
Effect of mechanical activation on thiosulfate leaching of gold from complex sulfide concentrate
Mohsen HASHEMZADEHFINI1, Jana FICERIOV?2, Emad ABKHOSHK3, Behrouz Karimi SHAHRAKI1
1. Iran Mineral Processing Research Center, Karaj, Iran;
2. Institute of Geotechnics of Slovak Academy of Sciences, Watsonova 4504353 Ko?ice, Slovakia;
3. Department of Mining Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran
Received 14 March 2011; accepted 8 June 2011
Abstract:
The use of mechanical activation to enhance gold recovery from a CuPbZn complex sulfide concentrate was investigated. The effects of milling time, ball size, sample to ball ratio and milling speed on thiosulfate leaching were studied. Under optimum conditions of milling time 1 h, ball size 20 mm, sample to ball ratio 1/15 and mill speed 600 r/min, nearly 78% of sample is amorphized, particle size decreases from d100=30 μm to d100=8 μm, specific surface area increases from 1.3 m2/g to 4.6 m2/g and gold recovery enhances from 17.4 % in non-activated sample to 73.26 %.
Key words:
mechanical activation; thiosulfate leaching; refractory ore; extraction of gold;
1 Introduction
Dissolution of gold from raw materials is mainly performed using cyanide leaching [1], though at present interest in the use of noncyanide processes for recovery of gold is a target due to the increasing concern regarding the hazardous character of cyanide. Thus, several leachants for gold recovery based on the stability of the corresponding gold complexes such as thiosulfate, thiourea, chloride, thiocyanate, ferric chloride and bromide have been proposed. Among them, ammoniacal thiosulphate leaching is considered a promising and nontoxic alternative to cyanidation [2]. The thiosulphate leaching and recovery of gold have been reviewed [2]. With depletion of the oxidized free-milling gold reserves close to the earth surface, most of the important new deposits being mined today do not respond to direct leaching. It is found that the gold is very finely disseminated and encapsulated in host matrices that are inert and/or impermeable to the leaching solution. In many cases, the host matrices are sulfide minerals, which exhibit a strong association with finely disseminated gold particles in many ore bodies [3]. Several attempts have been made to process efficiently those raw materials [4-9].
In order to overcome the refractory character, a pre-treatment is required to breakdown the sulfide matrix and renders the gold amenable for recovery prior to the application of any conventional treatment. The traditional route to treat these types of raw materials is by oxidative roasting of the sulfides before leaching. Alternative viable methods of oxidation such as pressure oxidation, bio-oxidation and electrooxidation have been developed to eliminate pollution problems caused by the emission of toxic gases (SO2 and As2O3) during oxidative roasting [10].
The relatively new process of mechanochemical pretreatment is being successfully applied in both fundamental research and plant operations [11]. In this process, which is also called mechanical activation, the minerals are subjected to high-intensity grinding. This grinding results in particle size reduction and causes chemical or physicochemical transformations, which significantly affect the subsequent hydrometallurgical process [12-17].
Currently, mechanical activation has been widely applied to the pretreatment of minerals [18-21]. Several investigators studied the effect of mechanical activation on sulfide minerals dissolution [22-26]. Many researchers studied the effect of mechanical activation on the extraction of metals in refractory minerals [27-28], which indicated that mechanical activation can efficiently accelerate the process of hydrometallurgical extraction. FICERIOV? et al [29-30] investigated the effect of mechanical activation on gold recovery by thiosulfate leaching from refractory ores and showed that mechanical activation enhanced the gold recovery.
The aim of this work was to investigate the effect of mechanical activation by planetary mill on thiosulfate leaching of the gold from complex sulfide concentrate.
2 Experimental
2.1 Materials
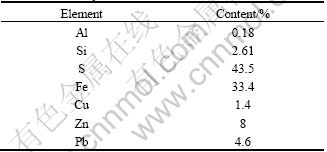
The CuPbZn concentrate was received from the North of Iran. The as-received concentrate after blending was rifled and the samples were collected for chemical analysis, size distribution and mineralogical characterization. Fire assay indicated that the concentrate contained 46 g/t gold. Chemical compositions of the concentrate shown in Table 1 were analysed by atomic absorption spectrometry (Varian 55B, Australia).
Table 1 Chemical compositions of studied concentrate

The XRF analysis of the concentrate was carried out (Philips magix Pro. 2002, Netherland). The results shown in Table 2 illustrate that the majority of sample is made up of Fe and S which shows the presence of pyrite as a main mineral.
Table 2 XRF analysis of concentrate
Mineralogical determinations were performed using X-ray diffraction (X’Pert, Philips, Netherland), EPMA (CAMECA SX100, France) and diagnostic leaching (series of acid leaching stages aimed to destroy specific minerals, followed by thiosulfate leaching of the residue from each stage).
Semi-quantitative X-ray diffraction analysis shown in Table 3 and Fig. 1, showed that pyrite and sphalerite are the major minerals, and chalcopyrite, galena, quartz, anglesite, barite and calcite are the minor ones.
Table 3 XRD analysis of concentrate
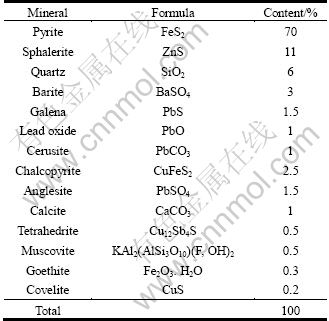
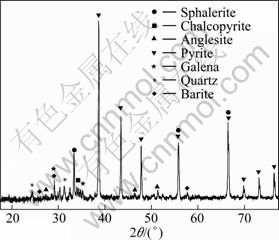
Fig. 1 X-ray diffraction pattern of as-received concentrate
EPMA analysis and diagnostic leaching showed that 82.6 % of gold occurred as invisible gold (solid solution) in sphalerite and 17.4 % of that as free milling filling up the intergrain space of sulfides and quartz. Table 4 shows the average values of 10 points of chalcopyrite, sphalerite, pyrite and sulphosalt minerals carried out by EPMA analyzer. It demonstrates that gold is associated with sphalerite. Figure 2, taken by EPMA, illustrates that free gold (2-25 μm) links to the pyrite, sphalerite and quartz minerals. The diagnostic leaching conditions were beyond of the scope of this work.
Prior to using mechanical activation, the as-received concentrate was ground to 100% passing 30 μm by rod mill. Screen analysis of as-received concentrate shown in Fig. 3 was performed by mechanically shaken Tyler sieves.
Table 4 EPMA analyses of concentrate
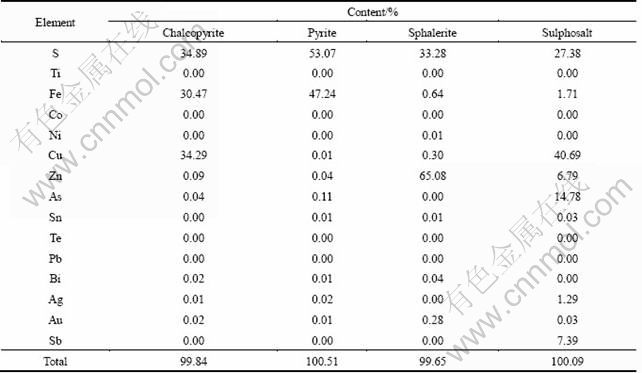
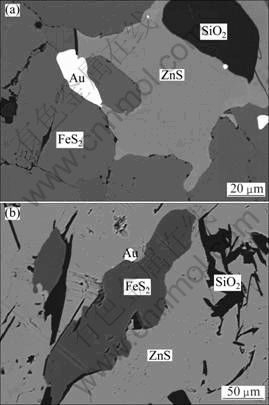
Fig. 2 BSE images of sample
2.2 Structural disordering calculations
The effect of mechanical activation was assessed using the increase in the X-ray-amorphous portion of mineral compared with the nonactivated (reference) sample [11], which is assumed to correspond to crystallinity X as
![]() (1)
(1)
where U0 and Ux are the background counts for the reference sample and activated sample, respectively; I0 and Ix are the integral intensities of the diffraction lines of the reference sample and activated sample, respectively. The extent of amorphization A is simply calculated using Eq. (2) and used for the evaluation of degree of minerals disordering.
A=100-X (2)
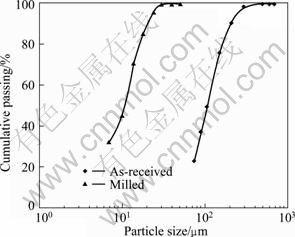
Fig. 3 Size distribution of milled and as-received concentrate
In this concentrate, gold is primarily associated with the sphalerite and it is logical to evaluate the degree of structural disordering of sphalerite to investigate the effect of mechanical activation on the gold dissolution.
2.3 Particle size and surface area analysis
In order to determine the size of particles and surface area of mechanical activated samples, a particle size analyzer (Malvern, United Kingdom) was used. Also, scanning electron micrographs were obtained on a scanning electron microscope (Zeiss, LEO 435vp, United Kingdom).
2.4 Mechanical activation
Dry mechanical activation of the concentrate was performed in a Fritsch Pulverizette planetary mill having ceramic bowl and ball (Fritsch, Germany). The mass of 20 mm and 10 mm balls are 15.2 g and 2.4 g, respectively. The effects of mill speed, milling time, ball size and ore to ball ratio were investigated under conditions shown in Table 5.
Table 5 Mechanical activation tests condition
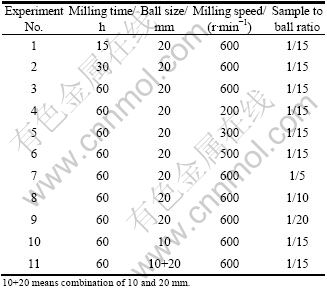
In all experiments, 50% of the planetary mill was filled with sample and grinding charge, and in experiment No. 11, the mass ratio of 10 mm ball to 20 mm ball was 50%.
2.5 Leaching
The leaching was investigated using a 250 mL glass reactor, in which 100 mL of leaching solution (1.5 mol/L (NH4)2S2O3 and 30 g/L CuSO4) and 10 g of concentrate were added. A mechanical stirrer was used to provide agitation speed of 600 r/min. Leaching was performed at 70 ?C and pH 7 for up to 16 h. 2 mL aqueous solutions were withdrawn at 1, 4, 8 and 16 h for determination of the contents of the dissolved gold by atomic absorption.
3 Results and discussion
3.1 Physicochemical changes of mechanically activated sample
The fraction of fine particles and specific surface area increase and the crystallinity of mineral components decreases as the consequence of intensive grinding. Figure 4 shows the XRD patterns of the concentrate samples mechanically activated for 0, 30 and 60 min (experiments 2 and 3 in Table 5). The results from the XRD analysis show that as the activation time increases, line broadening of both the pyrite and sphalerite peaks occurs.
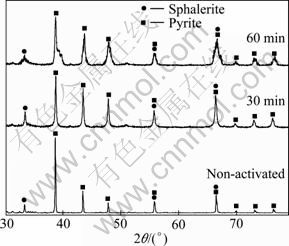
Fig. 4 XRD patterns of non-activated and mechanically activated for 30 and 60 min samples
The SEM micrographs of the activated samples after grinding for 30 and 60 min (experiments 2 and 3 in Table 5), respectively, are depicted in Fig. 5. The activated samples comprise of smooth subrounded to subangular particles in the size range of submicron to micron, which is in conformity with the particle size analysis.
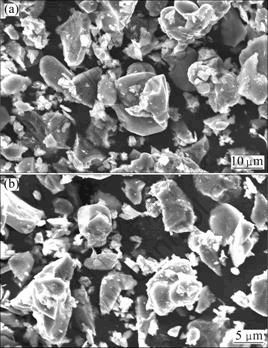
Fig. 5 SEM images of complex sulfide sample: (a) After mechanical activation for 30 min; (b) After mechanical activation for 60 min
3.2 Effects of mechanical activation parameters
3.2.1 Effect of milling time
Figure 6 shows the effect of milling time on sphalerite structural disordering, specific surface area and particle size of the sample. It is clear that increasing milling time enhances the specific surface area and the degree of sphalerite amorphization and reduces the particle size of the sample. For a sample with a surface area of 4.6 m2/g, 78% of sphalerite was amorphized and the particle size was 8 μm.
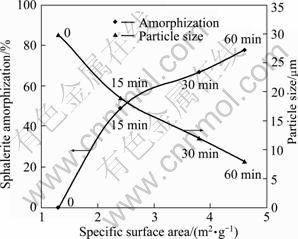
Fig. 6 Effect of milling time on parameters of sphalerite (Milling speed: 600 r/min; sample to ball ratio: 1/15; ball size: 20 mm)
The effect of milling time on gold recovery is shown in Fig. 7. It is obvious that gold recovery is improved due to the parameters like specific surface area and structural disordering by mechanical activation. In non-mechanical activated sample, only 17.4% of gold was recovered after 16 h leaching. This amount was increased to 73.26 % in a sample mechanically activated for 60 min.
3.2.2 Effect of mill speed
The effect of milling speed on parameters of the sample is illustrated in Fig. 8. The specific surface area and structural disordering increased in the whole interval of activation, and the highest value of amorphization and specific surface area were obtained in the case of the sample activated at the milling speed of 600 r/min.
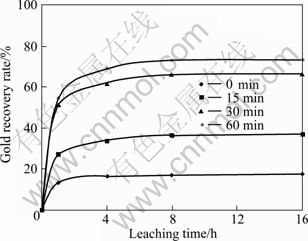
Fig. 7 Effect of milling time on gold recovery (Milling speed: 600 r/min; sample to ball ratio: 1/15; ball size: 20 mm)
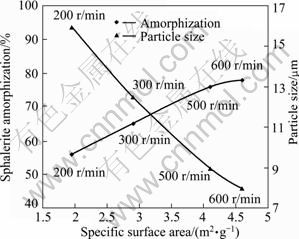
Fig. 8 Effect of milling speed on physical changes (Milling time: 60 min; sample to ball ratio: 1/15; ball size: 20 mm)
In fact, we input more energy by increasing the milling speed and the milling time, which results in finer products with higher surface area. In some studies, aggregation was observed in long milling time or high milling speed [31-32], but in this study, this formation was not observed. This fact is supported by the particle size distribution as well as the results of the SEM analysis (Fig. 5), which clearly shows that prolonging milling time does not result in formation agglomeration.
Gold recovery is raised with increasing the milling speed (Fig. 9). The gold recovery was 26.1% at milling speed of 200 r/min and increased to 73.26% at milling speed of 600 r/min. The results for the mechanically activated samples indicate that the changes in structural disordering of the gold-bearing sphalerite brought about an increase in gold recovery in the process of thiosulfate leaching.
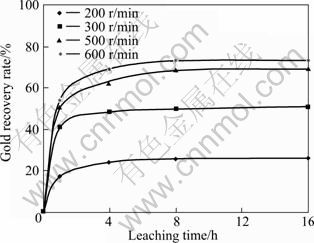
Fig. 9 Effect of milling speed on gold recovery (Milling time: 60 min; sample to ball ratio: 1/15; ball size: 20 mm)
3.2.3 Effect of ball size
From Fig. 10, there is a significant difference between amorphization in experiment which used 20 mm balls (experiment 3 in Table 5) and in experiments which used 10 mm and combination of 10 and 20 mm balls (experiments 10 and 11 in Table 5). In experiment 3, the amorphization percentage reached 78% but this amount dropped to 31% and 57% in experiments 10 and 11, respectively.
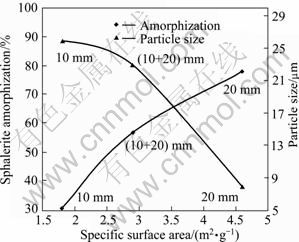
Fig. 10 Effect of ball size on parameters (Milling time: 60 min; sample to ball ratio: 1/15; milling speed: 600 r/min)
The largest ball has the most significant effect on both particle size reduction and surface area increase. The surface area reached 4.6 m2/g and the particles size (d100) reduced to 8 μm. In this research, just two ball sizes which were available and the combinations of them were used, and showed that the largest one had better results. But in order to complete the study, it needs to use a range of ball sizes and combination of them.
It can be seen from Fig. 10 that the ball size has a considerable effect on the parameters of concentrate. This effect is confirmed by considering the effect of ball size on the gold recovery (Fig. 11). In experiment 3 (Table 5), when 20 mm balls were used the gold recovery reached 73.26%, but when 10 mm balls and combination of 10 mm and 20 mm balls were used, it dropped to 28.9% and 57.6 % respectively.
3.2.4 Effect of sample to ball ratio
Figure 12 describes the effect of sample to ball ratio on the sphalerite amorphization percentage, specific surface area and particle size of the sample. It shows that with the decrease of sample to ball ratio from 1/5 to 1/15, the structural disordering and specific surface area increase and then reach a plateau. It may be attributed to the decrease in the contact between the particles and balls.
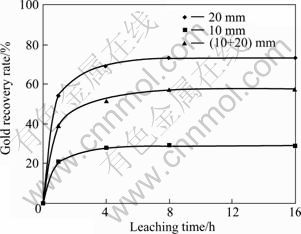
Fig. 11 Effect of ball size on gold recovery (Milling time: 60 min; sample to ball ratio: 1/15; milling speed: 600 r/min)
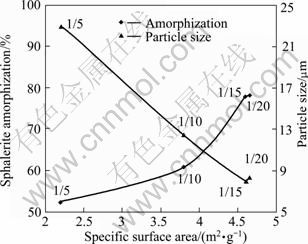
Fig. 12 Effect of sample to ball ratio on parameters (Milling time: 60 min; ball size: 20 mm; milling speed: 600 r/min)
The effect of sample to ball ratio on the gold recovery was investigated. Figure 13 shows the influence of sample to ball ratio on the parameters of the concentrate. The gold recovery was raised by decreasing the sample to ball ratio but with the ratio of 1/15 the gold recovery slightly decreased.
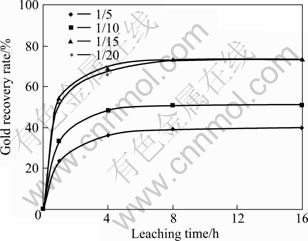
Fig. 13 Effect of sample to ball ratio on gold recovery (Milling time: 60 min; ball size: 20 mm; milling speed: 600 r/min)
4 Conclusions
1) Mechanical activation has an influence on both the rates of extraction and recovery of gold as a result of the physiochemical changes on gold-bearing CuPbZn concentrate. It is possible to achieve 73.26% gold recovery after 16 h leaching of a mechanically activated sample, which is very favorably with 17.4 % of that from non-activated sample.
2) Milling time, ball size, milling speed and sample to ball ratio all affect the degree of sphalerite amorphization, particle size and specific surface area. Thiosulfate leaching of gold shows the dependence on the degree of amorphization and surface area. The enhanced reactivity of the mill products is predominantly a function of their structural disordering and specific surface area.
Acknowledgments
This work was supported by Iran Mineral Processing Research Center (IMPRC). The authors thank the IMPRC for the financial support of this work.
References
[1] MARSDEN J, HOUSE I. The chemistry of gold extraction [M]. London: Ellis Horwood, 1992.
[2] AYLMORE M G, MUIR D M. Thiosulfate leaching of gold—A review [J]. Minerals Engineering, 2001, 14(2): 135-174.
[3] LI J, DABROWSKI B, MILLER J D, ACAR S, DIETRICH M, LEVIER K M, WAN R Y. The influence of pyrite pre-oxidation on gold recovery by cyanidation [J]. Minerals Engineering, 2006, 19(9): 883-895.
[4] DESCHENES G, XIA C, FULTON M, CABRI L J, PRICE J. Evaluation of leaching parameters for a refractory gold ore containing aurostibite and antimony minerals: Part I—Central zone [J]. Minerals Engineering, 2009, 22(9-10): 799-808.
[5] MA S J, LUO W J, MO W, SU X J, LIU P, YANG J L. Removal of arsenic and sulfur from a refractory gold concentrate by microwave heating [J]. Minerals Engineering, 2010, 23(1): 61-63.
[6] MURTHY D S R, KUMAR V, RAO K V. Extraction of gold from an Indian low-grade refractory gold ore through physical beneficiation and thiourea leaching [J]. Hydrometallurgy, 2003, 68(1-3): 125-130.
[7] CHEN A, ZHAO Z W, JIA X, LONG S, HUO G, CHEN X. Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore [J]. Hydrometallurgy, 2009, 97(3-4): 228-232.
[8] GONEN N, KORPE E, YILDIRIM M E, SELENGIL U. Leaching and CIL processes in gold recovery from refractory ore with thiourea solutions [J]. Minerals Engineering, 2007, 20(6): 559-565.
[9] NANTHAKUMAR B, PICKLES C A, KELEBEK S. Microwave pretreatment of a double refractory gold ore [J]. Minerals Engineering, 2007, 20(11): 1109-1119.
[10] COSTA M C. Portugal: Faculty of Sciences of Lisbon, 1996.
[11] BALAZ P. Extractive metallurgy of activated minerals [M]. Amsterdam: Elsevier, 2000.
[12] BALAZ P, SEKULA F, JAKABSKY S, KAMMEL R. Application of attrition grinding in alkaline leaching of tetrahedrite [J]. Minerals Engineering, 1995, 8(11): 1295-1308.
[13] BALAZ P, FICERIOVA J, SEPELAK V, KAMMEL R. Thiourea leaching of silver from mechanically activated tetrahedrite [J]. Hydrometallurgy, 1996, 43(1-3): 367-377.
[14] LINGE H G, WELHAM N J. Gold recovery from arsenopyrite ore by in situ slurry oxidation [J]. Minerals Engineering, 1997, 10(6): 557- 566.
[15] TKACOVA K. Mechanical activation of minerals [M]. Amsterdam: Elsevier, 1989.
[16] WELHAM N J. Mechanochemical processing of gold-bearing sulfides [J]. Minerals Engineering, 2001, 14(3): 341-347.
[17] WELHAM N J. The effect of extended milling on minerals [J]. Can Inst Metall Bull, 1997, 90: 64-68.
[18] LI C, LIANG B, GUO L H, WU Z B. Effect of mechanical activation on the dissolution of Panzhihua ilmenite [J]. Minerals Engineering, 2006, 19(14): 1430-1438.
[19] WEI L, HU H, CHEN Q, TAN J. Effects of mechanical activation on the HCl leaching behavior of plagioclase, ilmenite and their mixtures [J]. Hydrometallurgy, 2009, 99(1-2): 39-44.
[20] SASIKUMAR C, RAO D S, SRIKANTH S, MUKHOPADHYAY N K, MEHROTRA S P. Dissolution studies of mechanically activated manavalakurichi ilmenite with HCl and H2SO4 [J]. Hydrometallurgy, 2007, 88(1-4): 154-169.
[21] AMER A M. Alkaline pressure leaching of mechanically activated Rosetta ilmenite concentrate [J]. Hydrometallurgy, 2002, 67(1-3): 125–133.
[22] ACHIMOVICOVA M, BALAZ P. Influence of mechanical activation on selectivity of acid leaching of arsenopyrite [J]. Hydrometallurgy, 2005, 77(1-2): 3–7.
[23] ZHAO Z, ZHANG Y, CHEN X, CHEN A, HUO G. Effect of mechanical activation on the leaching kinetics of pyrrhotite [J]. Hydrometallurgy, 2009, 99(1-2): 105-108.
[24] BALAZ P, ACHIMOVICOVA M, BASTL Z, OHTANI T, SANCHEZ M. Influence of mechanical activation on the alkaline leaching of enargite concentrate [J]. Hydrometallurgy, 2000, 54(2-3): 205–216.
[25] HU H, CHEN Q, YIN Z, ZHANG P, WANG G. Effect of grinding atmosphere on the leaching of mechanically activated pyrite and sphalerite [J]. Hydrometallurgy, 2004, 72(1-2): 79-86.
[26] XIAO Z, CHEN Q, YIN Z, HU H, ZHANG P. Calorimetric studies on leaching of mechanically activated sphalerite in FeCl3 solution [J]. Thermochimica Acta, 2004, 416(1-2): 5-9.
[27] BALAZ P, ACHIMOVICOVA M. Mechano-chemical leaching in hydrometallurgy of complex sulfides [J]. Hydrometallurgy, 2006, 84(1-2): 60-68.
[28] GODOCIKOVA E, BALAZ P, BOLDIZAROVA E. Structural and temperature sensitivity of the chloride leaching of copper, lead and zinc from a mechanically activated complex sulfide [J]. Hydrometallurgy, 2002, 65(1): 83–93.
[29] FICERIOVA J, BALAZ P, BOLDIZAROVA E, STANISLAV J. Thiosulfate leaching of gold from a mechanically activated CuPbZn concentrate [J]. Hydrometallurgy, 2002, 67(1-3): 37-43.
[30] FICERIOVA J, BALAZ P, VILLACHICA C L. Thiosulfate leaching of silver, gold and bismuth from a complex sulfide concentrates [J]. Hydrometallurgy, 2005, 77(1-2): 35–39.
[31] BALAZ P. Influence of solid state properties on ferric chloride leaching of mechanically activated galena [J]. Hydrometallurgy, 1996, 40(3): 359-368.
[32] MULAK W, BALAZ P, CHOJNACKA M. Chemical and morphological changes of millerite by mechanical activation [J]. International Journal of Mineral Processing, 2002, 66(1-4): 233-240.
机械活化对从复杂硫化精矿中
硫代硫酸盐浸取金的影响
Mohsen HASHEMZADEHFINI1, Jana FICERIOV?2, Emad ABKHOSHK3, Behrouz Karimi SHAHRAKI1
1. Iran Mineral Processing Research Center, Karaj, Iran;
2. Institute of Geotechnics of Slovak Academy of Sciences, Watsonova 4504353 Ko?ice, Slovakia;
3. Department of Mining Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran
摘 要:采用机械活化提高复杂硫化矿CuPbZn中金的回收。研究研磨时间、球尺寸、球料比和球磨转速对金的硫代硫酸盐浸取的影响。在最优条件下(球磨时间1 h,球尺寸20 mm,球料比15/1,球磨速度600 r/min),矿石的非晶化度达到78%,颗粒尺寸从30 μm下降到8 um,比表面积从1.3 m2/g 增加到 4.6 m2/g,金的回收率从7.4%提高到73.26%。
关键词:机械活化;硫代硫酸盐浸取;难处理矿;提金
(Edited by LI Xiang-qun)
Corresponding author: Mohsen HASHEMZADEHFINI; Tel: +98-9376715705, Fax: +98-2619208360; E-mail: hashemzadehmohsen@gmail.com
DOI: 10.1016/S1003-6326(11)61118-7
Abstract: The use of mechanical activation to enhance gold recovery from a CuPbZn complex sulfide concentrate was investigated. The effects of milling time, ball size, sample to ball ratio and milling speed on thiosulfate leaching were studied. Under optimum conditions of milling time 1 h, ball size 20 mm, sample to ball ratio 1/15 and mill speed 600 r/min, nearly 78% of sample is amorphized, particle size decreases from d100=30 μm to d100=8 μm, specific surface area increases from 1.3 m2/g to 4.6 m2/g and gold recovery enhances from 17.4 % in non-activated sample to 73.26 %.
[1] MARSDEN J, HOUSE I. The chemistry of gold extraction [M]. London: Ellis Horwood, 1992.
[10] COSTA M C. Portugal: Faculty of Sciences of Lisbon, 1996.
[11] BALAZ P. Extractive metallurgy of activated minerals [M]. Amsterdam: Elsevier, 2000.
[15] TKACOVA K. Mechanical activation of minerals [M]. Amsterdam: Elsevier, 1989.


