
Evolution of precipitates of Al-Cu alloy during equal-channel angular pressing at room temperature
XU Xiao-chang(许晓嫦)1, LIU Zhi-yi(刘志义)1, LI Yun-tao(李云涛)1,
DANG Peng(党 朋)1, ZENG Su-min(曾苏明)1, 2
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. Department of Mechanical Engineering, Shaoyang Institute, Shaoyang 422004, China
Received 29 October 2007; accepted 28 May 2008
Abstract:
The evolution of precipitates and hardness changes in Al-Cu alloys during equal-channel angular pressing(ECAP) at room temperature were investigated by hardness measurement, X-ray diffraction analysis and transmission electron microscopy. The results show that with the increase of the total equivalent strain during ECAP from 0 to 8.4, the hardness of specimens with metastable θ″ phase increases first and decreases in later period. The hardness increases successively in specimens containing metastable θ′ phase and equilibrium θ phase. It is believed that the evolutions of hardness are related to the mechanism of re-dissolution of precipitates. A critical nuclei size concept is provided to express the mechanism of such re-dissolution of three precipitates in Al-Cu alloys.
Key words:
Al-Cu alloy; re-dissolution; ECAP; precipitated phase;
1 Introduction
Large plastic deformation has been studied widely for aluminum alloys. The popular methods include equal-channel angular pressing(ECAP)[1-8], accumula- tive roll bonding(ARB)[9-10], multi-axial compression (MAC)[11-12] and high pressure torsion(HPT)[13-14]. Many reports showed that the microstructures of aluminum alloys after large plastic deformation are different form those gone through normal deformation. For example, several researchers[1-5] found that the hardness of multi-phase alloys containing precipitates increased gradually during ECAP. When the total equivalent strain(TES) surpassed about 3, the hardness starts to decrease due to the dissolution of precipitated phases. The hardness is then increased again for larger strain. Other researchers[6-8] showed that the hardness in this typical alloy increases successively with ECAP passes. There was no evidence showing the occurrence of decrease. However, those authors did not give the reasons for each difference.
In this work, we try to explain the reason behind the inconsistency of the hardness. Based on the result of Ref.[15], the evolution of precipitates during ECAP has no relation with temperature. We will take hardness measurement, X-ray diffraction pattern analysis and transmission electron microscopy to study the evolution of precipitates phase in Al-Cu alloys during ECAP at room temperature.
2 ExperimentalRod-shaped samples of an Al-4.11%Cu (mass fraction) alloy, 10 mm in diameter and 40 mm in length, were prepared by traditional casting and machining. The samples were solid solution treated at 540 ℃ for 1 h and water-quenched to room temperature, subsequently. The processing parameters of aging heat treatment are listed in Table 1.
The aged samples were subjected to ECAP at room temperature with MoS2 as lubricant. The ECAP die is illustrated in Fig.1. This die has a channel angle of 120?, where the two channels intersect 45? at the outer arc of curvature. It creates an equivalent strain of 0.7 after one pass. The deformed samples were prepared in axial direction by wire cutting for hardness measurement, transmission electron microscopy observation and X-ray diffractive pattern analysis. Brinell hardness was measured in Brinell hardness tester. The TEM samples were first mechanically polished and then polished in MTP-1 twin-jet machine at -25 ℃ with the nitric acid and methyl alcohol electrolyte. TEM observation was operated on Philips TECNAI-G2 20 TEM microscope at 200 kV.
Table 1 Parameters of heat-treatment
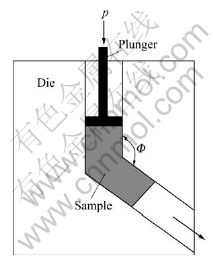
Fig.1 Schematic diagram of equal-channel angular pressing
Bulk-shaped samples, 5 mm in length and 5 mm in width and 5 mm in height, were prepared for D/max- 2550/PC X-ray diffractometry with Cu Kα radiation and 36 kV accelerated voltage.
3 Results3.1 Hardness variation
Fig.2 shows the hardness change of the samples containing θ″, θ′ and θ precipitates during ECAP. Before the deformation, the sample with θ″ phase shows the highest hardness value, followed by the samples with θ′ phase and θ phase. This is because that θ″ phase is completely coherent with matrix and strengthens the sample remarkably.
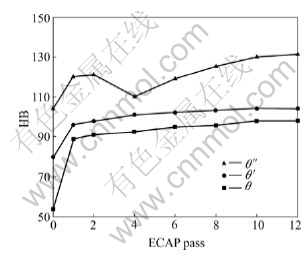
Fig.2 Hardness as function of ECAP passes
When the samples with θ phase run through one pass, the hardness increases drastically by more than 60%. After that, the hardness increases slightly and reaches a steady value after 10 passes. The hardness of θ′ phase strengthened samples increases less than that of θ phase after one pass. After then, they behave similar.
The hardness of the sample with θ″ phase increases less than that with θ phase and is similar to that of the θ′ phase. But it decreases after four passes. With the ECAP pass increasing, the hardness increases again and reaches a steady value. During ECAP, the sample with θ″ phase shows the highest hardness value and the sample with θ phase the lowest at all tests.
3.2 Microstructure of Al-Cu alloy during ECAP
Fig.3 shows the transmission electron micrographs of three samples with θ″, θ′ and θ phase, respectively. Fig.3(a) shows the similar morphology characteristic of the θ″ phase and matrix as Ref.[15]. After four passes, the amount of θ″ phase decreases rapidly and becomes fragments, as shown in Fig.3(b). The interfaces between precipitated phase and matrix become clearer, indicating partial incoherent interfaces occur. After 12 passes, the θ″ disappears and many fine grains are presented. The average grains size is 0.36 μm, as shown in Fig.3(c).
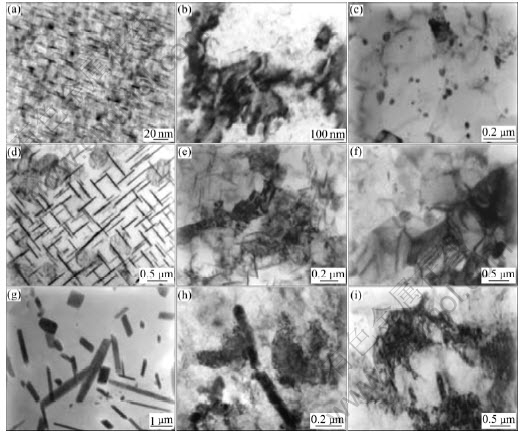
Fig.3 TEM images of samples containing θ″ phase (a, b, c), θ′ phase (d, e, f) and θ phase (g, h, i) as function of ECAP passes: (a), (d) and (g) At initial state; (b), (e) and (h) After 4 passes; (c), (f) and (i) After 12 passes
Widmanstatten structures are observed in Fig.3(d) due to the semi-coherent relationship between the θ′ phase and matrix. After four passes, as shown in Fig.3(e), the plate-like precipitated phase is crushed and refined. With increasing ECAP pass, the volume fraction of θ′ phase decreases. After 12 passes, there are still some precipitated phases present, as shown in Fig.3(f).
θ phase presents in a strip shape or block shape, as shown in Fig.3(g). This morphology proves the θ phase is rod-like. Fig.3(h) shows the plastic deformation and crushing of θ phase. The size of crushed θ phase is about 200 nm. Many precipitations are still observable after 12 passes, as shown in Fig.3(i).
3.3 X-ray diffraction analysis
The diffraction peak of θ″ phase almost disappears after four passes, as shown in Fig.4(b). This indicates that θ″ phase has almost re-dissolved. While the diffraction peak of θ′ phase is reduced and that of θ phase does not change. It is indicated that most of θ′ phase has re-dissolved. But θ phase almost does not re-dissolve, as shown in Fig.5(b) and Fig.6(b). The diffraction peaks of θ″ and θ′ phase disappear after 12 passes, as shown in Fig.4(c) and Fig.5(c). This indicates that θ″ and θ′ phases have re-dissolved. While the diffraction peak of θ phase still shows low intensity, meaning there are still some amounts of precipitated phase, as can be seen from Fig.6(c).

Fig.4 XRD patterns of θ″ phase as function of ECAP pass: (a) At initial state; (b) After 4 passes; (c) After 12 passes
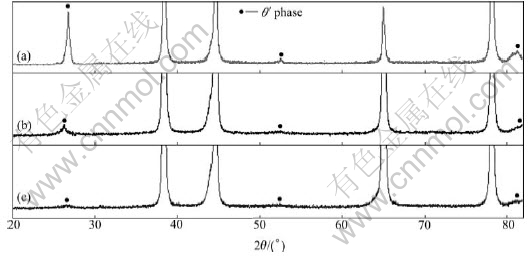
Fig.5 XRD patterns of θ′ phase as function of ECAP pass: (a) At initial state; (b) After 4 passes; (c) After 12 passes
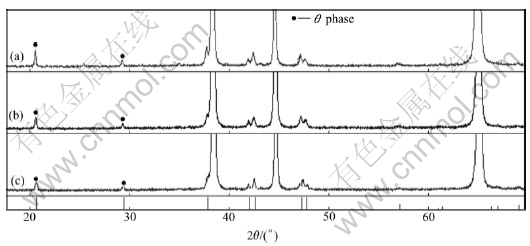
Fig.6 XRD patterns of θ phase as function of ECAP passes: (a) After 1 pass; (b) After 4 passes; (c) After 12 passes
4 Discussion4.1 Hardness variation during ECAP
Some researchers[1-5] found that the hardness decreases during ECAP and identified the re-dissolution of precipitated phase by TEM and X-ray analysis. But others[6-8] didn’t observe the hardness decreasing. FATAY et al[6] considered that the precipitated phase congregated at the grain boundary, rather than redissolved into the matrix. UNIHIRO et al[7] believed that, although the precipitated phases were thoroughly re-dissolved, Ag atoms aggregated where the particles stayed, prohibiting the dislocations movement.
Those two views do not exactly explain the hardness change during ECAP. On one hand, the powder-like particles at grain boundary may prevent the dislocations movement, and the effect is slighter than that of dispersion strengthening of precipitated phases in grain. On the other hand, the effect of Ag atoms inhibiting the dislocations movement is not prominent.
So it is hard to explain why the dissolving of the precipitated phases during ECAP does not lead to the decrease of hardness.
We believe that there are two opposite processes taking place during ECAP. One is the inhibition of dislocations movement which is weakened by the redissolution of the precipitated phases. The other is the strain hardening by continuous ECAP process, which increases the hardness.
In summary, the hardness of the samples during ECAP is controlled by the combined action of two processes mentioned above, as shown in Fig.7. Curves a, b and c in Fig.7, show the decrease of hardness due to re-dissolving of precipitated phases, while curve d shows the increase of hardness due to the severe plastic deformation.
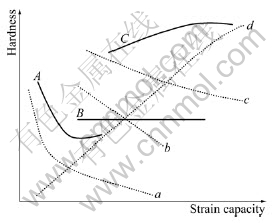
Fig.7 Schematic of interpreting evolution of hardness
If the precipitated phases make great contribution to the increase of hardness, the decrease of hardness will happen because of the re-dissolving of precipitated phases. When most of precipitated phases are redissolved, as shown in curve a, the hardness of samples will decrease. After that, with the strain becoming larger, the increase of hardness due to strain hardening dominants. Therefore, the hardness of samples increases again. This is the case of samples with θ″ phase as shown in Fig.2.
If the precipitated phases do not make great contribution to the increase of hardness as shown above, the increase of hardness due to severe plastic deformation counteracts the decrease of hardness due to re-dissolving of precipitated phases. The absolute value of slope of curve b equals almost that of curve d. The change of hardness is denoted as curve B.
If the precipitated phases contribute little to the increase of hardness, the increase of hardness due to severe plastic deformation is greater than the decrease of hardness due to re-dissolving of precipitated phases. As a result, the hardness increases with strain. The change of hardness is denoted as curve C. This is the case of samples with θ′ and θ phase shown in Fig.2 and similar to the results reported in Ref.[6-8].
4.2 Mechanism of re-dissolution of precipitated phases
At present, there are several different views about the mechanism of re-dissolving of precipitated phases. MURAYAMA et al[3] believed that θ′ phase is broken up into small particles in nanoscale due to the severe plastic deformation. The surface energy of those broken particles are very large. This makes the precipitated phases re-dissolved into the matrix, forming the supersaturated solid solution. FATAY et al[6] considered that the precipitated phases congregated at the grain boundary, but did not re-dissolve into the matrix. HIDAKA et al[9] thought that the cementites in eutectoid steel completely disappeared due to ball milling, and the carbon atoms segregated at the grain boundary of the nanosize (20 nm) ferrite. By Gibbs-Thompson equation, MARTIN et al[10] believed that the fine particles with diameters smaller than the average ones would re-dissolve because the surrounding solubility surpassed the mean concentration.
We consider that such four views mentioned above were not enough to explain the re-dissolution of the precipitated phases at room temperature. The grain boundary energy increases rapidly when the matrix grains are refined to the size of 0.05-0.5 μm after severe plastic deformation. If MURAYAMA et al were right, the grain boundary would not be able to exist while they did exist in a large number in the matrix. Studies from Refs.[8-9] reported that the value of matrix lattice parameters increase by the re-dissolving of the precipitated phases. This means that the viewpoint of FATAY et al[6] has no universal significance. Moreover, this point could not explain why the second phase particles precipitated again by heating in the alloys gone through ECAP[8-10]. HIDAKA et al only suggested that there are cementites but didn’t mention why cementites dissolved. MARTIN et al only explained the mechanism of the aggregation and growth of the precipitated phases, but didn’t give a reasonable explaination why the precipitated phases vanished.
We propose a “critical nuclei size theory” to interpret the relationship between the change of hardness and the re-dissolution of the precipitates during severe plastic deformation at room temperature.
As the classic theory of solid phase transformation [16], we know that the necessary condition for precipitates to grow and become stable is that their radii should be larger than the critical radius rc in solid-solid transformation. rc is decided by the driving force and resistance of the transformation. Following this theory, we get where σ is the surface free energy, ?G is the difference of the volume free energy between parent phase and precipitated phase, U is the strain energy, including the elastic strain energy resulting from the lattice distortion and the strain energy resulting from the increase of the density of the dislocations and other crystal defects. If we change the influencing factors, the value of rc will change accordingly. During the severe plastic deformation at room or low temperature, the precipitates are fragmentated due to the severe stress. Then the σ, ?G and U change, resulting in the change of rc, then the lattice aberrance appears. The coherence relationship disappears and the new interfaces appears which are incoherent with the matrix.

θ″ phase is completely coherent with the matrix. During ECAP, the severe deformation induces the distortion of lattice, then the coherent relationship of θ″ phase and matrix disappears, producing new phase interfaces. The values of σ and U increases remarkablely. The ?G between θ″ phase and matrix is originally small. From the formula, we can see that the value of rc increases. When the value of rc is larger than that of θ″ phase, it will re-dissolve into matrix. In summary, the re-dissolution of θ″ precipitates results from the increasing of the value of rc. This is mainly because the value ?G of θ″ phase is originally small and the boundary energy σ raises significantly due to disruption of complete coherent relation between θ″ phase and the matrix.
θ phase is incoherent with the matrix. During ECAP, the increase of the value of U is larger than that of θ″ phase and the value of ?G is the largest. From the formula, we can see that the value of rc increases, but is not larger than that of the θ″ phase. In summary, the re-dissolution of θ precipitates results from the increase of the value of rc, which is due to the increase of the U value of θ phase.
θ′ phase is semi-coherent with the matrix. During ECAP, the increase of the value of σ is not larger than that of θ″ phase. The increase of the value of U is not larger than that of θ phase, and the value of ?G is middle. From the formula, we can see that the value of rc increases in a scale not larger than that of the θ″ phase, but not smaller than that of θ phase. In summary, the re-dissolution of θ′ precipitates results from the increase of the value of rc, which is primarily due to the increase of the value of σ and U.
As discussed above, we can conclude that the change of hardness of the precipitates corresponds to the mechanism of the re-dissolution of the precipitates during ECAP. If the value of rc increases on a large scale, the amount of the re-dissolving precipitates is large and the rate of the re-dissolving process is fast. The decrease of hardness due to the re-dissolving of precipitated phases is larger than the increase of hardness due to strain hardening, as the case of θ″ phase shown in Fig.2. If the value of rc increases on a small scale, the amount of the re-dissolving precipitates is small and the rate of the re-dissolving process is slow. The decrease of hardness due to the re-dissolving of precipitated phases is smaller than the increase of hardness due to strain hardening, as the cases of θ′ and θ phase shown in Fig.2. After 12 passes, there are still some amounts of precipitates, as shown in Figs.3(f) and (i).
5 Conclusions
1) During ECAP at room temperature, the hardness of the specimens of Al-Cu alloy containing θ, θ′ and θ″ strengthening precipitates decreases due to the re-dissolving of precipitated phases and then increases due to the strain hardening simultaneously.
2) The hardness variation can not be used to judge re-dissolution of the precipitates due to the different re-dissolving rate of precipitated phases.
3) The re-dissolution of θ″ precipitates is mainly because the increase of the value of the surface free energy. The re-dissolution of θ′ precipitates primarily comes from the enhancement of the value of the surface free energy and strain energy. The re-dissolution of θ precipitates results from the increase of the value of the strain energy.
References
[1] PATRICK B, FURUKAWA, HORITA Z. Influence of pressing speed on microstructural development in equal channel angular pressing [J]. Metallurgical and Materials Transactions, 1999, A30: 33-42.
[2] OH-ISHI K, HASHI Y, SADAKATA A, KANEKO K, HORITA Z, LANGDON T G. Microstructural control of an Al-Mg-Si alloy using equal-channel angular pressing [J]. Materials Science Forum, 2002, 396/402: 333-338.
[3] MURAYAMA M, HORITA Z, HONO K. Microstructure of two-phase Al-1.7at% Cu alloy deformed by equal-channel angular pressing [J]. Acta Mater, 2001, 49: 21-29.
[4] YUNTIAN T Z, TERRY C L, TERENCE G L. Performance and application of nanostructural materials produced by severe plastic deformation [J]. Scripta Materials, 2004, 51: 825-830.
[5] KANG S B, LIM C Y, KIM H W, MAO J. Microstructure evolution and hardening behavior of 2024 aluminum alloy processed by the severe plastic deformation [J]. Materials Science Forum, 2002, 396/402: 1163-1168.
[6] FATAY D, BASTARASH E, NYILAS K, DOBATKIN S. X-ray diffraction study on the microstructure of an Al-Mg-Sc-Zr alloy deformed by high-pressure torsion [J]. Z Metallkd, 2003, 94(7): 842-847.
[7] UNIHIRO K, HASHI O, AKESHI T, UJITA F, EIICHIRO K. Microstructural control of a precipitate-hardenable Al-Ag alloy using severe plastic deformation [J]. Materials Science Forum, 2003, 426/432: 2637-2642.
[8] APPS P J, BOWEN J R, PRANGNELL P B. The effect of coarse second-phase particles on the rate of grain refinement during severe deformation processing [J]. Acta Materialia, 2003, 51: 2811-2822.
[9] HIDAKA M, TSUCHIYAMA T, TAKAKI S. Microstructural change during mechanical milling treatment in Fe-C alloy powders with different initial microstructure [J]. Mater Sci Forum, 2003, 426/432: 2717-2722.
[10] MARTIN J W, DOHER R D, CANTOR B. Stability of microstructure in metallic systems [M]. Cambridge: Cambridge University Press, 1997.
[11] DU Zhong-ze, WU Lai-zhi, WEI Fa-ming, WANG Jing-tao. Nanocrystalline 2J4 alloy processed by equal channel angular pressing [J]. Trans Nonferrous Met Soc China, 2005, 15(3): 212-215.
[12] ZHOU Zhi-min, SUN Yan-rui, ZHOU Hai-tao. Evolution of dislocation cells during plastic deformation [J]. Trans Nonferrous Met Soc China, 2005, 15(1): 149-151.
[13] GENKI S, AZNJI H T, ERENCE G L. Grain refinement and superplasticity in an alloy processed by high-pressure torsion [J]. Materials Science and Engineering, 2005, A393: 344-351.
[14] SCHAFLER E, PIPPAN R. Effect of thermal treatment on microstructure in high pressure torsion(HPT) deformed nickel [J]. Materials Science and Engineering A, 2004, 387/389: 799-804.
[15] XU Xiao-chang, LIU Zhi-yi, ZHANG Kun, ZHENG Qing-chun. Re-ageing behavior of retrogressive precipitated phase caused by severe plastic deformation [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(5): 759-764. (in Chinese)
[16] CHEN Jing-rong. The solid transformation in metals and alloys [M]. Beijing: Metallurgical Industry Press, 1997. (in Chinese)
Foundation item: Project(50571069) supported by the National Natural Science Foundation of China; Project(05JJ40005) supported by the Natural Science Foundation of Hunan Province, China
Corresponding author: XU Xiao-chang; Tel: +86-731-8836011; E-mail: xxc12@126.com
Abstract: The evolution of precipitates and hardness changes in Al-Cu alloys during equal-channel angular pressing(ECAP) at room temperature were investigated by hardness measurement, X-ray diffraction analysis and transmission electron microscopy. The results show that with the increase of the total equivalent strain during ECAP from 0 to 8.4, the hardness of specimens with metastable θ″ phase increases first and decreases in later period. The hardness increases successively in specimens containing metastable θ′ phase and equilibrium θ phase. It is believed that the evolutions of hardness are related to the mechanism of re-dissolution of precipitates. A critical nuclei size concept is provided to express the mechanism of such re-dissolution of three precipitates in Al-Cu alloys.


