
Microstructure of spray formed Al-Zn-Mg-Cu alloy with Mn addition
CAI Yuan-hua1, LIANG Rui-guang2, SU Zhan-pei2, ZHANG Ji-shan1
1. State Key Laboratory for Advanced Metals and Materials,
University of Science and Technology Beijing, Beijing 100083, China;
2. Agricultural Machine Management Office of Rencheng District of Jining City, Jining 272000, China
Received 26 March 2010; accepted 5 July 2010
Abstract:
With the aim to improve the strength of Al-Zn-Mg-Cu alloy, the alloy billet containing Mn was produced by spray forming method, and the microstructural features were investigated using X-ray diffraction (XRD), optical microscopy (OM), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and differential scanning calorimetry (DSC). The results show that the billet mainly consists of fine equiaxial grains of MgZn2 and Al6Mn with size ranging from 5 μm to 25 μm. Nano-scaled MgZn2 is dispersed in the as-sprayed alloy, primary Al6Mn particles are precipitated at grain boundaries with an average size of 5 μm. A few CuAl2, Al3Zr and eutectic are also found in as-sprayed Al alloy. The volume fraction of the porosity is about 12%. DSC result indicates that most of the solutes are precipitated during spray forming process, and no obviously thermal effects occur below 450 °C. Both matrix grains and Al6Mn particles grow monotonously with the increase of annealing temperature, but the growth rate of Al6Mn particles is markedly lower than that of Al grains, and the matrix grains grow rapidly when the annealing temperature is above 375 °C.
Key words:
Al-Zn-Mg-Cu alloy; spray forming; manganese; microstructure;
1 Introduction
Al-Zn-Mg-Cu series high strength aluminum alloys are widely used in aerospace and transportation industries[1]. Attempts including composition modification and employing new processing methods were made to further increase the properties of the alloys. Researches continuously performed on the composition modification by alloying with induced transition elements such as Li[2], Mn[1, 3-5], Ni[6] and Sc[7]. At the same time, new processing routes like low frequency electromagnetic casting (LFEC)[8], equal-channel angular pressing (ECAP)[9] and spray forming[3, 6] were also used to produce high strength Al-Zn-Mg-Cu series alloys.
As a promising technique of rapid solidification, spray forming has been successfully applied to produce high strength 7000 series Al alloys containing high content of transition elements like Mn[3-4]. But porosity is always present in spray-deposited materials regardless of the experimental conditions selected or the materials being synthesized. For practical applications, post thermo-mechanical processing, such as hot rolling and hot extrusion, must be invited to eliminate the porosity and completely densify the spray-deposited materials. Obviously, the initial microstructures and their evolution during heating have important influence on the selection of subsequent process for microstructures and properties to control. So a detailed understanding of the microstructural characteristics of spray formed Al-Zn-Mg-Cu billet containing Mn and their evolution during subsequent heat processing is necessary. However, up to now, such an understanding is still lacking.
In this work, to further increase the mechanical properties of high strength 7000 series alloys, Al-Zn- Mg-Cu-Mn alloy was fabricated by spray forming, and the microstructures and stability of the billet were evaluated.
2 Experimental
The aluminum based alloy used in this study had a composition of Al-8.8%Zn-3.0%Mg-1.7%Cu-1.0%Mn- 0.12%Zr (mass fraction). The binary master alloys and elemental metals used were obtained from several commercial sources. The details of spray deposition process can be seen in Ref.[4]. The molten metal was atomized at 800-850 °C by N2 with a dynamic pressure of 0.8 MPa, and deposited on a rotating substrate at a certain withdraw speed to maintain a constant deposition distance of 380 mm. In order to achieve a round billet and ensure a relatively flat deposition surface, the substrate was displaced off-centered at a distance of 20 mm and maintained an angle of 30° from the center line of spray cone during spray deposition.
The microstructures were studied by means of OM, SEM, XRD, TEM and DSC. Specimens for OM and SEM observations were prepared by the standard metallographic procedures using SiC abrasive paper, 2.5 μm diamond polishing paste and Keller’s reagent. The thin foils for TEM studies were mechanically polished and electro-polished using a twinjet machine with a 10% (volume fraction) nitric acid solution in methanol at -30 °C and (15-25) V, and then they were examined on a HITACHI H-800 TEM operated at 200 kV. X-ray diffraction (XRD) analyses were carried out with Philips APD-10 diffractometer using Cu Kα radiation (40 kV/150 mA). The DSC experiments were performed on a NETZSCH STA 409 C/CD DSC instrument at a heating rate of 10 K/min. The mass of each specimen for DSC analysis was 5-6 mg. For microstructural stability test, the spray-formed alloy was annealed at different temperatures for 2 h, respectively.
3 Results and discussion
3.1 Microstructure of as-sprayed alloy
The microstructures of all specimen obtained from different locations of the billet were qualitatively similar using optical microscope, all the specimen exhibited a fine, homogeneous microstructure with spherical grain morphology, which was typically observed in spray formed billets[4], as shown in Fig.1. Optical microstructure revealed that the grain size ranged from 5 to 25 μm, and the average grain size of the billets was about 18 μm. The only difference observed among the specimens was the grain size. Along the radial direction, the grain size decreased gradually towards the periphery of the billet, which was attributed to the difference of heat transfer conditions. A few grey short rod-like or block-shaped particles with size of about 5 μm were precipitated at grain boundaries. Obviously, there was still a distribution of about 12% (volume fraction) round gas pores with size of 10-50 μm. This distribution of porosity in the billet was consistent with the result obtained by UNDERHILL et al[10], they found the fraction of porosity varying from 5% to 30%. Those large, round pores were likely to originate from the atomization gas entrapment during the deposition process.
XRD analysis revealed that all the samples cut from
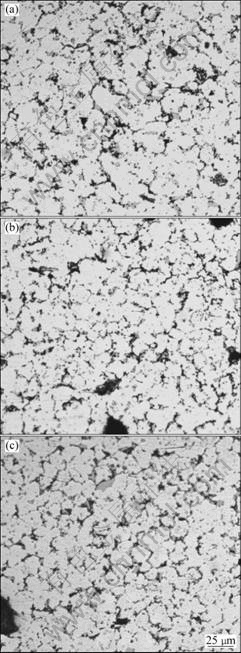
Fig.1 Microstructures of as-spray-deposited billet in different locations: (a) Central part; (b) Middle part; (c) Outer part
every location of deposited billet were composed of α(Al), MgZn2 and Al6Mn. There was no evidence of other phases, as shown in Fig.2.
Through the thickness, apart from the transitional regime of chill zone, the grain size nearly kept constant because each layer reached billet away from substrate experienced similar cooling. As a result, the grain size of the area away from the substrate did not increase monotonously as that in chill zone. When the deposition was finished, the top part had the best heat extraction condition, so the grain size in this area could be smaller than that in inner part of the billet.
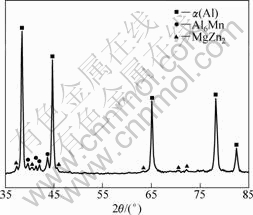
Fig.2 XRD pattern of as spray-formed billet
Fig.3(a) represents a typical SEM image of the as-sprayed billet. It reveals that the as-sprayed Al alloy consisted of several phases. According to XRD and EDS results, the large block shaped particles which precipitated at grain boundaries and had a composition of Al87.65Mn11.67Zn0.68 were ascertained as Al6Mn, the large amount of nano-scaled grey particles dispersed in grain were identified as MgZn2. Some Al3Zr particles were precipitated both at grain boundaries and inner matrix grains, and the bright particulates identified as CuAl2 were precipitated at grain boundaries. Those Cu-bearing precipitates and Al3Zr particles were not detected by XRD due to their low content.
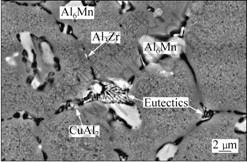
Fig.3 SEM image of as-spray-deposited billet
Intergranular eutectic phase was also found at grain boundaries, as shown in Fig.3. EDS analysis indicates that these eutectic phase contained Al, Zn, Mg and Cu, according to Ref.[11], the eutectic phase appeared to be T(AlZnMgCu). The presence of the intergranular eutectic phase in the as-sprayed material may be induced by the relatively low cooling rate during deposition. LIANG et al[12] demonstrated that the deposition stage was characterized by a very low cooling rate that was several orders of magnitude lower than that generally present during atomization stage (103-105 K/s). As a result, the deposited material would experience a high temperature anneal for a short time during deposition. So segregation of alloying elements would take place, leading to the formation of eutectic phase.
Fig.4 shows the TEM image of as-spray-deposited billet and its corresponding SAED pattern, which exhibited typical morphologies of fine particles in matrix. It could be seen that all the particles had a relatively uniform distribution in Al matrix. From the SAD patterns, the particles could be identified as Al6Mn and MgZn2 (η).
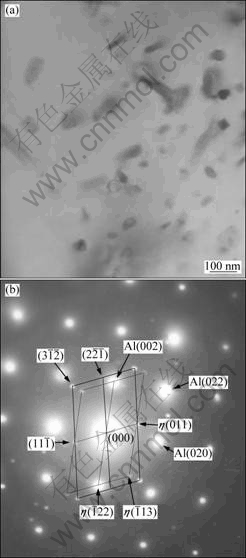
Fig.4 TEM image (a) and corresponding SAED pattern along [001]Al zone axis (b) of as-spray-deposited alloy
From Figs.3 and 4, it could be seen that Al6Mn particles had a large size range from tens of nanometers to several micrometers. The nano-scaled Al6Mn particles are helpful to the further improvement of strength and microstructural stability, and have no negative effect on the toughness and fatigue. But those primary coarse Al6Mn particles larger than 1 μm would not dissolve during post processing such as homogenization and solid solution, and cause severe deterioration to the mechanical properties, especially to the fracture toughness and fatigue properties[13]. Thus, the alloy composition or spray forming parameters should be modified to avoid the formation of such coarse primary particles.
3.2 Microstructural evolution during post heat treatment
The DSC curve which shows the thermal events exhibited by the as-sprayed preform during heating is shown in Fig.5. Notice that three exothermal reactions are apparent in the DSC curve at peak temperature of about 474 °C, 564 °C and 622 °C, respectively. The endothermic reactions at 474 °C and 622 °C may be due to the melting of S phase[14] and the melting of as-deposited Al alloy, respectively. HOWE[15] reported that the endothermic reaction at peak temperature of 564 °C was perhaps induced by the slight dissolution of Al6Mn, however, no attempt was made to verify this reaction experimentally. It should also be noted that exothermic reaction did not appear in the DSC curve. The absence of exothermic peak indicates that most of the solutes were precipitated and no alloying elements further precipitated during post heating, or at least the thermal effect caused by the precipitation of second phases was too little to be detected.
As the samples were heated at different temperatures from 325 to 475 °C with an interval of 50 °C, the amounts of Mn dispersoids and MgZn2 precipitates in Al matrix were changed. Those changes could be qualitatively analyzed from the relative intensity of their corresponding XRD patterns. As the anneal temperature increases, the diffraction intensity of Al6Mn phase increases (Fig.6) due to the further precipitation of Mn atoms from Al matrix, which was consistent with our previous study[1] that dissolved Mn atoms were precipitated at temperature of 350 °C or above. As to the MgZn2 precipitates, the diffraction
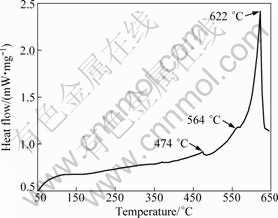
Fig.5 DSC thermogram of as-sprayed billet
intensity changed slightly when the sample was heated below 375 °C, when the sample was heated to temperature of 425 °C or above, the diffraction intensity decreased obviously because of the dissolution of MgZn2, which is in agreement with ROBSON’s work[16].
Fig.7 shows the typical SEM image of the annealed sample, which indicates the grain sizes of Al matrix and Al6Mn particles change during the heating. The average size increment of Al matrix and Al6Mn was plotted in Fig.8. It could be seen that the coarsening of the non- dissolvable Al6Mn phase was little and slower than that of Al alloy because of the low diffusion coefficient of Mn atoms in Al. This would be beneficial to the final properties of the Al alloy because the increase of high
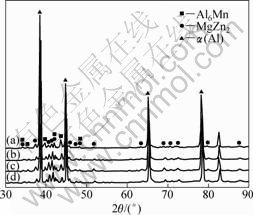
Fig.6 XRD patterns of as-sprayed alloy heated at 475 °C (a), 425 °C (b), 375 °C (c) and 325 °C (d) for 2 h

Fig.7 SEM images of spray formed Al alloy annealed at 375 °C (a) and 425 °C (b)
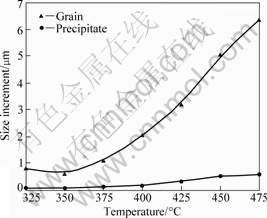
Fig.8 Effect of annealing temperature on size increment of matrix grain and Al6Mn particle
strength and high ductility needed both fine matrix grains and dispersed fine intermetallics. The fine grains microstructure could be achieved through recrystallization of heavily deformed grains induced by subsequent thermo-mechanical processing such as hot extrusion and hot rolling. As for the non-dissolvable Al6Mn particles, the best way to gain uniformly fine dispersoids is to ensure the precipitation of Mn atoms from Mn-supersaturated Al solid solution instead of Al melt.
4 Conclusions
1) The spray formed Al-Zn-Mg-Cu-Mn alloy mainly consisted of equiaxial grains with size ranging from 5 to 25 μm, Al6Mn and nano-scaled MgZn2. The primary Al6Mn particles were precipitated at grain boundaries with an average size of 5 μm. CuAl2 and Al3Zr particles were also precipitated at grain boundaries. A few eutectic was also found in as-sprayed Al alloy due to the actual low cooling rate during deposition process. Further efforts including composition modification and adjustment of spray forming parameters should be applied to avoid the formation of large primary Al6Mn particles.
2) DSC result indicated that no obviously thermal effects occurred below 450 °C. Both matrix grains and Al6Mn particles grew monotonously with the increase of annealing temperature, but the growth rate of Al6Mn particles was much lower than that of Al grains because of the low diffusion coefficient of Mn atoms in Al. When the annealing temperature was above 375 °C, the matrix grains grew rapidly.
References
[1] CAI Yuan-hua, CUI Hua, ZHANG Ji-shan. Microstructures and precipitation behaviour of cryomilled Al-Zn-Mg-Cu-Mn powders [J]. Materials Science and Technology, 2010, 26(3): 352-355.
[2] WEI Fang, LI Jin-shan, ZHOU Tie-tao, LIU Pei-ying. Influence of lithium on the kinetics of phase transformation in 7000 series aluminum alloy by SAXS investigation [J]. Acta Aeronautica et Astronautica Sinica, 2008, 29(4): 1037-1043. (in Chinese)
[3] SHARMA M. M. Microstructural and mechanical characterization of various modified 7XXX series spray formed alloys [J]. Materials Characterization, 2008, 59(1): 91-99.
[4] SHARMA M M, AMATEAU M F, EDEN T J. Mesoscopic structure control of spray formed high strength Al-Zn-Mg-Cu alloys [J]. Acta Materialia, 2005, 53(10): 2919-2924.
[5] SHIMIZU H, OSAMURA K, ADACHI H, KUSUI J, KIKUCHI K. Influence of additional Mn and Cu contents on mechanical properties of Al-Zn-Mg ternary based alloys [J]. Journal of Japan Institute of Light Metals, 2004, 54(1): 2-8.
[6] LI Zhen-liang, XIE Jian-xin, CHEN Wei, ZHAI Jing, REN Hui-ping, WANG Yu-feng. Effects of solid-solution on microstructure and property of high strength spray deposited Al-Zn-Mg-Cu alloy modified by Ni [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(12): 2099-2105. (in Chinese)
[7] ZOU Liang, PAN Qing-lin, HE Yun-bin, WANG Chang-zhen, LIANG Wen-jie. Effect of minor Sc and Zr addition on microstructures and mechanical properties of Al-Zn-Mg-Cu alloys [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(2): 340-345.
[8] DONG J, CUI J Z, YU F X, ZHAO Z H, ZHUO Y B. A new way to cast high-alloyed Al-Zn-Mg-Cu-Zr for super-high strength and toughness [J]. J Materials Processing Technology, 2006, 171(3): 399-404.
[9] WANG Shun-cai, STARINK M J, GAO Nong, QIAO Xiao-guang, XU Cheng, LANGDON T G. Texture evolution by shear on two planes during ECAP of a high-strength aluminum alloy [J]. Acta Materialia, 2008, 56(15): 3800-3809.
[10] UNDERHILL R P, GRANT P S, CANTOR B. Microstructure of spray formed Al alloy 2618 [J]. Materials and Design, 1993, 14(1): 45-47.
[11] MONDOLFO L F. Aluminum alloys: Structure and properties [M]. London: ButterWorths, 1976: 652-654, 857.
[12] LIANG X, EARTHMAN J C, LAVERNIA E J. On the mechanism of grain formation during spray atomization and deposition [J]. Acta Metall Mater, 1992, 40(11): 3003-3016.
[13] THOMPSON D S. Metallurgical factors affecting high strength aluminum alloy production [J]. Metall Mater Trans A, 1975, 6(4): 671-683.
[14] LI Xiao-mei, STARINK M J. Analysis of precipitation and dissolution in overaged 7xxx aluminium alloys using DSC [J]. Mater Sci Forum, 2000, 331-337: 1071-1076.
[15] HOWE J M. Metallographic and differential scanning calorimetry analyses of precipitation and recrystallization in an Al-Mn alloy [J]. Metall Mater Trans A, 1986, 17(4): 593-605.
[16] ROBSON J D. Microstructural evolution in aluminium alloy 7050 during processing [J]. Materials Science and Engineering A, 2004, 382(1-2): 112-121.
喷射成形含锰Al-Zn-Mg-Cu合金的显微组织
蔡元华1,梁瑞光2,苏占培2,张济山1
1. 北京科技大学 新金属材料国家重点实验室,北京 100083;
2. 济宁市任城区农业机械管理局,济宁 272000
摘 要:为提高Al-Zn-Mg-Cu合金的强度,利用喷射成形的方法制备了含锰Al-Zn-Mg-Cu合金锭,并利用X射线衍射(XRD)、光镜(OP)、扫描电镜(SEM)、透射电镜(TEM)和示差扫描热分析(DSC)研究了其微观组织特征。结果表明:喷射沉积坯主要由晶粒尺寸为5~25 μm的细小等轴晶粒、MgZn2 和Al6Mn相组成。纳米级的MgZn2颗粒弥散分布于基体,而平均尺寸为5 μm的Al6Mn一次相颗粒沿晶界析出。沉积合金中也发现了少量的CuAl2, Al3Zr 和共晶组织。沉积坯中缩孔疏松的体积分数约为12%。DSC分析结果说明大部分溶质原子在喷射成形过程中析出,在450 °C以下的加热过程中没有明显的热反应发生。随着退火温度的升高,基体晶粒和Al6Mn颗粒单调长大,但Al6Mn颗粒的长大速率显著低于基体晶粒的长大速率。当退火温度高于375 °C时,基体晶粒迅速长大。
关键词:Al-Zn-Mg-Cu合金;喷射成形;锰;显微组织
(Edited by FANG Jing-hua)
Foundation item: Project (2006CB605204) supported by the National Basic Research Program of China
Corresponding author: CAI Yuan-hua; Tel: +86-10-62332244; Fax: +86-10-62333447; E-mail: yhcaiustb@163.com
DOI: 10.1016/S1003-6326(11)60671-7


