V are presented in Fig.3. Curve 1 in Fig.3 shows the polarisation curve for oxygen evolution in the absence of added Co2+. The current increases exponentially in the potential range of 1.65 to 1.75 V. In the presence of 0.131 5 g/L Co2+ (curve 2 in Fig.3) the oxygen-evolution potential depolarises and current density appears exponentially in the potential range of 1.55-1.65 V. A further increase in Co2+ to 0.263 g/L (curve 3 in Fig.3) depolarises the oxygen potential more prominently at low current density (≤0.01 A/cm2). Higher concentrations of Co2+ (≥0.394 g/L) have a “catalytic effect” but reach saturation at around 0.789 g/L.
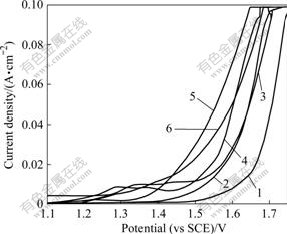
Fig.3 Anodic potentiodynamic curves on Pani anode in the presence of added Co2+: 1—0 g/L; 2—0.1315 g/L; 3—0.263 g/L; 4—0.394 g/L; 5—0.789 g/L; 6—1.578 g/L (ρ(Cu2+)=40 g/L; ρ(H2SO4)=170 g/L; θ=(45±1) ℃; v=10 mV/s)
However, the anodic polarisation behaviour of Co2+ on the Pb-Ag anode during copper electrowinning was studied in the potential range of 1.5-2.2 V and is presented in Fig.4. Curve 1 in Fig.4 shows the polarisation curve for oxygen evolution in the absence of added Co2+. The current density increases exponentially in the potential range of 2.0-2.15 V. In the presence of 0.526 g/L Co2+ (curve 3 in Fig.4) the oxygen-evolution potential depolarises and current density appears exponentially in the potential range of 1.87-1.97 V. A further increase in Co2+ to 1.315 g/L (curve 3 in Fig.4) depolarises the oxygen potential more prominently and have also a “catalytic effect”.
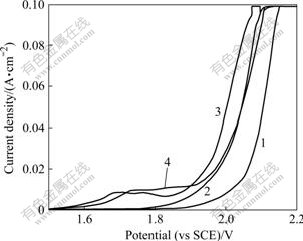
Fig.4 Anodic potentiodynamic curves on Pb-Ag anode in the presence of added Co2+: 1—0 g/L; 2—0.526 g/L; 3—1.315 g/L; 4—2.104 g/L (Other conditions same as in Fig.3)
3.4 Current efficiency and power consumption
The effect of added Co2+ on current efficiency and the corresponding power consumption with the anode systems was examined. It was found that the current efficiency remained between 96% and 98% for all additions of Co2+ up to 5.260 g/L and the cathode remained smooth, bright and compact. Slightly lower power consumption was calculated using the Pani anode due to the lower overall cell voltage of this cell. The decrease in power consumption of Cu was about 93 kW?h/t for the Pani anode with 0.789 g/L Co2+ additive and about 57 kW?h/t for the Pb-Ag with 1.315 g/L Co additive.
3.5 Crystal orientations
The copper electrowinning, obtained on the stainless steel cathode in the absence and the presence of cobalt ion was examined by XRD to determine the preferred crystal orientations and the relative growth of copper on the preferred planes. Representative XRD traces are redrawn and shown in Fig.5. The XRD patterns of all the copper deposits show a face centered cubic (FCC) structure, with the 2θ positions of the peaks remaining constant.
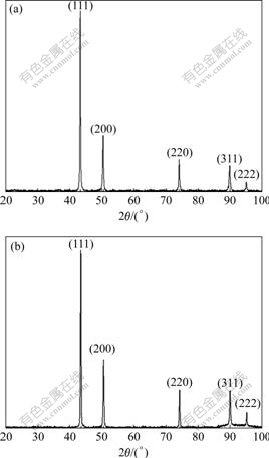
Fig.5 XRD patterns of copper electrowinning in the presence of added Co2+: (a) Nil; (b) 0.789 g/L (ρ(Cu2+)=20 g/L; ρ(H2SO4)= 30 g/L; θ=(30±1) ℃; anode substrate, Pani; td=3 h)
It was observed that in the absence of added Co2+, the order of preferred (hkl) planes of the copper deposits was (111), (200), (220), (311) and (222) (Fig.5(a)). In the presence of 0.789 g/L Co2+ (Fig.5(b)), the preferred order unchanged with the latter two planes exhibiting higher relative intensity. Similar observations were made on the copper electrowinning in the cell with the Pb-Ag anode although the relative intensities of the peaks were not exactly reproducible. This observation is at variance with the reported results[11].
3.6 Interpretations
The change in anode potential with respect to the absence and the presence of added Co2+(aq) can be best explained on the basis of reactions taking place at the anode. In the absence of Co2+, oxygen is evolved from the electrolysis of water taking place on an oxidized Pani surface which exhibits a high over-potential. In the presence of Co2+, oxidation to Co3+(Eq.(2)) allows the facile oxidation of H2O in accordance with Eq.(3).
These two processes lead to lower oxygen over-potential. The catalytic effect of cobalt ion has also been attributed to a lower oxygen over-potential through the reactions in Eqs.(2) and (3):
Co2+→Co3++e,![]() (2)
(2)
4Co3++2H2O→4Co2++4H++O2 (3)
These two processes lead to lower oxygen over-potential. The catalytic effect of cobalt ion has also been attributed to a lower oxygen over-potential through the reactions in Eqs.(4) and (5):
2Co3++2H2O→2Co2++H2O2+2H+ (4)
2H2O2→2H2O+O2 (5)
This accounts for the decrease in the anode potential with ρ(Co2+)o≤0.131 5 g/L and possibly up to ρ(Co2+)o≈0.789 g/L at higher current densities. However, saturation at higher Co2+ concentrations hints at competing inhibiting processes. One could speculate about the formation of a reactive CoO2 or Co3O4 surface layer[18]. A recent study by CACHET et al[19] on the influence of Co2+ or Mn2+ ion on the kinetics of lead anode assumes two opposing effects. A catalytic effect due to a stimulated rate of oxygen evolution on a CoO2 or MnO2 layer, whilst a transient inhibition process occurs which becomes more pronounced with increasing Co2+ concentration. It appears that the polarisation curve attains saturation when no more sites for oxygen evolution can be activated on the electrode surface.
4 Conclusions
1) The decrease in anode potential is more pronounced with the Pani electrode than the Pb-Ag(1%) electrode. The oxygen-evolution potential for Pani anode is depolarised at lower current densities (≤0.01 A/cm2) and attains saturation at ρ(Co2+)o≈0.789 g/L; whilst the oxygen-evolution potential for Pb-Ag(1%) anode is depolarised at higher current densities (≤0.02 A/cm2) and attains saturation at ρ(Co2+)o≈1.315 g/L.
2) The overall decrease in cell voltage results in a decrease in power consumption of Cu of about 93 kW?h/t for the Pani anode with 0.789 g/L Co2+ additive and and about 57 kW?h/t for the Pb-Ag with 1.315 g/L Co2+ additive.
3) The presence of added Co2+ has little effect on the current efficiency of copper but the deposit brightness is significantly improved which can be observed from measurements of reflectivity.
4) The preferred orientations of the copper deposits change from (220) to (111) with the addition of 0.394-0.789 g/L Co2+ but higher concentrations favor (220) orientation again.
References
[1] CLAASSEN J O, MEYRR E H O, RENNIE J, SANDENBERGH R F. Iron precipitation from zinc-rich solutions: Defining the zincor process[J]. Hydrometallurgy, 2002, 67(1): 87-108.
[2] MOSKALYK R R, ALFANTAZI A, TOMBALAKIAN A S, VALIC D. Anode effects in electrowinning[J]. Minerals Engineering, 1999, 12(1): 65-73.
[3] RASHKOV S, DOBREV T, NONCHEVA Z, STEFANOV Y, RASHKOVA B, PETROVA M. Lead-cobalt anodes for electrowinning of zinc from sulphate electrolytes[J]. Hydrometallurgy, 1999, 52(3): 223-230.
[4] XIA X L, ZHITOMIRSKY I, JOSEPH R M. Electrodeposition of zinc and composite zinc-yttria stabilized zirconia coatings[J]. Journal of Materials Processing Technology, 2009, 209: 2632-2640.
[5] REC?NDIZ A, GONZ?LEZ I, JOS? L N. Current efficiency studies of the zinc electrowinning process on aluminum rotating cylinder electrode (RCE) in sulfuric acid medium: Influence of different additives[J]. Electrochimica Acta, 2007, 52: 6880-6887.
[6] RASHKOV S, DOBREV T, NONCHEVA Z, STEFANOV Y, RASHKOVA B, PETROVA M. Lead-cobalt anodes for electrowinning of zinc from sulphate electrolytes[J]. Hydrometallurgy, 1999, 52: 223-230.
[7] GIANNOPULOU I, PANIAS D. Copper and nickel recovery from acidic polymetallic aqueous solutions[J]. Minerals Engineering, 2007, 20: 753-760.
[8] ARMSTRONG R D, TODD M, ATKINSON J W, SCOTT K. Selective electrodeposition of metals from simulated waste solution [J]. J Appl Electrochem, 1996, 26: 379-384.
[9] PANDA B, DAS S C. Electrowinning of copper from sulfate electrolyte in presence of sulfurous acid[J]. Hydrometallurgy, 2001, 59: 55-67.
[10] OISHI T, KOYAMA K, KONISHI H, TANAKA M, LEE J C. Influence of ammonium salt on electrowinning of copper from ammoniacal alkaline solutions[J]. Electrochimica Acta, 2007, 53: 127-132.
[11] PANDA B, DAS S C, PANDA R K. Effect of added cobalt ion on electr-deposition of copper from sulfate bath using graphite and Pb-Sb anode[J]. Hydrometallurgy, 2009, 95: 87-91.
[12] PANDA B, DAS S C, PANDA R K. Synergistic effects of added bivalent aqua cobalt ion, bivalent aqua iron and aqueous sulfurous acid on a graphite anode during electrodeposition of copper from a sulfate bath[J]. Hydrometallurgy, 2004, 72: 149-158.
[13] PANDA B, DAS S C, PANDA R K. Cathodic deposition of copper in the presence of aqueous sulfurous acid, bivalent aqua cobalt ion, or both using a stainless steel cathode and a graphite anode[J]. Metall Mater Trans B, 2003, 34: 813-819.
[14] YU O K. Evaluation of lead anode reactions in acid sulfate electrolytes. I: Lead alloys with cobalt additives[J]. J Electrochem Soc, 1999, 146: 1361-1369.
[15] THANPITCHA T, SIRIVAT A, JAMIESON A M, RUJIRAVANIT R. Synthesis and characterization of pH switching electrical conducting biopolymer hybrids for sensor applications[J]. Synth Met, 2008, 15: 695-703.
[16] JANG J, HA J, KIM K. Organic light-emitting diode with polyaniline-poly(styrene sulfonate) as a hole injection layer[J]. Thin Solid Films, 2008, 516: 3152-3156.
[17] BHADRA S, KHASTGIR D, SINGHA N K, LEE J H. Progress in preparation, processing and applications of polyaniline[J]. Progress in Polymer Science, 2009, 34: 783-810.
[18] MILAZZO G, CAROLI S. Table of standard electrode potentials[M]. Chichester: Wiley, 1978: 336-345.
[19] CACHET C, LE P R C, WIART R. Influence of Co2+ and Mn2+ ion on the kinetics of lead anodes for zinc electrowinning[J]. J Appl Electrochem, 1999, 29: 811-818.
___________________________
Foundation item: Project(50974065) supported by the National Natural Science Foundation of China; Project(2009009) supported by the Analysis and Testing Foundation of Kunming University of Science and Technology, China
Corresponding author: GUO Zhong-cheng; Tel: +86-871-8352598; Fax: +86-871-8352599; E-mail: guozhch@vip.163.com


