DOI:10.19476/j.ysxb.1004.0609.2019.06.17
含砷废水水热法臭葱石沉砷
张 俊,李存兄,魏 昶,樊 刚,李兴彬,邓志敢,李旻廷,张 鹏
(昆明理工大学 冶金与能源工程学院,昆明 650093)
摘 要:
本文以铜冶炼过程所产生的含砷废水为研究对象,研究了Fe/As摩尔比、初始pH值、氧分压、反应时间以及反应温度等宏观技术参数对水热臭葱石沉砷过程及沉砷渣物相转变的影响规律。结果表明:在Fe/As摩尔比1.5、初始pH1.0、反应温度160 ℃、搅拌转速500 r/min、氧分压0.6 MPa和反应时间3 h的优化技术条件下,砷与铁的沉淀率分别为98.09%和87.64%,获得了纯度较高的臭葱石沉砷渣;沉砷渣中砷、铁及硫的含量分别为22.21%、25.36%及3.34%,其中硫主要以亚稳态铁矾的形式存在;降低Fe/As摩尔比和初始pH值、延长反应时间均有利于亚稳态铁矾的返溶、重结晶,进而形成性质稳定的臭葱石物相。
关键词:
文章编号:1004-0609(2019)-06-1279-10 中图分类号:TF09 文献标志码:A
砷的毒性极强,具有生物积累性和致癌性。自然界中极少见自然砷或砷金属化合物,大多以硫化物的形式夹杂在铜、金、铅及锌等有色金属矿产资源中[1]。矿业活动是导致砷污染的重要原因之一[2],铜冶炼是产生含砷废水的主要行业,所产生的含砷废水砷浓度高,富含铁、锌、铜等重金属,成分极为复杂[3]。当前,含砷物料的无害化处理已成为决定产业生存与发展的重大问题[4-6]。
当前,含砷废水的处理主要有两种途径,一是将砷转化成相应的砷酸盐废渣如砷酸铁、砷酸钙等[7-9],由于这些废渣性质不稳定,易导致二次污染,国家已将其列入危险固体废弃物行列;二是将砷转化为砷产品回收利用,由于砷化合物毒性高,砷产品利用范围非常有限[10-12]。臭葱石(FeAsO4·2H2O)是一种天然的含砷矿物,晶体结构多为双锥状或者葡萄状,具有浸出毒性小及稳定性高的特点,被认为是最适合堆存的含砷固体废弃物[13],是目前砷固化的研究热点。许多研究者在常压下开展了As(Ⅴ)和Fe(Ⅲ)共沉淀臭葱石的研究[1, 8, 14-17],发现臭葱石沉砷渣易被非晶型砷酸铁物相及铁矾物相等污染。FUJITA等[18-19]改进了上述常压沉砷工艺,以FeSO4盐为铁源,利用氧化剂将含砷铁溶液中的Fe(Ⅱ)氧化与溶液中的As(Ⅴ)形成臭葱石沉淀。与DEMOPOULOS[16]的方法相比,后续的研究中溶液的初始Fe(Ⅲ)浓度较低,更利形成臭葱石沉淀[20-22]。
MAMBOTE等[23]和GOMEZ等[24]通过对水热条件下Fe-As-H2SO4系的热力学研究,阐述了臭葱石沉砷是可行的,其研究表明形成稳定臭葱石物相的温度范围为150~170℃。KITAMURA等[25]和余自秀等[26-27]研究发现,水热条件下形成的臭葱石与常压下形成的臭葱石相比,前者的稳定性较高,所需反应时间更短。以上常压或水热臭葱石沉砷研究均以含砷铁的纯溶液为研究对象,而冶金工业,特别是铜冶炼行业含砷废水成分极其复杂,因而本文以铜冶炼含砷废水为研究对象,研究了水热条件下Fe/As摩尔比、初始pH值、氧分压、反应时间以及反应温度等宏观技术参数对水热臭葱石沉砷过程及沉砷渣物相转变的影响规律。
1 实验
1.1 实验原料及设备
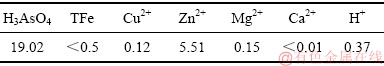
本研究所用含砷废水取自云南某炼铜企业,其化学组成如表1所示。七水硫酸亚铁(分析纯,FeSO4·7H2O,天津市凤船化学试剂科技有限公司生产)作为铁源、氢氧化钠(分析纯,NaOH,天津市大茂化学试剂厂生产)与98%(质量分数,成都市科龙化工试剂厂生产)硫酸调节溶液pH值、氧化剂为工业级氧气。
实验主体设备为2L-GSH型高压釜及温控仪(山东威海化工机械有限公司生产),辅助设备包括恒温干燥箱(上海一恒科学仪器有限公司生产),旋片真空泵(浙江台州求精真空泵有限公司生产),电子分析天平(梅特勒-托利多METTLER生产),PHSJ-5型实验室pH计(上海雷磁仪器有限公司生产),实验室用超纯水机(四川沃特尔水处理设备有限公司生产)等。
表1 含砷废水的主要化学组成
Table 1 Major chemical composition of arsenic-containing wastewater (g/L)
1.2 实验过程
向含砷废水中加入一定量的七水硫酸亚铁并调节pH之后,加入到高压釜内,工业氮气检查设备气密性后连接供氧设备。预设氧分压、搅拌转速和实验温度,待釜内温度达到预设值,开始供氧。过程取样方式:0~60 min内每10 min取一次样,60~180 min内每30 min取一次样,取样矿浆经液固分离后检测。到达反应时间后,关闭氧气阀快速降温,用旋片型真空泵抽出矿浆后进行液固分离,测量液样体积并保存待检。滤渣在干燥箱内于70 ℃下恒温烘烤24 h后送样分析。
1.3 分析检测
采用X射线衍射分析(XRD)(型号:D/max-2200)对实验渣物相进行分析,扫描电镜(SEM)(型号:VEGA 3 SBH)对渣样形貌进行分析。含砷废水化学组成及沉砷渣成分组成送云南有色冶金研究院分析。采用式(1)计算水热臭葱石沉砷过程的沉砷率和沉铁率。
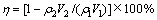 (1)
(1)
式中:η为沉铁率或沉砷率,%;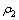 为滤液中铁或砷的浓度,g/L;
为滤液中铁或砷的浓度,g/L;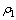 为初始溶液中铁或砷的浓度,g/L;V2为滤液的体积或反应前液的体积,L;V1为初始溶液体积,L。
为初始溶液中铁或砷的浓度,g/L;V2为滤液的体积或反应前液的体积,L;V1为初始溶液体积,L。
1.4 实验原理
在Fe-As-H2O系中形成臭葱石的反应为[11]
4H3AsO4+4FeSO4+O2+6H2O→4FeAsO4·2H2O+4H2SO4 (2)
式(2)由Fe(Ⅱ)的氧化反应及As(Ⅴ)与Fe(Ⅲ)共沉淀反应组成:
4FeSO4+O2+2H2SO4=2Fe2(SO4)3+2H2O (3)
Fe2(SO4)3+2H3AsO4+4H2O=2FeAsO4·2H2O+3H2SO4 (4)
当溶液中Fe(Ⅲ)达到过饱和状态及低酸度状态时,按如下反应(5)形成亚稳态铁矾:
M2SO4+3Fe2(SO4)3+12H2O=2MFe3(SO4)2(OH)6+6H2SO4 (M=K+, Na+, NH4+, H3O+等) (5)
随着反应体系温度升高、酸度增加,亚稳态铁矾亦按反应(6)返溶[28]:
2MFe3(SO4)2(OH)6+6H2SO4=M2SO4+3Fe2(SO4)3+12H2O (M=K+, Na+, NH4+, H3O+等) (6)
余自秀[26]绘制了160 ℃时Fe-As-H2O系的φ-pH优势图,如图1所示。在氧化条件下,溶液电位较低时,形成低价含砷物质。在低pH值及高电位时,臭葱石不能稳定存在,更易形成Fe3+及H3AsO4,因此合成臭葱石需避免在过低pH值和高电位下进行。在高pH值和高电位时,易水解形成Fe(OH)3,对于形成FeAsO4有不利影响,阻碍FeAsO4的形成。当氧化电位控制在区域A范围内,FeAsO4能够稳定存在,有利于溶液中的As(Ⅴ)以FeAsO4物相形成。
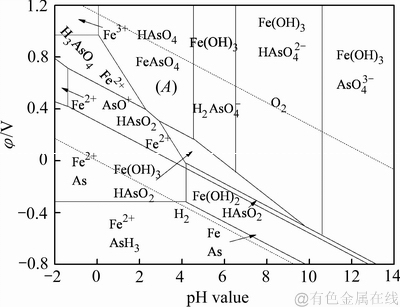
图1 160 ℃时As-Fe-H2O系电位-pH图[26]
Fig. 1 Potential-pH chart of As-Fe-H2O system at 160 ℃ (a=1)[26]
2 结果与讨论
2.1 Fe/As摩尔比的影响
在溶液初始pH值1、搅拌速度500 r/min,反应温度160 ℃,氧分压0.6 MPa和反应时间3 h条件下,考察了Fe/As摩尔比对沉砷率、沉铁率以及沉砷渣物相影响,沉砷渣的XRD谱、沉砷率与沉铁率及溶液铁浓度变化分别如图2和3所示。
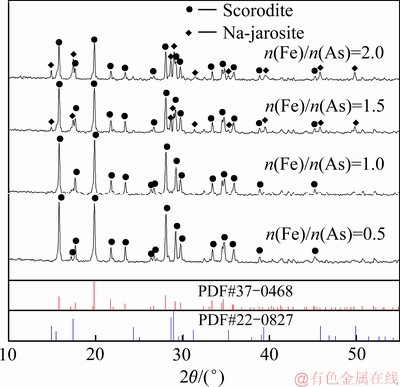
图2 不同Fe/As摩尔比沉砷渣的XRD谱
Fig. 2 XRD patterns of scorodite precipitate at various Fe/As molar ratio
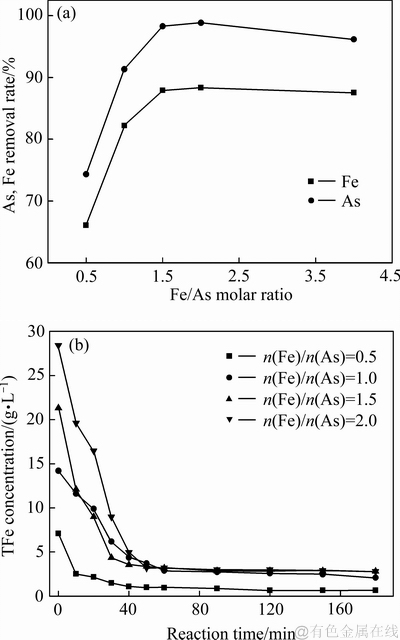
图3 Fe/As摩尔比对沉砷率、沉铁率及溶液铁浓度影响
Fig. 3 Effects of Fe/As molar ratio on As and Fe removal rates(a), and iron concentration(b)
由图2可知,当Fe/As摩尔比从0.5提升到1时,沉砷渣中只检测到臭葱石物相,表明Fe/As摩尔比为0.5至1时形成纯度高、结晶度良好的臭葱石沉砷渣。当Fe/As摩尔比提高至1.5时,沉砷渣除臭葱石物相外,开始出现铁矾物相,随着铁浓度的升高,开始发生副反应(5),臭葱石物相依然具有较强的衍射峰。当Fe/As摩尔比由1.5提高至2.0时,铁矾物相的衍射峰增强。由于Fe/As摩尔比升高,溶液中铁含量增加,将导致Fe(Ⅲ)过高达到过饱和状态,进而形成亚稳态铁矾物相[29-30]。
如图3所示,随着反应的进行,溶液中的铁浓度也在逐渐降低,说明沉砷反应(4)完成。反应时间设为3 h的目的是为了沉砷渣中其他的非臭葱石物相向臭葱石转化。当Fe/As摩尔比由0.5提高至1时,溶液中的铁浓度未达到过饱和状态,渣中只存在臭葱石物相,纯度较高,沉砷率约为90%,沉砷后液中仍残留部分砷。在Fe/As摩尔比为1.5时,物相中开始出现铁矾等物相,因发生反应(5),导致了沉铁率及沉砷率有所提高。在铁矾物相形成时,溶液中未反应的部分砷被形成的铁物相吸附沉淀[26],将沉砷率提高至98%,沉砷后液中砷残留量较低。
2.2 初始pH的影响
在Fe/As摩尔比1.5、搅拌速度500 r/min、反应温度160 ℃、氧分压0.6 MPa和反应时间3 h条件下,考察初始pH对沉砷率、沉铁率以及沉砷渣物相的影响。沉砷渣的XRD谱与沉砷率、沉铁率及溶液铁浓度变化分别如图4和5所示。
由图4可知,在沉砷渣中含有臭葱石与铁矾物相,初始pH值对沉砷渣物相的影响较大。当pH值在0.5和1时,沉砷渣中臭葱石物相衍射峰强度较强,而pH值为1.5与2时,臭葱石物相衍射峰强度相对较弱,表明在pH值为0.5至1范围更有利形成臭葱石,沉砷渣中臭葱石物相的纯度较高。随着初始pH 值的升高, 溶液中的铁浓度升高达到过饱和状态,导致副反应(5)发生,造成渣中含有大量的铁矾[19],由于溶液中的铁向铁矾物相转变,降低了臭葱石纯度。
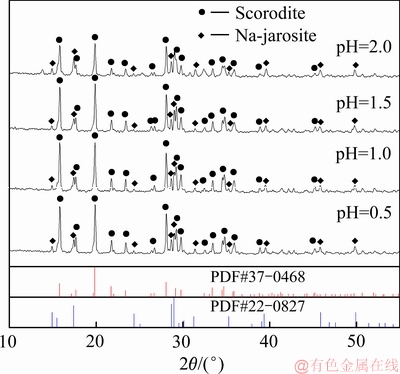
图4 不同pH值时沉砷渣的XRD谱
Fig. 4 XRD patterns of scorodite precipitate at various initial pH values
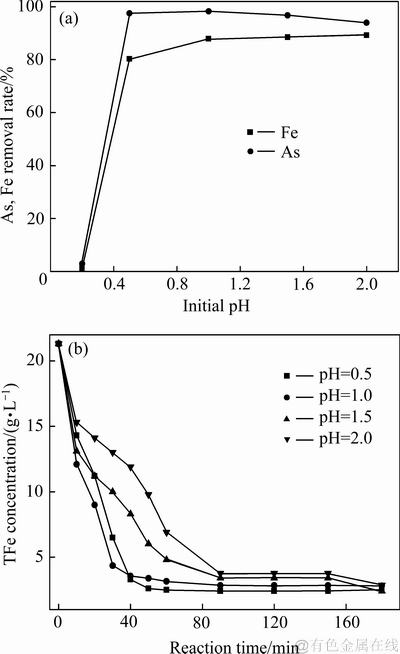
图5 不同初始pH值对沉砷率、沉铁率及溶液铁浓度变化影响
Fig. 5 Effects of initial pH value and reaction time on As and Fe removal rates(a), and iron concentration(b)
由图5(a)可知,在初始pH值为0.2时,较难生成臭葱石,因在水热条件下沉砷反应是产酸反应,会使沉砷后液pH值降低,此酸度明显高于臭葱石稳定存在的酸度范围。在初始pH值为0.5时,由于处在臭葱石稳定存在区内,形成了以臭葱石物相为主的沉砷渣。由图4和5(b)可知,沉砷后液中铁浓度变化率受初始pH值影响显著,初始pH值较低时,可促使Fe(Ⅲ)与As(Ⅴ)形成臭葱石,导致铁浓度变化率加快。然而初始pH值越高,体系中Fe(Ⅲ)更易水解,导致有利于Fe(Ⅲ)转化为铁矾物相。
2.3 氧分压的影响
在Fe/As摩尔比1.5、初始pH值1、搅拌速度500 r/min、反应温度160 ℃和反应时间3h条件下,考察氧分压对沉砷率、沉铁率以及沉砷渣物相的影响。沉砷渣的XRD谱与沉砷率、沉铁率及溶液铁浓度变化分别如图6和7所示。
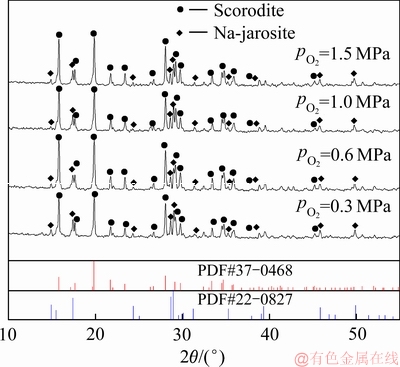
图6 不同氧分压沉砷渣的XRD谱
Fig. 6 XRD patterns of scorodite precipitate at various oxygen partial pressures
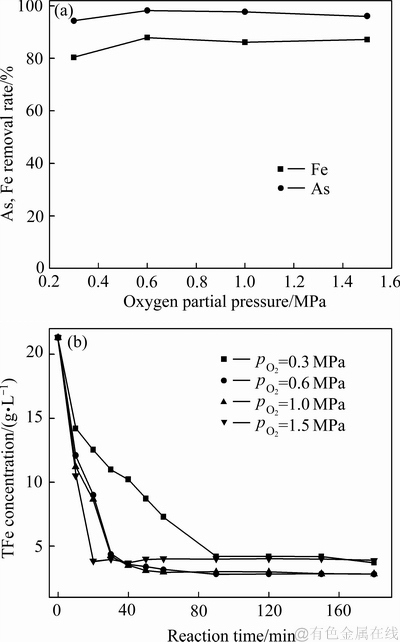
图7 不同氧分压对沉砷率、沉铁率及溶液铁浓度变化影响
Fig. 7 Effects of oxygen partial pressure and reaction time on As and Fe removal rates(a), and iron concentration(b)
当氧分压为0.3 MPa时,溶液中Fe(Ⅱ)的氧化速度较为缓慢,导致溶液中Fe(Ⅲ)浓度较低,不利于反应(3)向形成臭葱石物相的方向进行。由图6可知,该条件下形成的臭葱石物相的衍射峰强度明显较弱。由图7可知,当氧分压大于0.3 MPa时,沉砷率达95%以上,沉铁率约为80%。综上所述,为提高Fe(Ⅱ)氧化速率,为反应(3)提供充足的Fe(Ⅲ),形成结晶良好的臭葱石沉砷渣,控制反应过程的氧分压为0.6 MPa较为合理。
2.4 反应时间的影响
在Fe/As摩尔比1.5、初始pH值1、搅拌速度500 r/min、氧分压0.6 MPa和反应温度160 ℃条件下,考察反应时间对沉砷率、沉铁率以及沉砷渣物相的影响。沉砷渣的XRD谱与沉砷率、沉铁率及溶液铁浓度变化分别如图8和9所示。
由图8和9可知,当反应时间小于3 h时,反应时间对沉铁率、沉砷率有显著影响。反应时间从1h延长至3 h时,沉砷率和沉铁率分别提高了9.46%和8.35%,说明适当延长反应时间,有利于砷的沉淀。随着反应时间的延长,臭葱石物相的衍射峰强度增强,说明延长反应时间有利于非臭葱石物相转化为纯度较高的臭葱石物相。
2.5 反应温度的影响
在Fe/As摩尔比1.5、初始pH值1、搅拌速度500 r/min、氧分压0.6 MPa和反应时间3 h条件下,考察了反应温度对于沉砷率、沉铁率以及沉砷渣物相的影响。沉砷渣的XRD谱与沉砷率、沉铁率及溶液铁浓度变化分别如图10和11所示。
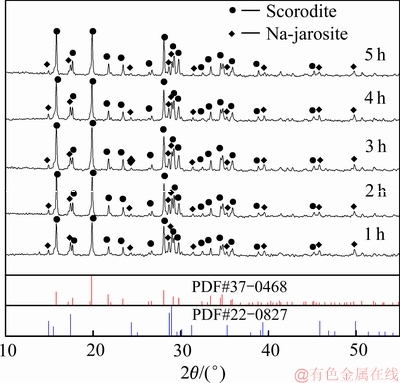
图8 不同反应时间沉砷渣的XRD谱
Fig. 8 XRD patterns of scorodite precipitate at various time
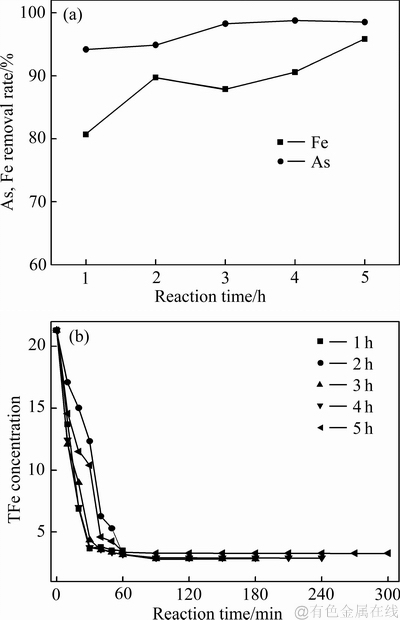
图9 不同反应时间对沉砷率、沉铁率及溶液铁浓度变化影响
Fig. 9 Effects of reaction time on As and Fe removal rates(a), and iron concentration(b)
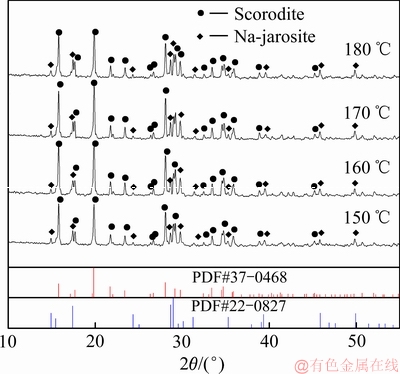
图10 不同反应温度沉砷渣的XRD谱
Fig. 10 XRD patterns of scorodite precipitate at various temperatures
由图10可知,在150~180 ℃的温度范围内,沉砷渣的主要物相为臭葱石,说明反应温度对臭葱石沉砷渣物相组成影响不大,但在160 ℃时臭葱石物相的衍射峰强度最强,继续升高温度至180 ℃过程,由于Fe(Ⅲ)达过饱和状态,导致有部分铁矾形成,从而臭葱石衍射峰强度减弱。由图11可知,当反应温度为150 ℃时,较低的反应温度延缓了反应(4)的进行,导致体系铁浓度缓慢降低;虽然沉铁率达到90%以上,但相当一部分Fe(Ⅲ)以铁矾物相形式沉淀。当反应温度升高至160 ℃时,沉砷率略有提高,但沉铁率有所降低,结合图10可知,因为反应温度处在臭葱石物相稳定温度区内[31-32],所以升高温度有利于臭葱石物相的形成。
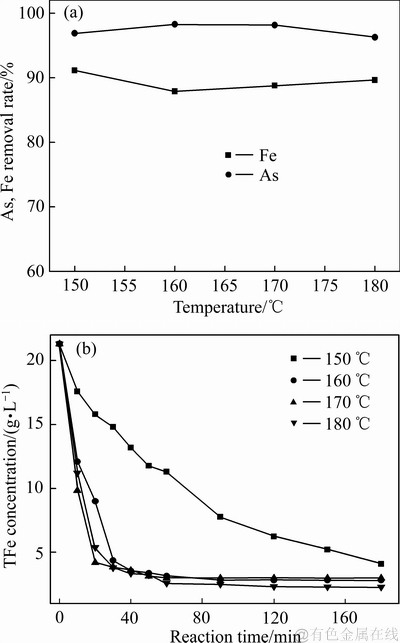
图11 不同反应温度对沉砷率、沉铁率及溶液铁浓度变化影响
Fig. 11 Effects of temperature and reaction time on As and Fe removal rates(a), and iron concentration(b)
3 平行验证实验
上述单因素实验获得的铜冶炼含砷废水水热臭葱石沉砷的优化技术条件为:Fe/As摩尔比1.5,初始pH值1,氧分压0.6 MPa,反应时间3 h及反应温度160 ℃。为验证优化技术条件的可靠性,在此条件下,开展了6次铜冶炼含砷废水水热臭葱石沉砷平行实验。实验结果分别如表2、图12和13所示。
表2 平行实验结果
Table 2 Results of parallel experiments
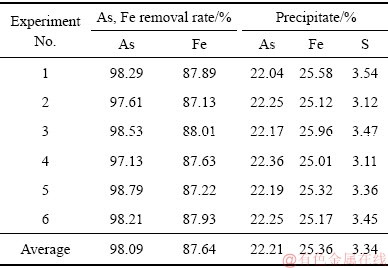
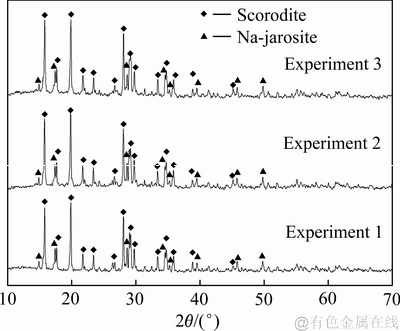
图12 平行实验沉砷渣XRD谱
Fig. 12 XRD patterns of scorodite precipitate obtained under parallel experiments
由表2可知,综合条件下各平行实验的结果重现性较好,砷与铁的平均沉淀率分别为98.09%和87.64%左右,沉砷渣中砷和铁的含量约为22.21%和25.36%。铜冶炼含砷废水经水热臭葱石处理后可将绝大部分砷固化为性质稳定的臭葱石,但沉砷后液中仍残留少量砷,其浓度约为0.5 g/L,并未达到国家直接排放标准,建议采用中和沉淀法或膜分离法进行二段深度除砷后达标排放。由平行实验1、2、3沉砷渣的XRD谱可知,在优化技术条件下获得了以臭葱石为主要物相的沉砷渣,并伴有微量的铁矾物相。
从沉砷渣的SEM像和EDS谱(见图13和14)可知,优化技术条件下形成的臭葱石颗粒晶体结构良好,表面较为光滑,是由正八面体晶粒聚集而成的大颗粒晶体。在臭葱石颗粒周围还存在一些不规则的细颗粒。
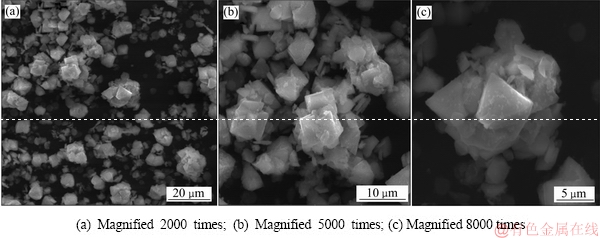
图13 沉砷渣的SEM像
Fig. 13 SEM images of scorodite precipitate at different magnanimities
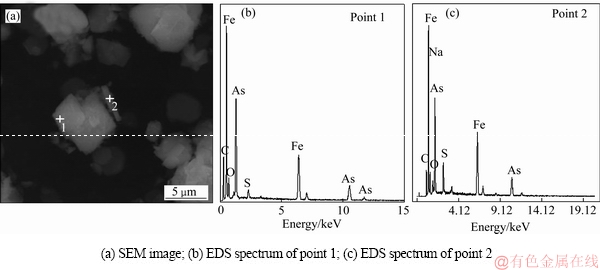
图14 沉砷渣的SEM-EDS谱
Fig. 14 SEM image and EDS spectra of scorodite precipitate
分别选取形状规则的臭葱石颗粒(见图14(a)中点1)和不规则的颗粒(见图14(a)中点2)进行能谱分析。由图13(b)可知,形状规则的臭葱石沉砷颗粒的主要元素为砷、铁、氧,由此推断其主要物相组成为臭葱石;图13(c)中主要元素除砷、铁外,还有较多硫、钠、氧存在,说明形状不规则的细小颗粒中有铁矾物相存在。
4 结论
1) 在实验条件范围内,适当地提高Fe/As摩尔比、降低反应体系初始pH值、延长反应时间都有利于臭葱石的形成,同时,有利于亚稳态铁矾等非臭葱石物相返溶、重结晶,进而形成性质稳定的臭葱石物相,提高沉砷率。
2) 含砷废水水热合成臭葱石沉砷的优化技术条件如下:Fe/As摩尔比1.5,初始pH值1.0,反应温度160 ℃,搅拌转速500 r/min,氧分压0.6 MPa,反应时间3 h。在此优化技术条件下,砷、铁的沉淀率分别为98.09%和87.64%左右;获得了以臭葱石为主要物相、并伴有微量铁矾的沉砷渣,其砷、铁及硫的含量分别约为22.21%、25.36%及3.34%。形成的臭葱石颗粒晶体结构良好,表面光滑,是由正八面体晶粒聚集而成的大颗粒晶体。铁矾以不规则小颗粒存在于臭葱石颗粒周围。
3) 含砷废水经水热沉砷工艺处理后可将98%以上的砷固化为性质稳定的臭葱石。该工艺对我国数量庞大的冶金含砷废水的无害化处置具有重要的借鉴和指导意义。
REFERENCES
[1] 张 旭, 刘志宏, 李玉虎, 刘智勇, 李启厚. 苛性碱溶液氧压浸出高砷锑烟尘[J]. 中南大学学报(自然科学版), 2014, 45(5): 1390-1396.
ZHANG Xu, LIU Zhi-hong, LI Yu-hu, LIU Zhi-yong, LI Qi-hou. Oxygen pressure leaching of arsenic and antimony bearing flue dust in NaOH solution[J]. Journal of Central South University(Science and Technology), 2014, 45(5): 1390-1396.
[2] 张焕然, 刘晓英, 衷水平, 伍赠玲, 蓝碧波, 王俊娥. 富铼砷滤饼加压浸出工艺研究[J]. 中国有色冶金, 2015, 44(5): 59-62.
ZHANG Huan-ran, LIU Xiao-ying, ZHONG Shui-ping, WU Zeng-ling, LAN Bi-bo, WANG Jun-e. Pressure leaching technique of smelter dust with high-copper and high-arsenic[J]. China Nonferrous Metallurgy, 2015, 44(5): 59-62.
[3] 徐志峰, 聂华平, 李 强, 卢秋虎, 王 巍, 月日辉. 高铜高砷烟灰加压浸出工艺[J]. 中国有色金属学报, 2008, 18(S1): s59-s63.
XU Zhi-feng, NIE Hua-ping, LI Qiang, LU Qiu-hu, WANG Wei, YUE Ri-hui. Study on pressure leaching process of arsenic-rhenium filter cake[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(S1): s59-s63.
[4] 易 宇, 石 靖, 田庆华, 郭学益. 高砷烟尘碱浸渣制备焦锑酸钠的新工艺[J]. 中国有色金属学报, 2015, 25(1): 241-249.
YI Yu, SHI Jing, TIAN Qing-hua, GUO Xue-yi. Novel technology for preparation of sodium pyroantimonate from alkali leaching residue of high arsenic dust[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(1): 241-249.
[5] 李 阔, 徐瑞东, 何世伟, 陈汉森, 朱 云, 华宏全, 舒 波. 采用碱性加压氧化浸出从高铋铅阳极泥中脱除砷锑[J]. 中国有色金属学报, 2015, 25(5): 1394-1402.
LI Kuo, XU Rui-dong, HE Shi-wei, CHEN Han-sen, ZHU Yun, HUA Hong-quan, SHU Bo. Arsenic and antimony removal from bismuth-rich lead anode slime by alkaline pressure oxidation leaching[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(5): 1394-1402.
[6] 袁铁锤, 周科朝, JIA Yong-feng, DEMOPOULOS G P. 结晶型砷酸镍的溶解性[J]. 化工学报, 2006, 57(1): 122-125.
YUAN Tie-chui, ZHOU Ke-chao, JIA Yong-feng, DEMOPOULOS G P. Solubility of annabergite[J]. CIESC Journal, 2006, 57(1): 122-125.
[7] 方兆珩, 石 伟, 韩宝玲, 夏光祥. 高砷溶液中和脱砷过程[J]. 化工冶金, 2000, 21(4): 359-362.
FANG Zhao-heng, SHI Wei, HAN Bao-ling, XIA Guang-xiang. Removal of arsenic from high arsenic solutions by scorodite precipitation[J]. Engineering Chemistry & Metallurgy, 2000, 21(4): 359-362.
[8] OEHMEN A, VALERIO R, LANOS J, FRADNHOJ, SERRAS, MARIAA, REIS M, JOAO, CRESPOG, SVETLOZAR V. Arsenic removal from drinking water through a hybrid ion exchange membrane—Coagulation process[J]. Separation and Purification Technology, 2011, 83: 137-143.
[9] 孙桂琴, 王见华, 殷 茵, 梁小敏, 胡尚义. 蜈蚣草湿地系统处理含砷废水的研究[J]. 江西化工, 2008(3): 102-105.
SUN Gui-qin, WANG Jian-hua, YIN Yin, LIANG Xiao-min, HU Shang-yi. Study on treatment of wastewater containing arsenic by centipede grassland wetland system[J]. Jiangxi Chemical Industry, 2008(3): 102-105.
[10] 郑雅杰, 张胜华, 龚 昶. 含砷污酸资源化回收铜和砷的新工艺[J]. 中国有色金属学报, 2013, 23(10): 2985-2992.
ZHENG Ya-jie, ZHANG Sheng-hua, GONG Chang. Novel technique for recovery of copper and arsenic from arsenic-containing waste acid[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(10): 2985-2992.
[11] 曹俊雅, 叶栩文, 杜 娟, 赵 欢, 张广积, 杨 超. 铁氧化菌对含砷溶液中砷沉淀和臭葱石晶体形成的影响[J].过程工程学报, 2015, 15(2): 307-312.
CAO Jun-ya, YE Xu-wen, DU Juan, ZHAO Huan, ZHANG Guan-ji, YANG Chao. Effects of iron oxidation bacteria on arsenic precipitation and the formation of crystalline scorodite from arsenic-containing solution[J]. The Chinese Journal of Process Engineering, 2015, 15(2): 307-312.
[12] 柯平超, 刘志宏, 刘智勇, 李玉虎, 刘付朋. 固砷矿物臭葱石组成与结构及其浸出稳定性研究现状[J]. 化工学报, 2016, 67(11): 4533-4540.
KE Ping-chao, LIU Zhi-hong, LIU Zhi-yong, LI Yu-hu, LIU Fu-peng. Research status on composition, structure, and leaching stability of an arsenic solidification mineral scorodite[J]. CIESC Journal, 2016, 67(11): 4533-4540.
[13] WANG S, MULLIFAN C N. Occurrence of arsenic contamination in Canada: Sources, behavior and distribution[J]. Science of the Total Environment, 2006, 366(2/3): 701-721.
[14] 徐根福. 处理高砷浓度工业废水的化学沉淀法[J]. 湿法冶金, 2009, 28(1): 12-17.
XU Gen-fu. Chemical precipitation methods for treatment of high-arsenic concentration industrial effluents[J]. Hydrometallurgy of China, 2009, 28(1): 12-17.
[15] 刘志宏, 潘庆琳, 刘智勇, 李玉虎, 李启厚. As(Ⅲ)在酸性水溶液中与金属铁的反应行为[J]. 中国有色金属学报, 2015, 25(10): 2945-2952.
LIU Zhi-hong, PAN Qing-lin, LIU Zhi-gong, LI Yu-hu, LI Qi-hou. Reactive behaviors between As(Ⅲ) and metallic iron in acidic aqueous solution[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(10): 2945-2952.
[16] DEMOPOULOS G P. On the preparation and stability of scorodite[C]// REDDY R G, RAMAEHANDRAN eds. Arsenic Metallurgy. Warrendale, PA: TMS, 2005: 25-50.
[17] MIN Xiao-bo, LIAO Ying-ping, CHAI Li-yuan, YANG Zhi-hui, XIONG Shan, LIU Lin, LI Qing-zhu. Removal and stabilization of arsenic from anode slime by forming crystal scorodite[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1298-1306.
[18] FUJITA T R, TAGUDHI R, ABUMIYA M, MATSUMOTO M, SHIBATA E, NAKAMURA T. Novel atmospheric scorodite synthesis by oxidation of ferrous sulfate solution: Part Ⅰ[J]. Hydrometallugy, 2008, 90(2): 92-102.
[19] FUJITA TR, TAGUDHI R, ABUMIYA M, MATSUMOTO M, SHIBATA E, NAKAMURA T. Effect of pH on atmospheric scorodite synthesis by oxidation of ferrous ions: Physical properties and stability of the scorodite[J]. Hydrometallurgy, 2009, 96(3): 189-198.
[20] BERRE J F L, GAUVIN R, DEMOPOULOS G P. A study of the crystallization kinetics of scorodite via the transformation of poorly crystalline ferric arsenate in weakly acidic solution[J]. Colloids & Surfaces A Physicochemical & Engineering Aspects, 2008, 315(1/3): 117-129.
[21] MAJZLAN J, DRAHOTA P, FILIPPI M, GREVEL K D, KAHL W A, PLASIL J, BOERIO-GOATES J, WOODFIELD B F. Thermodynamic properties of scorodite and parascorodita (FeAsO4·3.5H2O), kankite (FeAsO4·2H2O) and FeAsO4[J]. Hydrometallugy, 2012: 117/118: 47-56.
[22] ITOU H, TAKASU T, UENO Y. Crystallization of the amorphous ferric arsenate in the saturated steam atmosphere[J]. High Temp Mater Processes (London), 2011, 30(4/5): 473-483.
[23] MAMBOTE R C M, KRIJGSMAN P, REUTER M A. Hydrothermal precipitation of arsenic compounds in the ferric–arsenic (III)–sulfate system: Hermodynamic modeling[J]. Minerals Engineering, 2003, 16(5): 429-440.
[24] GOMEZMA, BECZE L, CUTLERJN, DEMOPOULOSGP. Hydrothermal reaction chemistry and characterization of ferric arsenate phases precipitated from Fe2(SO4)3-As2O5- H2SO4 solutions[J]. Hydrometallurgy, 2011, 107(3/4): 74-90.
[25] KITAMURA Y, OKAWA H, KATO T, KATO T, SUGAWARA K. Size and morphology of scorodite particles synthesized using ultrasound irradiation[J]. Jpn J Appl Phys, 2014, 53(7): 467-468.
[26] 余自秀. 含砷铁溶液水热臭葱石沉砷研究[D]. 昆明: 昆明理工大学, 2017: 30-90.
YU Zi-xiu. Hydrothermal precipitation of arsenic in solution containing arsenic and iron based on scorodite formation[D]. 2017: 30-90.
[27] 余自秀, 李存兄, 魏 昶, 杨晨年, 邓志敢, 李兴彬, 樊 刚. 含砷铁溶液水热臭葱石沉砷研究[J]. 昆明理工大学学报(自然科学版), 2017, 42(1): 1-8.
YU Zi-xiu, LI Cun-xiong, WEI Chang, YANG Chen-nian, DENG Zhi-gan, LI Xing-bin, FAN Gang. Hydrothermal precipitation of arsenic in solution containing arsenic and iron based on scorodite formation[J]. Journal of Kunming University of Science and Technology (Natural Science Edition). 2017, 42(1): 1-8.
[28] 李存兄, 魏 昶, 邓志敢, 李兴彬, 樊 刚, 王益昭, 易烁文, 李旻廷. FeSO4-H2O体系中水热赤铁矿沉铁及亚稳态铁物相转变行为[J]. 中国有色金属学报, 2018, 28(3): 628-636.
LI Cun-xiong, WEI Chang, DENG Zhi-gan, LI Xing-bin, FAN Gang, WANG Yi-zhao, YI Shuo-wen, LI Min-ting. Hydrothermal hematite precipitation and conversion behavior of metastable iron phase in FeSO4-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(3): 628-636.
[29] FUJITA T, TAGUDHI R, ABUMIYA M. Effects of zinc, copper and sodium ions on ferric arsenate precipitation in a novel atmospheric scorodite process[J]. Hydrometallurgy, 2008, 93(1): 30-38.
[30] FUJITA T, TAGUDHI R, ABUMIYA M, MATSUMOTO M, SHIBATA E, NAKAMURA T. Novel atmospheric scorodite synthesis by oxidation of ferrous sulfate solution. Part Ⅱ. Effect of temperature and air[J]. Hydrometallurgy, 2008, 90(2/4): 85-91.
[31] DUTRIZAC J E, JAMBOR J L. Characterization of the iron arsenate–sulphate compounds precipitated at elevated temperatures[J]. Hydrometallurgy, 2007, 86(3): 147-163.
[32] PAPANGELAKIS V G, BLAKEY B C, LIAO H. Hematite solubility in sulfate process solution[C]//Hydrometallurgy '94. Dordrecht: Springer, 1994: 159-175.
Scorodite precipitation from arsenic-containing wastewater by hydrothermal method
ZHANG Jun, LI Cun-xiong, WEI Chang, FAN Gang, LI Xing-bin, DENG Zhi-gan, LI Min-ting, ZHANG Peng
(Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Abstract: In this paper, the arsenic-containing wastewater produced by the copper smelting process was studied. The effects of macroscopic technical parameters, such as Fe/As molar ratio, initial pH, reaction temperature, oxygen partial pressure and reaction time, on the arsenic precipitate process and the phase transformation of scorodite precipitate were investigated. The experimental results indicate that, under hydrothermal conditions of initial Fe/As molar ratio of 1.5, initial pH of 1, temperature of 160 ℃, agitation speed of 500 r/min, oxygen partial pressure of 0.6 MPa and reaction time of 3 h, the arsenic and iron removal rates are 98.09% and 87.64%, respectively, the contents of As, Fe and S in scorodite precipitate are 22.21%, 25.36% and 3.34%, respectively, and the sulfur is mainly in the form of jarosite. Reducing the Fe/As molar ratio and initial pH, and extending the reaction time are all favorable for re-dissolution of metastable jarosite and recrystallization to form the stable scorodite phase.
Key words: arsenic-containing wastewater; scorodite; arsenic removal rate; iron removal rate; jarosite
Foundation item: Projects(51664038, 51474117, 51364022) supported by the National Natural Science Foundation of China
Received date: 2018-05-25; Accepted date: 2018-12-10
Corresponding author: LI Cun-xiong; Tel: +86-13518764748; E-mail: licunxiong@126.com
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51664038,51474117,51364022)
收稿日期:2018-05-25;修订日期:2018-12-10
通信作者:李存兄,教授,博士;电话:13518764748;E-mail:licunxiong@126.com
摘 要:本文以铜冶炼过程所产生的含砷废水为研究对象,研究了Fe/As摩尔比、初始pH值、氧分压、反应时间以及反应温度等宏观技术参数对水热臭葱石沉砷过程及沉砷渣物相转变的影响规律。结果表明:在Fe/As摩尔比1.5、初始pH1.0、反应温度160 ℃、搅拌转速500 r/min、氧分压0.6 MPa和反应时间3 h的优化技术条件下,砷与铁的沉淀率分别为98.09%和87.64%,获得了纯度较高的臭葱石沉砷渣;沉砷渣中砷、铁及硫的含量分别为22.21%、25.36%及3.34%,其中硫主要以亚稳态铁矾的形式存在;降低Fe/As摩尔比和初始pH值、延长反应时间均有利于亚稳态铁矾的返溶、重结晶,进而形成性质稳定的臭葱石物相。
[1] 张 旭, 刘志宏, 李玉虎, 刘智勇, 李启厚. 苛性碱溶液氧压浸出高砷锑烟尘[J]. 中南大学学报(自然科学版), 2014, 45(5): 1390-1396.
[2] 张焕然, 刘晓英, 衷水平, 伍赠玲, 蓝碧波, 王俊娥. 富铼砷滤饼加压浸出工艺研究[J]. 中国有色冶金, 2015, 44(5): 59-62.
[3] 徐志峰, 聂华平, 李 强, 卢秋虎, 王 巍, 月日辉. 高铜高砷烟灰加压浸出工艺[J]. 中国有色金属学报, 2008, 18(S1): s59-s63.
[4] 易 宇, 石 靖, 田庆华, 郭学益. 高砷烟尘碱浸渣制备焦锑酸钠的新工艺[J]. 中国有色金属学报, 2015, 25(1): 241-249.
[5] 李 阔, 徐瑞东, 何世伟, 陈汉森, 朱 云, 华宏全, 舒 波. 采用碱性加压氧化浸出从高铋铅阳极泥中脱除砷锑[J]. 中国有色金属学报, 2015, 25(5): 1394-1402.
[6] 袁铁锤, 周科朝, JIA Yong-feng, DEMOPOULOS G P. 结晶型砷酸镍的溶解性[J]. 化工学报, 2006, 57(1): 122-125.
[7] 方兆珩, 石 伟, 韩宝玲, 夏光祥. 高砷溶液中和脱砷过程[J]. 化工冶金, 2000, 21(4): 359-362.
[9] 孙桂琴, 王见华, 殷 茵, 梁小敏, 胡尚义. 蜈蚣草湿地系统处理含砷废水的研究[J]. 江西化工, 2008(3): 102-105.
[10] 郑雅杰, 张胜华, 龚 昶. 含砷污酸资源化回收铜和砷的新工艺[J]. 中国有色金属学报, 2013, 23(10): 2985-2992.
[11] 曹俊雅, 叶栩文, 杜 娟, 赵 欢, 张广积, 杨 超. 铁氧化菌对含砷溶液中砷沉淀和臭葱石晶体形成的影响[J].过程工程学报, 2015, 15(2): 307-312.
[12] 柯平超, 刘志宏, 刘智勇, 李玉虎, 刘付朋. 固砷矿物臭葱石组成与结构及其浸出稳定性研究现状[J]. 化工学报, 2016, 67(11): 4533-4540.
[14] 徐根福. 处理高砷浓度工业废水的化学沉淀法[J]. 湿法冶金, 2009, 28(1): 12-17.
[15] 刘志宏, 潘庆琳, 刘智勇, 李玉虎, 李启厚. As(Ⅲ)在酸性水溶液中与金属铁的反应行为[J]. 中国有色金属学报, 2015, 25(10): 2945-2952.
[26] 余自秀. 含砷铁溶液水热臭葱石沉砷研究[D]. 昆明: 昆明理工大学, 2017: 30-90.
[27] 余自秀, 李存兄, 魏 昶, 杨晨年, 邓志敢, 李兴彬, 樊 刚. 含砷铁溶液水热臭葱石沉砷研究[J]. 昆明理工大学学报(自然科学版), 2017, 42(1): 1-8.


