Trans. Nonferrous Met. Soc. China 24(2014) 1596-1603
Extraction of alumina from fly ash by ammonium hydrogen sulfate roasting technology
Ruo-chao WANG, Yu-chun ZHAI, Xiao-wei WU, Zhi-qiang NING, Pei-hua MA
School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 27 May 2013; accepted 10 November 2013
Abstract:
NH4HSO4 roasting technology was used for preparing Al2O3 from fly ash. First, Al and Fe were extracted from fly ash by NH4HSO4 roasting and deionized water leaching. Then, the Al and Fe in the leached liquid were precipitated by adding NH4HCO3 solution. After the mixed precipitations of Al(OH)3 and Fe(OH)3 were leached by NaOH solution, the NaAl(OH)4 solution was decomposed by carbonation. Finally, the pure Al(OH)3 was calcined to α-Al2O3. The optimal conditions of the whole technology were determined by experiments. The quality of α-Al2O3 product is up to the technical indicator of YS/T 274-1998 standard.
Key words:
fly ash; ammonium hydrogen sulfate; alumina; extraction;
1 Introduction
Fly ash is a fine ash separated from the flue gas of coal-fired power plant burning pulverized coal [1], and it is the main by-product from power plant. By 2020, total amount of fly ash in China will reach more than 3 billion tons. A large proportion of fly ash is used by the building industry, highway road bases and grout mixes [2-6]. However, despite these positive uses, the production rate of fly ash is much greater than its consumption [7]. There is still a proportion which is disposed of in ponds or landfill, so fly ash has become the main waste of power plant. Thus, it will be necessary to improve value-added utilization of fly ash. Al2O3 industry has been developed rapidly in China in recent years. Therefore, there is an increasing focus on extracting Al2O3 from fly ash, along with the reduction of bauxite reserves and the decrease of minable ore grade.
At present, the typical metallurgical methods for extracting Al2O3 from fly ash include soda-lime sintering [8-10] and acid leaching [11-13]. Soda-lime sintering is considered to have great potential for industrial use, as it is similar to the major technology currently used in the Al2O3 plants nowadays [14]. Whereas, this method has some defects, such as high energy consumption [15], large amount of waste residue and more Al2O3 loss. Acid leaching method can effectively separate Al and Si, but due to the high requirement for equipment [16] and high cost, this method has not been applied in industrial practice.
In this work, a novel technology for extracting Al2O3 from fly ash by NH4HSO4 roasting technology was established. Compared with the above methods, NH4HSO4 roasting technology has lower energy consumption, higher Al extraction rate and better operating environment. The technology includes roasting process, aluminum precipitation, alkali dissolution, carbonation decomposition and calcination. First, Al and Fe were extracted from fly ash by NH4HSO4 roasting and deionized water leaching. Second, the leached liquid containing Al and Fe was used for aluminum precipitation by NH4HCO3 solution. Third, the mixed precipitations of Al(OH)3 and Fe(OH)3 were leached by NaOH solution to remove Fe. Fourth, the NaAl(OH)4 solution was decomposed by carbonation. At last, the pure Al(OH)3 was prepared for α-Al2O3 by calcination. The Al2O3-extracted slag was used as raw material to recover SiO2. The present work mainly focuses on preparing α-Al2O3 product and optimizing conditions of the whole process. Moreover, the optimal operation parameters were determined by experiments.
2 Experimental
NH4HSO4 and NaOH were industrial grade; ZnSO4, EDTA-2Na, K2Cr2O7 and other reagents were analytical grade. Deionized water was used throughout the experiments whenever needed. Fly ash used in this study was got from a coal-fired power plant in Inner Mongolia, China, the size fraction of which mainly ranged from 61 to 74 μm. The detailed chemical composition was examined by ICP. The analytical result, which is listed in Table 1, indicates that the main components of fly ash are Al2O3 and SiO2. The XRD analysis was performed using Cu Kα radiation (λ=1.5406 nm) at 40 kV and 30 mA. From the XRD result shown in Fig. 1, it can be seen that the major mineral phases of fly ash are mullite and α-SiO2.
Table 1 Composition of fly ash from a power plant in Inner Mongolia, China (mass fraction, %)
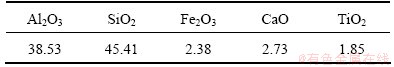
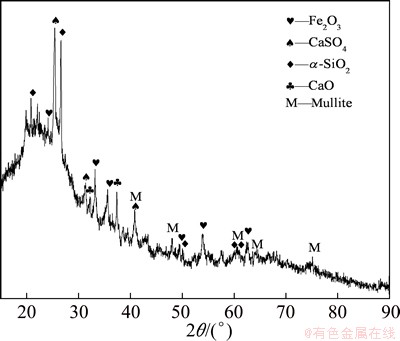
Fig. 1 XRD pattern of raw fly ash
2.1 Procedure of NH4HSO4 roasting
Fly ash was mixed with NH4HSO4 at a suitable mole ratio of Al2O3 in fly ash and NH4HSO4. The resistance wire furnace was used, and the temperature was controlled by a programmable temperature controller with a precision of ±2 °C. The reaction began at a required temperature. After a selected time, the roasted clinker was immediately taken out. It was leached by deionized water, and then filtered. The contents of Al and Fe in the leached liquid were detected by EDTA titration and K2Cr2O7 titration, respectively. The Al2O3-extracted slag was used for raw material of recovering SiO2.
The main chemical reactions taking place between fly ash and NH4HSO4 are as follows:
3Al2O3·2SiO2+12NH4HSO4=6NH4Al(SO4)2+6NH3+9H2O+2SiO2 (1)
3Al2O3·2SiO2+9NH4HSO4=3Al2(SO4)3+9NH3+9H2O+2SiO2 (2)
Fe2O3+4NH4HSO4=2NH4Fe(SO4)2+2NH3+3H2O (3)
Fe2O3+3NH4HSO4=Fe2(SO4)3+3NH3+3H2O (4)
Three key roasting parameters, namely roasting temperature, mole ratio of Al2O3/NH4HSO4 and roasting time, were optimized by orthogonal experiment.
2.2 Procedure of aluminum precipitation
The concentrations of Al and Fe in the leached liquid were as follows: CAl≈5.5 g/L, CFe≈0.3 g/L. 250 mL leached liquid was used for each experiment.
The leached liquid was heated from 25 to 99 °C. When the temperature was reached, 10% NH4HCO3 solution was dropped into the leached liquid under stirring, and then the Al and Fe were precipitated. When the pH value reached 4-7, the reaction began. After a certain time, the reaction solution was filtered. The filter cake, which was the mixed precipitations of Al(OH)3 and Fe(OH)3, was used for alkali dissolution. The filtrate can be used to prepare the crude product of (NH4)2SO4 by the evaporation crystallization process.
The main chemical reactions are as follows:
NH4HCO3=NH4++OH-+CO2 (5)
Al3++3OH-=Al(OH)3↓ (6)
Fe3++3OH-=Fe(OH)3↓ (7)
Three key parameters including temperature, pH value and holding time were optimized by single factor experiments.
2.3 Procedure of alkali dissolution
Fe, which is the impurity of strong interference, can decrease the quality of α-Al2O3 product. Therefore, the key problem of preparing α-Al2O3 product from fly ash is deironing. NaOH solution can be used to separate Al and Fe, because Al(OH)3 is dissolved while Fe(OH)3 is not dissolved in NaOH solution.
According to the Al content in the mixed precipitations of Al(OH)3 and Fe(OH)3, the dosage of NaOH (solid) can be calculated. The alkali dissolution process was investigated at Al(OH)3 to NaOH mass ratio of 1:1-1:5. These NaOH (solid) and 250 mL deionized water were mixed, and then heated to the temperature of 25-99 °C. When the temperature reached and remained stable, the mixed precipitations were added to the NaOH solution under the condition of continuous stirring. After a period of time, the reaction solution was filtered. The residue was the precipitation of Fe(OH)3, and the filtrate was NaAl(OH)4 solution.
The following reaction could occur in this process:
Al(OH)3+NaOH=NaAl(OH)4 (8)
Three key parameters, which include temperature, mass ratio of Al(OH)3/NaOH and holding time, were determined by single factor experiments.
2.4 Procedure of carbonation decomposition
The NaAl(OH)4 solution was heated to 25-99 °C. At the selected temperature, CO2 was blew into the solution at a gas-flow rate of 20-100 mL/min. The reaction solution was stirred under atmospheric pressure. After a certain time, the reaction solution was filtered. The filter cake was the precipitation of Al(OH)3, and the filtrate was Na2CO3 solution in which CaO can be added for causticizing.
The chemical reactions occurred can be written as [17,18]:
2NaOH+CO2=Na2CO3+H2O (9)
NaAl(OH)4=Al(OH)3↓+NaOH (10)
There were two essential processes in carbonation decomposition. One was the neutralization reaction between NaOH and CO2 (Reaction (9)); the other was the process of Al(OH)3 precipitation (Reaction (10)).
Three key parameters, namely temperature, gas-flow rate of CO2 and holding time, were examined by single factor experiments.
2.5 Procedure of calcination
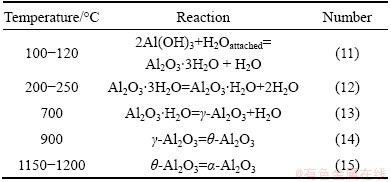
Al(OH)3 was calcinated at 1200 °C for 2 h, then the α-Al2O3 product was obtained.
The chemical reactions occurred are listed in Table 2 [19,20]:
Table 2 Chemical reactions occurred at different temperatures
The whole technological flow of extracting Al2O3 from fly ash is shown in Fig. 2.
3 Results and discussion
3.1 Procedure of NH4HSO4 roasting
Table 3 lists the schedule of the orthogonal experiment, in which the key parameters including roasting temperature (A), Al2O3/NH4HSO4 mole ratio (B) and roasting time (C) are selected as three factors. And every factor has three levels to be optimized. The Al extraction rate is taken as the index point to evaluate the roasting performance under different factors and levels.
The orthogonal experiment results are listed in Table 4. From the mathematical processing of the data, some results can be concluded. First, the factor of Al2O3/NH4HSO4 mole ratio shows the most notable influence on Al extraction rate. Then, roasting temperature is the relatively remarkable factor compared with roasting time. The optimal conditions of roasting process were determined as 400 °C, 1:8 and 45 min, for roasting temperature, Al2O3/NH4HSO4 mole ratio and roasting time, respectively. Moreover, 90.11% of Al extraction rate was achieved under the optimal roasting parameters.
The Al2O3 extracted slag was characterized by chemical analysis, XRD and SEM. The chemical compositions of Al2O3-extracted slag are shown in Table 5. Compared with Table 1, it is evident that the Al2O3 content is lowered, while the SiO2 content is improved. The purposes of extracting Al2O3 and cumulating SiO2 from fly ash are realized. The XRD pattern of Al2O3- extracted slag is shown in Fig. 3, indicating that the major mineral phase is α-SiO2. The morphologies of fly ash and Al2O3-extracted slag were analyzed by SEM. It can be seen from Fig. 4 that the contact surface of fly ash is eroded by NH4HSO4.
3.2 Procedure of aluminum precipitation
The effects of temperature, pH value and holding time on Al and Fe precipitation rates are shown in Fig. 5. These results manifest that increasing pH value and holding time while decreasing temperature could increase Al and Fe precipitation rates. The decomposability of NH4HCO3 is enhanced with the increase of temperature. Thereby, high temperature is unfavorable to the reaction. However, Al(OH)3 and Fe(OH)3 are amorphous precipitates, which need high temperature to accelerate the precipitation rate and decrease the adsorption. For this reason, 80 °C is chosen (Fig. 5(a)). It is found from Fig. 5(b) that the precipitation rate of Al at pH value of 7 is lower than that at pH value of 6. This is because a small amount of Al(OH)3 is transformed into 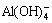 at pH value of 7, that is, Al3+ returns to the solution. As seen from Fig. 5(c), Al is precipitated completely after 40 min.
at pH value of 7, that is, Al3+ returns to the solution. As seen from Fig. 5(c), Al is precipitated completely after 40 min.
The optimal conditions for the process of aluminum precipitation are temperature of 80 °C, pH value of 6 and holding time of 40 min. Under these conditions, the precipitation rates of Al and Fe are up to 99% and 98%, respectively.
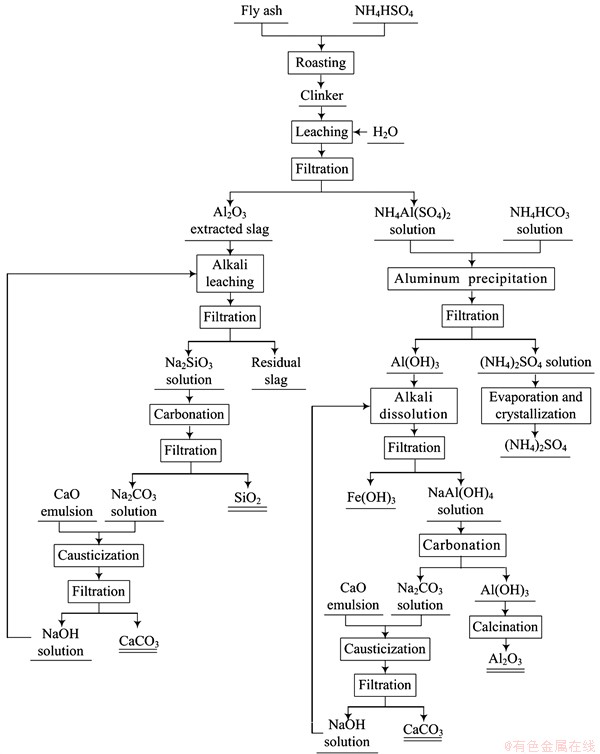
Fig. 2 Flow diagram of extracting Al2O3 from fly ash
Table 3 Factors and levels selected for orthogonal experiment
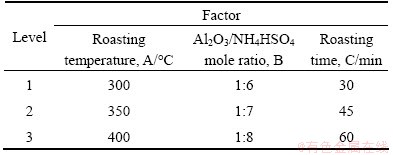
Table 4 Orthogonal experiment results
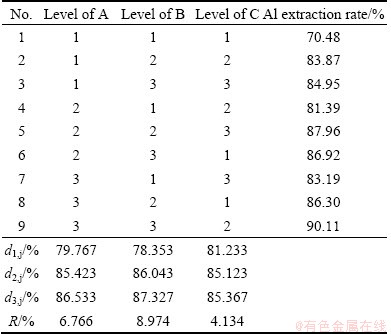
Table 5 Composition of Al2O3-extracted slag (mass fraction, %)
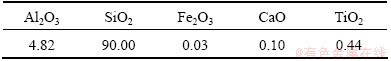
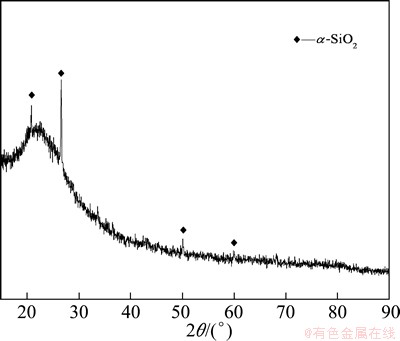
Fig. 3 XRD pattern of Al2O3-extracted slag
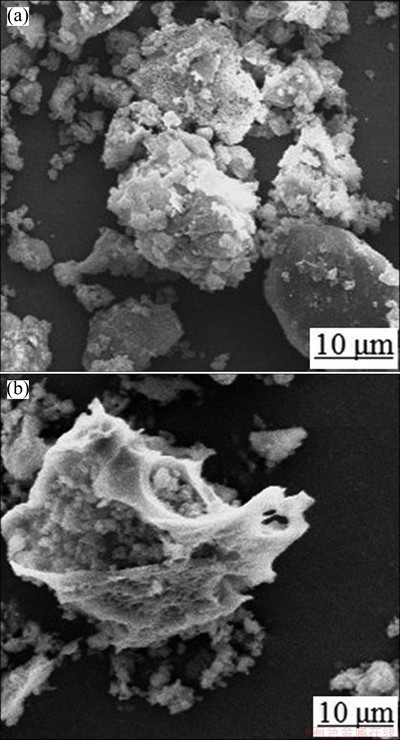
Fig. 4 SEM images of fly ash (a) and Al2O3-extracted slag (b)
3.3 Procedure of alkali dissolution
The influences of temperature, Al(OH)3/NaOH mass ratio and holding time on Al alkali dissolution rate and deironing rate are shown in Fig. 6. It is evident that increasing temperature, Al(OH)3/NaOH mass ratio and holding time increase the alkali dissolution rate of Al and deironing rate. The increase of temperature would improve the solubility of Al(OH)3. When the temperature is 80 °C, the alkali dissolution process can achieve a better effect (Fig. 6(a)). Additionally, excess NaOH can make the reactive molecules contact better and ensure sufficient reaction, so Al(OH)3/NaOH mass ratio of 1:4 is chosen (Fig. 6(b)). It can be seen from Fig. 6(c) that the alkali dissolution rate of Al could reach the maximum when the reaction time is fixed at 30 min.
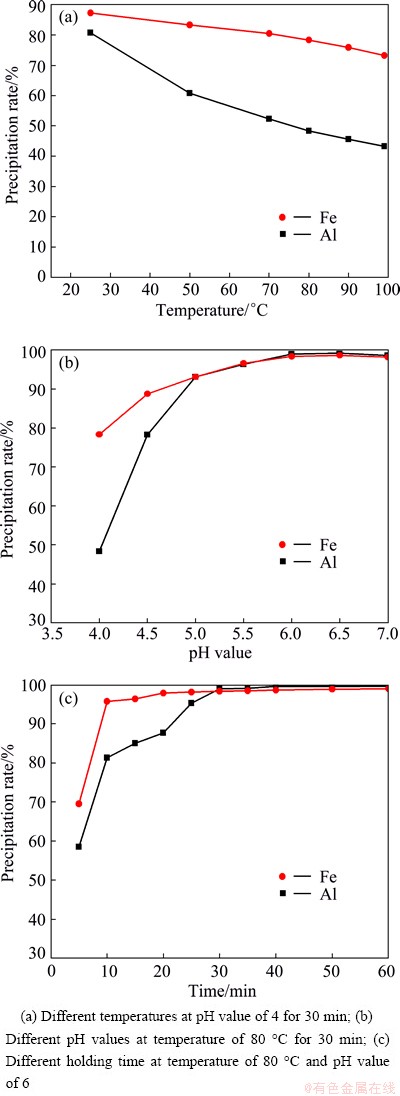
Fig. 5 Effects of different conditions on Al and Fe precipitation rates
As stated above, the optimal conditions for the process of alkali dissolution are temperature of 80 °C, Al(OH)3/NaOH mass ratio of 1:4 and holding time of 30 min. Under these conditions, the alkali dissolution rate of Al and the deironing rate are more than 99% and 96%, respectively.
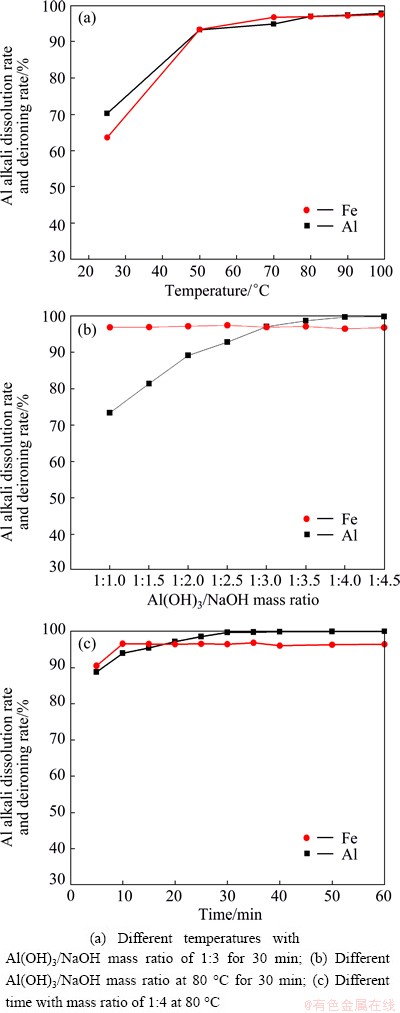
Fig. 6 Effects of different conditions on Al alkali dissolution rate and deironing rate
There are mainly two reasons responsible for incomplete deironing. First, a small part of Fe, which is absorbed by Al(OH)3 during the process of aluminum precipitation, is released when this Al(OH)3 is leached by NaOH solution; second, massive NaOH makes a small amount of Fe(OH)3 dissolve. In this experiment, the mixed precipitations of Al(OH)3 and Fe(OH)3 are leached directly by NaOH solution without process of drying. Thus, the mixed precipitations have a high reaction activity, which induce the relatively small dosage of NaOH (solid). Considering the above analysis, the reason of incomplete deironing is the first case.
3.4 Procedure of carbonation decomposition
The effects of temperature, CO2 gas-flow rate and holding time on Al precipitation rate are shown in Fig. 7.
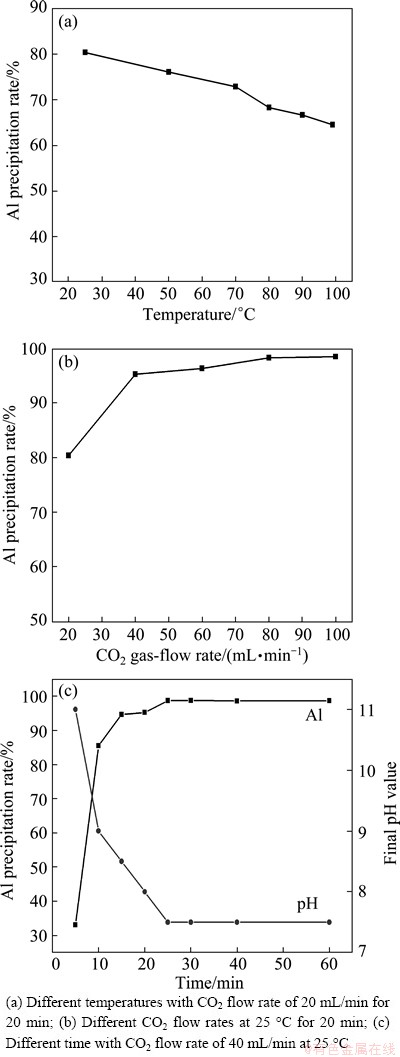
Fig. 7 Effects of different conditions on Al precipitation rate
The results show that increasing CO2 gas-flow rate and holding time and decreasing temperature improve the Al precipitation rate. The solubility of CO2 decreases with the increase of temperature, indicating that high temperature is adverse for the process of carbonation decomposition. Thus 25 °C is determined (Fig. 7(a)). It is evident from Fig. 7(b) that the reaction of carbonation decomposition is accelerated with increasing CO2 gas-flow rate. However, excess CO2 could affect the economy of the whole process, so CO2 gas-flow rate is chosen as 40 mL/min. As shown in Fig. 7(c), Al precipitation rate can reach a maximum after 25 min. Also the final pH value decreases with the increase of Al precipitation rate. This manifests that the final pH value is also an index point to evaluate the Al precipitation performance.
According to the experiments result, the optimal conditions for the process of carbonation decomposition are determined as temperature of 25 °C, CO2 gas-flow rate of 40 mL/min and holding time of 25 min. Under these conditions, Al precipitation rate is up to 98%.
3.5 Characterization of product
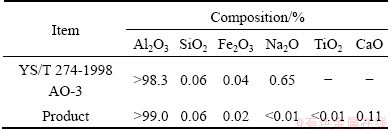
After the process of calcination, the α-Al2O3 product was obtained, and it was characterized by chemical analysis, XRD and SEM. From the XRD pattern in Fig. 8, it can be seen that the diffraction peaks of the product match with the standard diffraction peaks of α-Al2O3, which shows that the product is α-Al2O3. As shown in Fig. 9, the SEM image indicates that the fine particles of α-Al2O3 product are globular. Table 5 lists the chemical composition of α-Al2O3 product. It is found from Table 6 that the Al2O3 content of the product is more than 99%, and the quality of α-Al2O3 product is up to the technical indicator of YS/T 274-1998 standard.
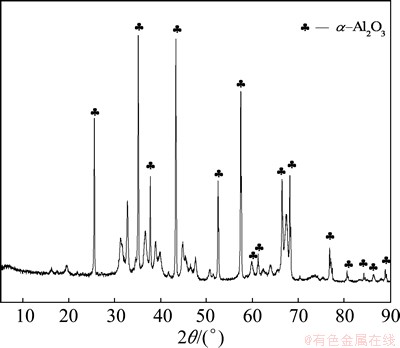
Fig. 8 XRD pattern of α-Al2O3 product

Fig. 9 SEM image of α-Al2O3 product
Table 6 Composition of α-Al2O3product (mass fraction, %)
4 Conclusions
1) A novel technology was proposed for comprehensive utilization of fly ash, which provides a new route for exploitation and utilization of fly ash.
2) The technology includes five parts: roasting process, aluminum precipitation, alkali dissolution, carbonation decomposition and calcination. The optimal conditions of the whole technology were determined. The Al extraction rate during the roasting process is up to 90.11% at 400 °C in mole ratio of Al2O3/NH4HSO4 of 1:8 for 45 min. The precipitation rates of Al and Fe are more than 99% and 98%, respectively, under the conditions of temperature 80 °C, pH value 6 and holding time 40 min. The alkali dissolution rate of Al and the deironing rate are 99% and 96%, respectively, and the optimal conditions are as follows: temperature 80 °C, Al(OH)3/NaOH mass ratio 1:4 and holding time 30 min. The carbonation decomposition rate of Al could reach 98% under normal temperature and pressure, CO2 gas-flow rate of 40 mL/min and holding time of 25 min.
3) The α-Al2O3 product was prepared, the quality of which was up to the technical indicator of YS/T 274-1998 standard.
4) The whole technology has many merits, such as low cost, low energy consumption and good operating environment. It turns waste into useful product and has good social benefit.
References
[1] SWANEPOEL J C, STRYDOM C A. Utilisation of fly ash in a geopolymeric material [J]. Applied Geochemistry, 2002, 17(8): 1143-1148.
[2] QUEROL X, MORENO N,  J C, ALASTUEY A,
J C, ALASTUEY A,  A, PLANA F. Synthesis of zeolites from coal fly ash: An overview [J]. International Journal of Coal Geology, 2002, 50(1-4): 413-423.
A, PLANA F. Synthesis of zeolites from coal fly ash: An overview [J]. International Journal of Coal Geology, 2002, 50(1-4): 413-423.
[3] IYER R S, SCOTT J A. Power station fly ash—A review of value-added utilization outside of the construction industry [J]. Resources, Conservation and Recycling, 2001, 31(3): 217-228.
[4] PHOO-NGERNKHAM T, CHINDAPRASIRT P, SATA V, PANGDAENG S, SINSIRI T. Properties of high calcium fly ash geopolymer pastes with Portland cement as an additive [J]. International Journal of Minerals, Metallurgy and Materials, 2013, 20(2): 214-220.
[5] ARENAS C G, MARRERO M, LEIVA C,  J, VILCHES ARENAS L F. High fire resistance in blocks containing coal combustion fly ashes and bottom ash [J]. Waste Management, 2011, 31(8): 1783-1789.
J, VILCHES ARENAS L F. High fire resistance in blocks containing coal combustion fly ashes and bottom ash [J]. Waste Management, 2011, 31(8): 1783-1789.
[6] AHMARUZZAMAN M. A review on the utilization of fly ash [J]. Progress in Energy and Combustion Science, 2010, 36(3): 327-363.
[7] IYER R. The surface chemistry of leaching coal fly ash [J]. Journal of Hazardous Materials, 2002, 93(3): 321-329.
[8] RAYZMAN V L, SHCHERBAN S A, DWORKIN R S. Technology for chemical-metallurgical coal ash utilization [J]. Energy & Fuels, 1997, 11(4): 761-773.
[9] PADILLA R, SOHN H Y. Sodium aluminate leaching and desilication in lime soda sinter process for alumina from coal wastes [J]. Metallurical and Materials Transaction B, 1985, 16(4): 707-713.
[10] BAI Guang-hui, TENG Wei, WANG Xiang-gang, QIN Jin-guo, XU Peng, LI Peng-cheng. Alkali desilicated coal fly ash as substitute of bauxite in lime-soda sintering process for aluminum production [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(s1): s169-s175.
[11] SEIDEL A, ZIMMELS Y. Mechanism and kinetics of aluminum and iron leaching from coal fly ash by sulfuric acid [J]. Chemical Engineering Science, 1998, 53(22): 3835-3852.
[12] DUTTA B K, KHANRA S. Leaching of elements from coal fly ash: assessment of its potential for use in filling abandoned coal mines [J]. Fuel, 2009, 88(7): 1314-1323.
[13] WU Cheng-you, YU Hong-fa, ZHANG Hui-fang. Extraction of aluminum by pressure acid-leaching method from coal fly ash [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(9): 2282-2288.
[14] LIU Kang, XUE Ji-lai, ZHU Jun. Extracting alumina from coal fly ash using acid sintering-leaching process [C]//SUAREZ C E. Light Metals 2012. Hoboken, NJ, USA: John Wiley & Sons Inc, 2012: 201-206.
[15] NAYAK N, PANDA C R. Aluminium extraction and leaching characteristics of Talcher thermal power station fly ash with sulphuric acid [J]. Fuel, 2010, 89(1): 53-58.
[16] MATJIE R H, BUNT J R, VAN HEERDEN J H P. Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal [J]. Minerals Engineering, 2005, 18: 299-310.
[17] LI Xiao-bin, CHEN Bin, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong, LIU Xiang-min. Kinetics of carbonation decomposition of sodium aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(5): 848-853. (in Chinese)
[18] LI Xiao-bin, LIU Xiang-min, GOU Zhong-ru, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng, DING An-ping, LI Ming, LIU Ye-xiang. Thermodynamics of carbonization of aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(4): 1005-1010. (in Chinese)
[19] ZIVAN D Z. Phase transformations of aluminium hydroxide in the calcination process [J]. Thermochimica Acta, 1977, 21(3): 391-398.
[20] GAO Xiao-lin, WANG Shi-fa, XIA Xiang, LIU Chun-ming, ZU Xiao-tao. Photoluminescence of macroporous α-alumina prepared by polyacrylamide gel technique [J]. Acta Physica Sinica, 2013, 62(1): 016105. (in Chinese).
硫酸氢铵焙烧粉煤灰提取氧化铝
王若超,翟玉春,吴晓卫,宁志强,马培华
东北大学 材料与冶金学院,沈阳 110819
摘 要:采用NH4HSO4焙烧法从粉煤灰中提取Al2O3。首先,通过NH4HSO4焙烧和去离子水浸出法提取粉煤灰中的Al和Fe;然后,加入NH4HCO3溶液沉淀浸出液中的Al和Fe,利用NaOH溶液浸出得到的Al(OH)3和Fe(OH)3混合沉淀,所得铝酸钠溶液经碳酸化分解得到纯净的Al(OH)3;最后,煅烧纯净的Al(OH)3制备α-Al2O3产品。通过实验确定各工艺流程的最佳条件。制备的α-Al2O3产品达到YS/T 274-1998标准的工艺指标。
关键词:粉煤灰;硫酸氢铵;氧化铝;提取
(Edited by Chao WANG)
Foundation item: Project (2007CB613603) supported by the National Basic Research Program of China; Project (2013M530934) supported by the China Postdoctoral Science Foundation
Corresponding author: Yu-chun ZHAI; Tel: +86-13709845210; E-mail: 370347814@163.com
DOI: 10.1016/S1003-6326(14)63230-1
Abstract: NH4HSO4 roasting technology was used for preparing Al2O3 from fly ash. First, Al and Fe were extracted from fly ash by NH4HSO4 roasting and deionized water leaching. Then, the Al and Fe in the leached liquid were precipitated by adding NH4HCO3 solution. After the mixed precipitations of Al(OH)3 and Fe(OH)3 were leached by NaOH solution, the NaAl(OH)4 solution was decomposed by carbonation. Finally, the pure Al(OH)3 was calcined to α-Al2O3. The optimal conditions of the whole technology were determined by experiments. The quality of α-Al2O3 product is up to the technical indicator of YS/T 274-1998 standard.


