Preparation of high-purity bismuth by sulphur deleadization in vacuum distillation
XIONG Li-zhi(熊利芝)1, HE Ze-qiang(何则强)1, LIU Wen-ping(刘文萍)1,
MA Cheng-jin(麻成金)1, DAI Yong-nian(戴永年)2
(1. College of Chemistry and Chemical Engineering, Jishou University, Jishou 416000, China;
2. College of Materials and Metallurgical Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Abstract:
The feasibility of separation of impurities in refined bismuth and sulphur deleadization with vacuum distillation was studied theoretically. Experimental studies on sulphur deleadization were carried out under vacuum. The influences of amount of sulphur, distillation temperature, vacuum degree and distillation time on deleadization were investigated and an optimal technical condition was achieved. The content of lead in refined bismuth can be decreased from 30μg/g to 0.21μg/g, which has reached the level of “5N” high-purity bismuth. Other impurities in refined bismuth can be also removed effectively under certain conditions.
Key words:
vacuum distillation; sulphur deleadization; refined bismuth; high-purity bismuth CLC number: TF121;
Document code: A
1 INTRODUCTION
Poisonless and high-purity bismuth can be widely used for chemical compound semiconductor, high-purity alloy, electric refrigeration components, thermoelectricity transformation components and the refrigeration of the atomic nucleus reactor carrier in liquid state[1]. The research and development of high-purity bismuth will have a bright future[2]. Vacuum distillation and regional smelt are regarded as the most effective methods for metal separation and purification[3, 4], which have been used for the preparation of many high-purity metals[5-18],such as tellurium, dysprosium, gallium, scandium, terbium, niobium, indium, bismuth, selenium, zinc, lithium and so on. By far vacuum distillation is the most conventional way to purify industrial rich bismuth to 99.999%(5N)[10-15]. But there still exist some problems in the conventional method due to the close saturation vapor pressure of bismuth and lead, i.e. the effect of deleadization is not very desirable and extra chloride deleadization is needed[1]. To solve these problems, we are considering the addition of sulphur for deleadization in vacuum condition, that is, to remove lead by turning it into volatile lead sulphide. Thus we can effectively remove lead without prolonging the technological process for “5N” high-purity Bi. It aims at high rate of recovery, less pollution, less energy consuming and more economic benefit. According to Yunnan Scientific and Technological Intelligence Research Institute, the preparation of high-purity Bi by addition of sulphur for deleadization is by far not reported.
2 THEORETICAL BASIS
2.1 Tthermodynamics basis for sulphur deleadization of refined Bi in vacuum distillation
The chemical reactions related are[19]:
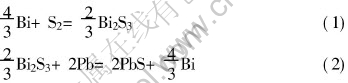
According to Ref.[20], as for reaction (1), ΔG544=-1.35×105(J/mol); to reaction (2), ΔG544=-1.55×105(J/mol), therefore the above reactions are fairly complete from the left to the right in vacuum condition.
The fundamental condition to determine whether substance is separable in vacuum distillation is the differences of the vapor pressure in pure state. The relationship of vapor pressure and temperature between PbS and pure Bi is shown in Fig.1. It shows when the temperature is higher than 900K, the vapor pressure of PbS is greater than that of Bi, that is, at appropriate vacuum distillation temperature, PbS will volatilize prior to Bi. But the degree of separation is the key to the effect of deleadization.
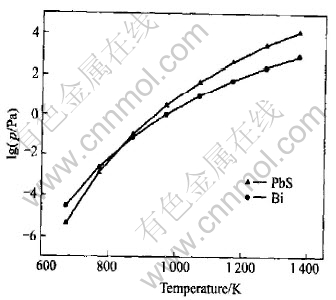
Fig.1 lgp—T curves of PbS and Bi in pure state
In order to determine the possibility and the separation degree of the two components in vacuum distillation, the separation coefficient β is introduced and according to Ref.[20]:
![]()
where γA and γB were the activity coefficients of component A and B, respecting; p*A and p*B are the saturation vapor pressures of A and B in pure state. As to rich Bi 99.999% with very few impurities, it can be regarded as the thin solution with the solvent Bi, hence the activity coefficient is close to 1, that is γ(Bi)=1. By far the document about the activity coefficient of PbS is not reported, supposing γ(Pb)=1, then β=pA/pB. Thus the separation coefficient(β) can be worked out as in Table 1.
Table 1 Saturated vapor pressure and separation coefficients of pure Bi and Bi2S3
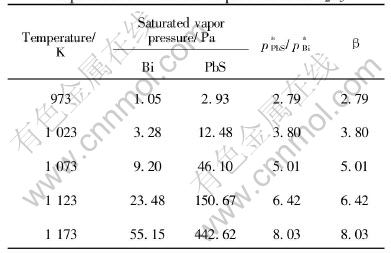
According to Table 1, the separation coefficient of pure Bi and PbS increased from 2.79 to 8.03 while the temperature increases from 973 to 1173K. The higher the temperature, the larger the β, which shows the possibility to separate Bi and PbS in vacuum distillation and it will be more favorable to separate with higher temperature.
According to the above analysis, theoretically it is feasible to purify Bi by the addition of sulphur for deleadization, and PbS can coacervate in the vapor prior to Bi. Besides, from reaction (1), impurity Bi2S3 will be produced together with PbS. The relationship of the saturated vapor pressure between Bi2S3 and the temperature is shown in Table 2. From Tables 1 and 2, the saturated vapor pressure of Bi2S3 is much greater than that of Bi at the same temperature, therefore, Bi2S3 will volatilize first. While the temperature is high enough to melt Bi2S3, it can be discomposed into liquid Bi and gaseous sulphur and it's beneficial to increase the rate of Bi recovery in vacuum.
Table 2 Relationship between saturated vapor pressure of Bi2S3 and temperature[1]

2.2 Thermodynamics basis for removal of other impurities in refined Bi in vacuum distillation
Fig.2 shows the saturated vapor pressure of impurities and bismuth in refined bismuth at different temperatures. The separation coefficients of impurities and bismuth in refined bismuth are shown in Table 3. From Fig.2 and Table 3, other impurities in refined bismuth can be separated with bismuth in vacuum distillation.
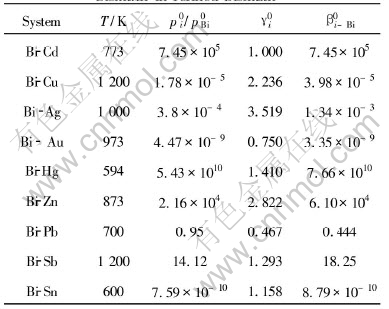
Table 3 Separation coefficients of impurities and bismuth in refined bismuth[20]
3 EXPERIMENTAL
3.1 Materials and equipments
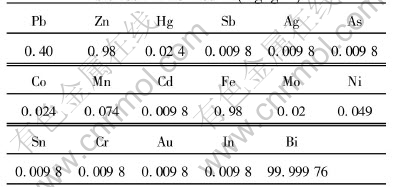
The material No.1 refined Bi is provided by Zhuzhou Smeltery. The components are determined with ICP-MASS by Jiangxi Analysis Testing Center. Table 4 lists the amount of impurities in No.1 refined Bi.
The sulphur used is sublimated with the content of 99.5%. The experiment is carried out with equipment made up of vacuum space, heater, system of controlling and taking temperature, the system of acquiring vacuum and measuring. Vacuum gauge is Mi-styled with measuring range from 0.1 to 150Pa. When the pressure is greater than 150Pa, U-shaped manometer is used. The temperature is taken with platinum platinum-rhodium thermocouple and controlled with controllable silicon temperature controller.
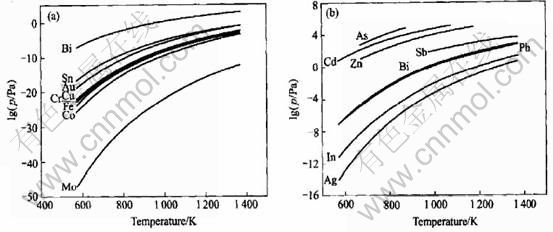
Fig.2 Saturated vapor pressure of impurities and bismuth in refined bismuth
Table 4 Chemical composition of refined bismuth(mass fraction, %)
3.2 Operating process
The refined Bi is ground to size of less than 45μm, and put into graphite crucible with sulphur in certain proportion, then heated with vacuum stove. The inner pressure will keep more or less the same by imputing nitrogen. When the temperature is high enough, make it vacuum and distillate for a while, then cut off the power, refrigerate. The condensate and residue are taken out and weighed. Make sure the amount of lead in the residue meets the requirement of high-purity Bi. Further distillation is carried out at another temperature and then the residue is analyzed.
4 RESULTS AND DISCUSSION
4.1 Influence of content of sulphur on effect of deleadization of refined Bi
Fig.3 shows the effect of deleadization when the sulphur amount ranges from 0.01-0.05g with the temperature of 800K, pressure of 5Pa and distillation time of 20min. It shows that the amount to lead deceases with the addition of sulphur and the volatilization rate increases. While the content of lead in the residue and the volatilization rate of Bi will remain the same with 0.02-0.05g/g sulphur. Thus the content of sulphur should be best at 0.02g/g.

Fig.3 Relationship between content of Pb in residue and volatilization rate of Pb and amount of sulphur added
4.2 Influence of distillation temperature on effect of deleadization of refined Bi
Fig.4 represents the fact of deleadization at different temperatures with the pressure of 5Pa, sulphur content of 0.05g/g, distillation time of 20min. It shows that the volatilization ratio of lead increases and the amount in Bi decreases when the temperature increases, which is up to the theoretical analysis. While it increases to 1123K or so, the content of sulphur added with the increasing temperature, so is the volatilization of PbS and Bi. When it reaches a certain temperature, the volatilization of Bi will also increase, a small portion of lead in the residue has not turned into PbS, so the content of lead in residue will increase at another high temperature. The experiment indicates the appropriate temperature should be 1073K or so.
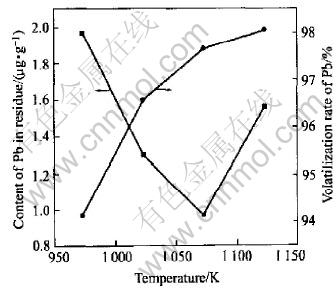
Fig.4 Relationship between content of Pb in residue and volatilization rate of Pb and distillation temperature
4.3 Influence of vacuum degree on effect of deleadization of refined Bi
Fig.5 shows the effect of deleadization with distillation temperature of 1073K, sulphur content of 0.05g/g, distillation time of 20min while the pressure is at 5Pa,10Pa, 16Pa, 20Pa, 30Pa respectively.
According to Fig.5, the vacuum degree will effect the results of deleadization more or less. The content of lead in residue increases and its volatilization ratio decreases by the addition of pressure when the temperature is at 1073K or so. But when the pressure is less than 16Pa, its influence is rare. According to Refs.[20, 21], at the temperature of 1073K, the critical pressure is 10Pa, thus the loss of Bi will be deceased without effecting the deleadization if the experiment is carried out with the pressure of 16Pa.
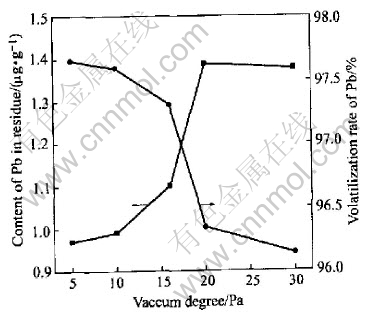
Fig.5 Relationship of content of Pb in residue and volatilization rate of Pb to vacuum degree
4.4 Influence of distillation time on effect of deleadization
Distillation time is considered for further investigating the volatilization of sulphur. The experiment is carried out with the temperature of 1073K, sulphur content 0.05g/g, vacuum degree of 5Pa. The results are shown in Fig.6 and Table 5. It shows the volatilization will speed up as distillation time lasts. While the speed of volatilization will keep unchanged when exceeding 15min, which is critical. Table 5 shows that the distillation time has little effect on deleadization.
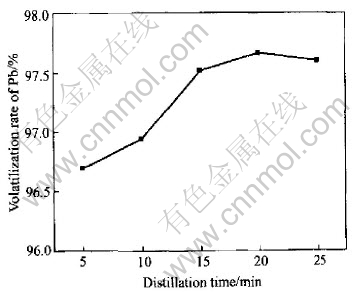
Fig.6 Relationship between distillation time and volatilization rate of Pb
Table 5 Effect of distillation time on sulphur deleadization

4.5 Influence of way of adding sulphur on effect of deleadization
With the same content of sulphur(0.02g/g), the influence on deleadization with a small amount of sulphur frequently is listed in Table 6. Decreasing the amount every time while increasing the time can improve the effect of deleadization.
4.6 Removal of other impurities in refined Bi
Table 6 Effect of distillation times on sulphur deleadization

From Table 7 we can see that the content of impurities(except for Fe) has attained the goal of “5N” refined Bi. The reasons why Fe is a little more than the others lie in the contact with Fe while grinding, sampling and refrigerating.
Table 7 Content of impurities and bismuth in refined Bi(μg·g-1)
5 CONCLUSIONS
1) It is feasible to prepare high-purity Bi with sulphur deleadization in vacuum distillation. Compared with traditional chloride deleadization, sulphur dedeadization is characterized by simple technological process, working condition and efficiency improvement.
2) With the temperature of 1073K, the addition of sulphur 0.02g/g, the content of lead can be decreased from 30 to 0.21μg/g and the deleadization rate is 99.3%.
3) “5N” high-purity Bi can be obtained by sulphur deleadization in vacuum distillation in industry.
REFERENCES
[1]WANG Li-guo. Bismuth Metallurgy [M]. Beijing: Metallurgical Industry Press, 1983. 193-195.(in Chinese)
[2]GUO Qing-wei. Prospect and status of studies on high purity metals [J]. World Nonferrous Metals, 1996, 4: 17-18. (in Chinese)
[3]WU Hong, YAN Hong, WANG Dan. Making the metal in high solidification by the way of regional smelt [J]. Chemical Engineer, 2001, 84(3): 16-17. (in Chinese)
[4]CHEN Hong-bin. Preparation and Purification of High Purity Regent [M]. Shanghai: Shanghai Science and Technology Press, 1983. (in Chinese)
[5]LEI Bo-chao, LIU Bin. Study and commercial operation of high-purity metal [J]. Rare Metals and Cemented Carbides, 2001, 147: 18-20. (in Chinese)
[6]SU Yi, LI Guo-bin, LUO Kang-bi, et al. Progress of technology on preparing high purity gallium [J]. Chinese Journal of Rare Metals, 2003, 27(4): 495-499. (in Chinese)
[7]HU Hua-ye. Preparation of high-purity scandium [J]. Chinese Rare Earths, 1999, 20(4): 70-72. (in Chinese)
[8]LI Zong-an, ZHANG Wei, XU Jing, et al. Study on the preparation process of high-purity terbium [J]. Chinese Rare Earths, 2000, 23(6): 36-39. (in Chinese)
[9]SHI Ying-jiang. Preparation of high-purity niobium [J]. Rare Metals and Cemented Carbides, 1995, 120(1): 41-48. (in Chinese)
[10]ZHOU Zhi-hua, MO Hong-bing, ZENG Dong-ming. Preparation of high-purity indium [J]. Mining and Metallurgical Engineering, 2003, 23(3): 40-43. (in Chinese)
[11]DING Wei-an, DAI Yong-nian. Desilver from crude bismuth by vacuum distillation [J]. Nonferrous Metals, 1994, 46(4): 49-53. (in Chinese)
[12]DING Wei-an, NIU Hui-xian, DAI Yong-nian. Vacuum refining of crude bismuth with silver impurity [J]. Vacuum Science and Technology, 1998, 18(2): 155-160. (in Chinese)
[13]CHEN Wen, DAI Yong-nian, TANG Wen-rong. A study on the regularity of vacuum distillation of metal bismuth [J]. Vacuum Science and Technology, 1994, 14(4): 254-259. (in Chinese)
[14]CHEN Wen, HU Cui. Studies on the critical pressure of bismuth in the vacuum distillation process [J]. Nonferrous Metals Mining and Metallurgy, 2000, 16(3): 25-29. (in Chinese)
[15]DENG Zhi-ming, DAI Yong-nian. New technique of desilverization from crude bismuth by vacuum distillation [J]. The Chinese Journal of Nonferrous Metals, 1997, 7(3): 52-55. (in Chinese)
[16]JING Shi-ping, YANG Bin, HUANG Zhan-chao, et al. Selenium removal by vacuum distillation [J]. Vacuum Science and Technology, 2003, 23(5): 369-372. (in Chinese)
[17]JIA Yong-zhong, ZHOU Yuan, JING Yan, et al. Preparation of pure lithium by vacuum thermal reduction and distillation [J]. Chinese Journal of Inorganic Chemistry, 2001, 17(5): 735-740. (in Chinese)
[18]LAN Hai-cang, ZHAO Wei, HU Chu-qian, et al. Preparation of high-purity lithium in industry scale by vacuum distillation [J]. Chinese Journal of Rare Metals, 1998, 22(4): 286-289. (in Chinese)
[19]QIU Zhu-xian. Metallurgical Thermodynamics [M]. Beijing: Metallurgical Industry Press, 1985. 47-61.(in Chinese)
[20]DAI Yong-nian. Vacuum Metallurgy [M]. Beijing: Metallurgical Industry Publishing Company, 2000. (in Chinese)
[21]YU Jue-qi. Collection of State Diagrams of binary alloys [M]. Shanghai Science and Technology Publishing Company, 1987. 223-240.(in Chinese)
Foundation item: Excellent Youth Project(04B016) supported by the Education Office of Hunan Province
Received date: 2004-05-13; Accepted date: 2004-07-19
Correspondence: XIONG Li-zhi; Tel:+86-743-6125318; E-mail: kmpanda99111@163.com


