Trans. Nonferrous Met. Soc. China 23(2013) 231-236
Interaction mechanism of Cu2+, Fe3+ ions and extracellular polymeric substances during bioleaching chalcopyrite by Acidithiobacillus ferrooxidans ATCC2370
Run-lan YU, Jing LIU, An CHEN, Dai-li ZHONG, Qian LI, Wen-qing QIN, Guan-zhou QIU, Guo-hua GU
Key Laboratory of Biometallurgy, Ministry of Education, School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 7 December 2011; accepted 16 April 2012
Abstract:
The extracellular polymeric substances (EPS) of Acidithiobacillus ferrooxidans ATCC 23270, and iron and copper enclosed in EPS were extracted by ultrasonication and centrifugation methods to determine the interaction mechanism of Cu2+, Fe3+ and EPS during bioleaching chalcopyrite. Generally, Cu2+ ions can stimulate bacteria to produce more EPS than Fe3+ ions. The mass ratio of Fe3+/Cu2+ enclosed in EPS decreased gradually from about 4:1 to about 2:1 when the concentration of Cu2+ ions increased from 0.01 to 0.04 mol/L. The amount of iron and copper bound together by EPS in ferrous-free 9K medium containing 1% chalcopyrite was about 2 times of that in 9K medium containing 0.04 mol/L Cu2+ ions. It was inferred that the EPS with jarosites on the surface of chalcopyrite gradually acted as a weak diffusion barrier for Cu2+, Fe3+ ions transference during bioleaching chalcopyrite.
Key words:
extracellular polymeric substances; iron ions; copper ions; bioleaching; chalcopyrite;
1 Introduction
The bioleaching of secondary copper sulphides has been applied successfully and commercially. However, the bioleaching of chalcopyrite, the most abundant copper mineral in nature, is still a major challenge due to slow kinetics and poor extraction [1,2]. Many researchers have focused on investigating the leaching kinetics, interfacial action mechanism, microorganism ecology and mineralogy during bioleaching of chalcopyrite [3,4]. Generally speaking, the reaction products [4,5], such as elemental sulphur, polysulphides and jarosites, cover the mineral surface to form a tight passivation layer which inhibits the bio-extraction of copper from chalcopyrite [1,2]. There is also a suitable potential range during bioleaching chalcopyrite. Some methods such as controlling pH and redox potential, using moderately thermophilic and/or thermophilic bacteria, can improve the bioleaching of chalcopyrite [6-8].
The extracellular polymeric substances (EPS) of microorganisms seem to play an important role in the bioleaching of sulphide minerals [9,10]. It is increasingly accepted that EPS mediates the attachment of microorganisms to mineral surfaces. Sulfide minerals are dissolved by “indirect mechanism” and “EPS contact leaching mechanism” [11]. Generally, the EPS of microorganisms consists of neutral sugars and lipids [12]. In the leaching environments of extremely acidic pH, many sulphide minerals have a net negative charge, at the same time, the EPS may facilitate the Fe3+ concentration by complexation through uronic acids or other residues, resulting in a net positive charge to cells. Therefore, the attachment of a positively charged bacterium to a negatively charged surface is due to electrostatic forces [13,14]. After the initial attachment of cells, the biofilm develops within a few days, covering the mineral surface with cells embedded in a continuous EPS layer [15-17]. The EPS containing iron(III) ions comprises a reaction space, in which the dissolution processes take place [18]. The behavior of attached bacteria is very dependent on the Fe3+/Fe2+ ratio in the EPS layer, which is, in turn, very dependent upon the redox potential in solution and the concentration of soluble iron [19-22]. Bacterial cells adapt the chemical composition of their exopolymers to the substrate. In contrast to the cells of A. ferrooxidans grown on sulphur, the cells grown on pyrite or iron(II) sulphate incorporate uronic acids and ferric ion in their EPS [12,23,24]. Reports on the amount of EPS produced are variable [12,17], but this may be due to the experimental differences such as the differences of bacterial strains and the time of incubation. At present, these studies on EPS in bioleaching have mainly focused on pyrite, the influence of Fe3+ ions and EPS components. Recently, ZENG et al [25] have reported the investigation of the EPS components and functions during the bioleaching of chalcopyrite. But the EPS interface action mechanism is sophisticated and still not fully understood. Particularly, there are few investigations on the effect of Cu2+ ions on EPS and the relationship among Cu2+ ions, Fe3+ ions and EPS during bioleaching chalcopyrite.
In this work, a comparative investigation method was employed to investigate the interaction mechanism of Cu2+, Fe3+ ions and EPS during bioleaching chalcopyrite. Acidithiobacillus ferrooxidans (ATCC23270) were cultured in 9K medium (A), 9K medium containing different concentrations of Cu2+ ions (B), ferrous-free 9K media containing 1% (w/v) chalcopyrite (C), respectively, and then, their EPS was extracted in different growth phases. They were analyzed by FTIR, and the amounts of EPS, iron and copper enclosed in EPS were determined. The interaction mechanism of Cu2+, Fe3+ and EPS during bioleaching chalcopyrite was discussed.
2 Experimental
2.1 Bacterium and culture medium
The bacteria used in these experiments were Acidithiobacillus ferrooxidans ATCC23270 conserved in our laboratory.
A. ferrooxidans ATCC23270 cells were incubated in 9K medium with an initial pH of 2.0 at 30 °C. The 9K medium contained: 3 g/L (NH4)2SO4, 0.1 g/L KCl, 0.5 g/L K2HPO4, 0.5 g/L MgSO4·7H2O, 0.01 g/L Ca(NO3)2, 30 g/L FeSO4·7H2O.
2.2 Chalcopyrite ore
The floatation concentrate of chalcopyrite was from Dexing Copper Mine in Jiangxi Province, China. Mineral phase of the concentrate analyzed by X-ray diffraction spectrometry revealed that its principal minerals are 80% chalcopyrite, 5% pyrite and 5% quartz and 10% other minerals.
2.3 Bacterial culture and EPS extraction
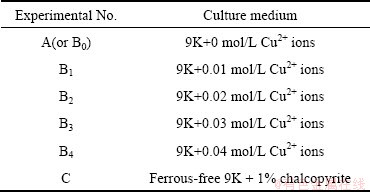
The experiments were carried out in 500 mL Erlenmeyer flasks containing 200 mL culture medium, as shown in Table 1. These experiments were divided into three groups: (A) the ferrous ions were added as bacterial energy source; (Bi) on the basis of the first group, different amounts of CuSO4·5H2O were added in order to form a gradient of copper ions; (C) on the basis of the first group, the chalcopyrite concentrate was added with 1% (w/v) pulp density instead of iron as the energy source. Prior to experiments, the concentrate was treated with sulfuric acid (pH=1) for 1 h at the room temperature and dried overnight at 60 °C. These flasks were inoculated with the A. ferrooxidans cells. The initial bacterial density was about 1.0×106 cell/mL. All the experiments were carried out in an orbital shaker with 170 r/min at 30 °C. Each set of experiments was carried out in duplicate.
Table 1 Initial concentration of Cu2+ ions in each flask
Ultrasonication (JY92-II ultrasonator) associated with centrifugation (J-E Avanti centrifuge, Beckman Coulter Inc.) was employed for extracting EPS [26]. Contamination by the membrane fragments of possibly damaged cells was checked by the detection of 2-keto-3-deoxyoctonate (KDO) and DNA. KDO is part of the cell membrane in Gram negative bacteria, and it can be used as a marker for their membrane compound contamination. Furthermore, the detection of DNA concentration could give more information to investigate the breaking extent of bacteria. Therefore, low levels of both DNA and KDO could indicate that the extracted EPS was not contaminated by a significant amount of intracellular materials in these experiments [12,27]. The investigation revealed that the rupture degree of the cells depends on the frequency and intensity of ultrasonication. As long as the operation conditions are controlled properly, not only can EPS be stripped from the cells completely, but also the damage degree of cells is as small as possible [28]. In these experiments, prior to ultrasonication, the samples of bacteria were suspended in ferrous-free 9 K medium for 1 h at 30 °C and shook at the same time, and then stripped by ultrasonication. During the process, the sample must be put in ice bath. At the same time, the ultrasonic power, interval and time were controlled at 80 W, 2 s and 9 min, respectively. At last, the sample was centrifugalized at 12000 r/min, 4 °C for 20 min, and the supernatant was the EPS solution.
2.4 Analysis methods
The bacterial density in each solution was determined by direct counting using an optical microscope with a Petroff–Hausser counting chamber. The properties of the functional groups of EPS were analyzed by Fourier transform infrared spectrometry (FTIR). The total iron as well as Cu2+ ions adsorbed by EPS or in EPS was determined by BSH9-D atomic absorption spectrometer.
The total concentration of the polysaccharides of EPS was determined using phenol-sulfuric acid method through measuring the absorbance at 490 nm by UV-9200 spectrophotometer. The content of total polysaccharides of EPS was determined with glucose as control. According to the standard curve, the content of the total polysaccharides can be calculated by absorbance of EPS solution. The content of total polysaccharides was used to evaluate the amount of EPS, because it is from the hydrolysis of EPS of the A. ferrooxidans cells.
3 Results and discussion
3.1 FTIR analysis of EPS
FTIR spectra of the EPS of A. ferrooxidans cells in 9K media were obtained at exponential growth phase (the 4th day), stable phase (the 6th day) and decline phase (the 12th day), respectively, as shown in Fig. 1. As shown in these FTIR spectra, the bands at around 3320 cm-1, 2940 cm-1 and 990-1250 cm-1, common to all polysaccharides, represent O—H stretching, C—H stretching of the —CH2 group and saccharide, respec- tively. A stronger band of EPS at around 1590-1630 cm-1 could be attributed to the ring stretching of galactose and mannose. It could also be attributed to the assymmetric stretching of —COO-. And the small band at about 1410 cm-1 represents the symmetric stretching of —COO-. The strong band at about 1720 cm-1 is attributed to acetyl groups, referring to the presence of acyl groups of acid groups in the EPS. As a result, it was confirmed that the main components of the EPS are polysaccharides and lipids. Comparing the FTIR spectra of EPS at three different stages, it could be found that they are nearly identical, except for their intensities of the bands which are approximatively proportional to the amount of EPS in the samples.
In the culture media 9K plus different concentrations of Cu2+ ions, namely, Bi (i=1, 2, 3, 4) media, the FTIR spectra of EPS in stationary phase are shown in Fig. 2. It is found that the spectra in Fig. 2 are nearly similar to that in Fig. 1.
So, it could be concluded that the superficial functional groups of the EPS produced by the bacteria had no essential change, but the content of some compositions of the EPS had some changes with the leaching time and media varying.
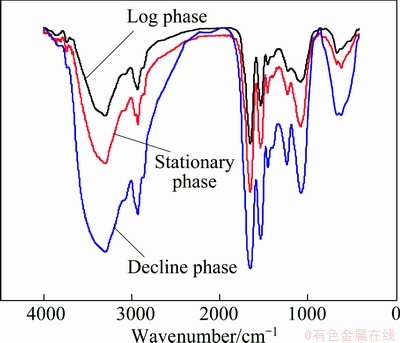
Fig. 1 FTIR spectra of EPS in different growth phases in medium A
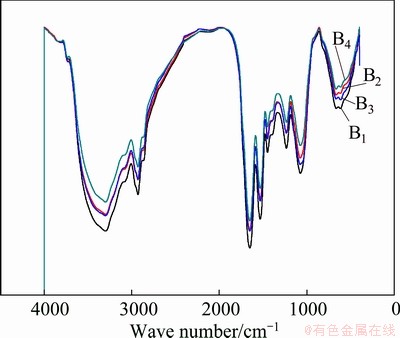
Fig. 2 FTIR spectra of EPS in stationary phases in media Bi
3.2 Evolvement of EPS amount with bioleaching time and different media
In 9K medium, the change of the extracted EPS amount with culturing time shown in Fig. 3 (Experiment A) was nearly in accordance with the typical growth curve of the microorganism. With the growth of the cells, more and more Fe2+ ions were bio-oxidized into Fe3+ ions, and leaching environment for the cells became deterioration gradually so that the cells had to produce more and more EPS against their disadvantageous environment, such as high ratio of Fe3+/Fe2+. After absolutely most of Fe2+ ions were oxidized into Fe3+ ions, the leaching environment for the cells became very adverse, bacteria came to death phase, and the extracted EPS amount decreased. The maximal value of the extracted EPS was 53 mg per 1012 cells.
In 9K medium containing different concentrations of Cu2+ ions, at first the extracted EPS also increased rapidly, and then decreased slowly with the increase of culturing time, as seen from Fig. 3 (Experiment C). Obviously, the extracted EPS was much more from medium C than from medium A. The possible reason is that there are a lot of Cu2+ ions in medium C during bioleaching chalcopyrite.
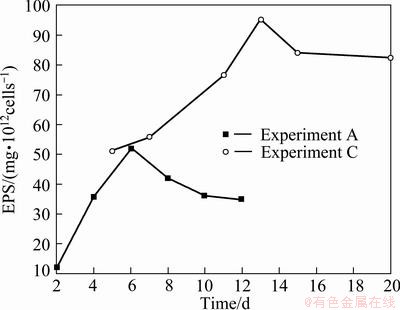
Fig. 3 Extracted EPS amonnts with culturing time and medium
Figure 4 shows a comparative diagram of the extracted EPS amount in stationary phase in different media. It was found that the extracted EPS from media Bi was always higher than that from 9K medium (medium, B0 namely, medium A). It increased with the concentration of Cu2+ ions added. When the concentration of Cu2+ ions was lower than 0.02 mol/L (the tolerant value of the cells) [29], the extracted EPS slightly increased from medium B0 to B2. But when the concentration of Cu2+ ions was more than 0.02 mol/L, it increased rapidly, up to 172 mg/1012cells at the concentration of 0.04 mol/L Cu2+ ions. The extracted EPS from chalcopyrite medium was between the values from 0.03 mol/L Cu2+ and 0.04 mol/L Cu2+ solution. Obviously, the amount of EPS in medium B4 was about three times of that in medium A, and the amount of EPS in medium C was about two times of that in medium A. It was concluded that Cu2+ ions could stimulate bacteria to produce more EPS than Fe3+ ions, especially, when it was over the bacterial tolerable concentration.
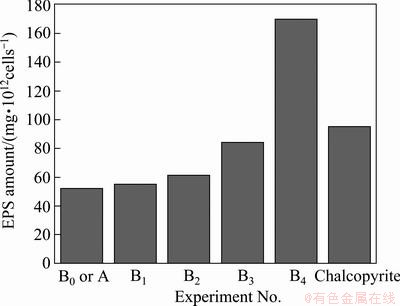
Fig. 4 Extracted EPS amount in stationary phases in different media
As mentioned above, it was found that the superficial functional groups of the EPS produced by the bacteria had no essential change but the content of some compositions of the EPS had some changes with culture time and media, and the EPS amount in medium C was much more than that in medium A. It could be deduced that bacteria resist these disadvantageous solution environments, such as high ratio of Fe3+/Fe2+ and the increase of the concentration of Cu2+ through excreting more EPS rather than through changing EPS essence during bioleaching of chalcopyrite.
3.3 Evolvement of copper and iron amount enclosed by EPS with leaching time and different media
As seen from Fig. 5, both copper and iron enclosed in EPS gradually increased to their maximal values, and then declined very slowly. Moreover, iron enclosed in EPS was always more than copper.
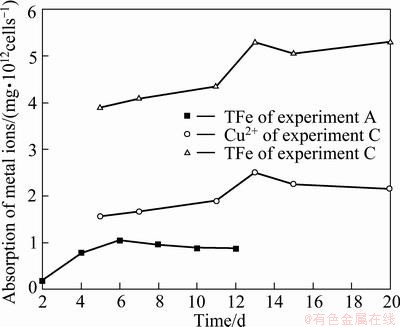
Fig. 5 Iron and copper amount enclosed in EPS layer in medium C with time
Figure 6 shows a comparative diagram of the amounts of iron and copper enclosed in EPS layer in media A, Bi and C in the stationary phases, respectively. In 9K medium, the maximal value of iron enclosed in EPS only was 1.08 mg/1012cells in the stationary phase. The values of iron and copper enclosed in EPS increased with the addition of Cu2+ ions, and the maximal values of iron and copper in medium C were about 5.3 and 2.6 mg/1012cells, respectively. In addition, it was found that, the mass ratio of Fe3+/Cu2+ decreased gradually from about 4:1 to about 2:1 when the media changed from B1 to B4, and the ratio of Fe3+/Cu2+ in medium B4 was almost the same as that in medium C as in Fig. 6. There were three kinds of imaginable causes: 1) the cells produced more EPS to combine with Cu2+; 2) these restrained bacteria by high concentration of Cu2+ ions in EPS space such as in medium B4 could oxidize Fe2+ ions to form jarosites or iron precipitations in the EPS space so that ion channels of the EPS space were gradually blocked and Cu2+ ions entered the EPS space through the ion channels were enclosed in the EPS space in medium B4; and 3) both of A and B mechanisms. The cause A seems to be correct from Fig. 4, but it could not explain why the iron enclosed in EPS also increased with the addition of Cu2+ ions and why the mass ratio of Fe3+/Cu2+ in medium B4 was almost the same as that in medium C according to Fig. 6.
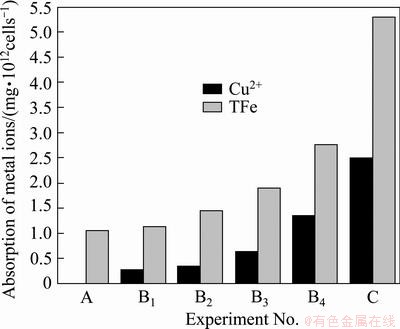
Fig. 6 Iron and copper amount enclosed in EPS in stationary phases in media A, Bi and C
It was interesting that the extracted EPS from medium B4 possessed the maximal value among experiments A, Bi and C in their stationary phases, the next was from ferrous-free 9K medium containing chalcopyrite (medium C) as seen from Fig. 4, but their maximal values of iron and copper enclosed in EPS were obtained from medium C as seen from Fig. 6. The amounts of iron and copper enclosed in EPS in medium C were about 2 times of that in media B4. It is indicated that interaction mechanism of EPS, iron and Cu2+ was different between solution phases (namely, media A and Bi) and medium C. Obviously, these differences were consanguineously relative with the attachment of EPS layer on chalcopyrite. In media A and Bi, bacterium only suspended in 9K solution and had to produce more EPS to incorporate Fe3+ or Cu2+ ions in the EPS layer by complexation through uronic acids or other residues [12,23]. However, in medium C, bacterial EPS layer with positive charge in the solution could rapidly attach to the surface of chalcopyrite, and the cells produced more EPS to prevent the environment of EPS space deteriorating, such as high ratio of Fe3+/Fe2+ and the increase of the concentration of Cu2+ during bioleaching chalcopyrite. It was testified that EPS could flocculate iron deposition such as jarosites and iron hydrate precipitations, and the jarosites formed by bio-oxidized Fe3+ ions were more easy to adhere to the outside and inside of EPS space, and the biofilm with a lot of jarosites was a weak diffusion barrier for ions transference [21,22,30]. So in media A and Bi, although jarosites and iron hydrate precipitations also adhered to outside of EPS space, iron and copper were not enclosed very seriously because bacterium only suspended oneself in 9K solution and the flask was shaking. The concentration of Cu2+ ions was much higher in medium B4 than in medium C, but the amounts of iron and copper bound together by EPS in medium C were about 2 times of that in medium B4. It was only explained that the EPS with jarosites on the surface of chalcopyrite acted as a weak diffusion barrier for ions transference so that more Cu2+ ions and iron matters from bio-oxidizing maybe were enclosed in EPS space.
4 Conclusions
1) Cu2+ ions can stimulate bacteria to produce more EPS than Fe3+ ions.
2) In EPS space, the mass ratio of Fe3+/Cu2+ decreased gradually from about 4:1 to about 2:1 when the concentration of Cu2+ ions increased from 0.01 to 0.04 mol/L.
3) The amounts of iron and copper bound together by EPS in medium C were about 2 times of that in medium B4. It was inferred that EPS with jarosites or iron precipitations on the surface of chalcopyrite acted as ion diffusion barrier in the end of bioleaching chalcopyrite.
References
[1] WATLING H R. The bioleaching of sulfide minerals with emphasis on copper sulphides—A review [J]. Hydrometallurgy, 2006, 84: 81-108.
[2] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part I: General aspects [J]. Hydrometallurgy, 2008, 93: 81-87.
[3] BRIERLEY J A. Acidophilic thermophilic archaebacteria: Potential application for metals recovery [J]. FEMS Microbiology Letters, 1990, 75(2-3): 287-292.
[4] GOMEZ E, BALLESTER A, BLAZQUEZ M L, GONZALEZ F. Silver-catalysed bioleaching of a chalcopyrite concentrate with mixed cultures of moderately thermophilic microorganisms [J]. Hydrometallurgy, 1999, 51(1): 17-46.
[5] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F, BALLESTER A. Passivation of chalcopyrite during its chemical leaching with ferric ion at 68 °C [J]. Minerals Engineering, 2009, 22(3): 229-235.
[6] WANG Li, WEN Jian-kang, LIU Me-lin. Biological effect of EPS on metal sulfides [J]. Metal Mine, 2008(11): 38-42. (in Chinese)
[7] PRADHAN N, NATHSARMA K C, SRINIVASA R K, SUKLA L B, MISHRA B K. Heap bioleaching of chalcopyrite: A review [J]. Minerals Engineering, 2008, 21: 355-365.
[8] JAVIER V, RYOICHI Y, CHIHIRO I. Effect of pH reduction and ferric ion addition on the leaching of chalcopyrite at thermophilic temperatures [J]. Hydrometallurgy, 2009, 96: 62-71.
[9] HIROYOSHI N, KITAGAWA H, TSUNEKAWA M. Effect of solution composition on the optimum redox potential for chalcopyrite leaching in sulfuric acid solutions[J]. Hydrometallurgy, 2008, 91: 144-149.
[10] WANG Li, WEN Jian-kang. Research on the leaching effect of extracellular polymers of acidithiobacillus ferrooxidans [J]. Metal Mine, 2011(7): 86-89. (in Chinese)
[11] SAND W, GEHRKE H. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacical processes involving iorn(III) ions and acidophilic bacteria [J]. Research in Microbiology, 2006, 157: 49-56.
[12] GEHRKE T, TELEGDI J, THIERRY D, SAND W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching [J]. Applied and Environmental Microbiology, 1998, 64(7): 2743-2747.
[13] BLAKE R, LYLES M M, SIMMONS R. Morphological and physical aspects of attachment of Thiobacillus ferrooxidans to pyrite and sulphur [C]//VARGAS T, JEREZ C A, WIERTZ J V, TOLEDO H. Biohydrometallurgical Processing, Vol. 1. Santiago: University of Chile, 1995: 13-22.
[14] KAR N, DASGUPTA A.The possible role of surface charge in membrane organization in an acidophile [J]. Indian Journal of Biochemistry and Biophysics, 1996, 33: 398-402.
[15] GEHRKE T, HALLMANN R, SAND W. Importance of exopolymers from thiobacillus ferrooxidans and leptospirillum ferrooxidans for bioleaching [C]//VARGAS T, JEREZ C A, WIERTZ J V, TOLEDO H. Biohydrometallurgical Processing, Vol. 1. Santiago: University of Chile, 1995: 1-11.
[16] ESCOBAR B, HUERTA G, RUBIO J. Influence of lipopolysaccharides on the attachment of Thiobacillus ferrooxidans to minerals [J]. World Journal of Microbiology and Biotechnology, 1997, 13: 593-594.
[17] POGLIANI C, DONATI E. The role of exopolymers in the bioleaching of a non-ferrous metal sulphide [J]. Journal of Industrial Microbiology and Biotechnology, 1999, 22: 88-92.
[18] KINZLER K, GEHRKEA T, TELEGDIB J, SAND W. Bioleaching- A result of interfacial processes caused by extracellular polymeric substances [J]. Hydrometallurgy, 2003, 71: 83-88.
[19] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion (Part II): Effect of redox potential [J]. Hydrometallurgy, 2008, 93: 88-96.
[20] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion (Part IV): The role of redox potential in the presence of mesophilic and thermophilic bacteria [J]. Hydrometallurgy, 2008, 93: 106-115.
[21] YU Run-lan, TAN Jian-xi, YANG Peng, SUN Jing, OUYANG Xiong-jing, DAI Yun-jie. EPS-contact-leaching mechanism of chalcopyrite concentrates by A. Ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 1427-1432.
[22] YU Run-lan, TAN Jian-xi, GU Guo-hua, HU Yue-hua, QIU Guan-zhou. Mechanism of bioleaching chalcopyrite by acidithiobacillus ferrooxidans in agar simulated extracelluar polymeric substances media [J]. Journal of Central South University of Technology, 2010, 17(1): 56-51.
[23] AGATE A D, KORCZYNSKI M S, LANDGREN D G. Extracellular complex from the culture filtrate of Thiobacillus ferrooxidans [J]. Microbiol, 1969, 15(3): 259-264.
[24] SKLODOWSKA A, MATLAKOWSKA R. Influence of exopolymers produced by bacterial cells on hydrophobicity of substrate surface [J]. Biotechnology Techniques, 1997, 11: 837-840.
[25] ZENG Wei-min, QIU Guan-zhou, ZHOU Hong-bo, LIU Xue-duan, CHEN Miao, CHAO Wei-liang, ZHANG Cheng-gui, PENG Juan-hua. Characterization of extracellular polymeric substances extracted during the bioleaching of chalcopyrite concentrate [J]. Hydrometallurgy, 2010, 100: 177-180.
[26] COMTE S, GUIBAUD G, BAUDU M. Relations between extraction protocols of the activated sludge extracellular polymeric substances (EPS) and EPS complexation properties (Part I): Comparison of the efficiency of eight EPS extraction properties [J]. Enzyme and Microbial Technology, 2006, 38: 237-245.
[27] KARKHANIS Y D, ZELTNER J Y, JACKSON J J, CARLO D J. A New and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of gram-negative bacteria [J]. Analytical Biochemistry, 1978, 85: 595-601.
[28] YU Run-lan, OU Yang, TAN Jian-xi, WU Fa-deng, SUN Jing, MIAO Lei, ZHONG Dai-li. Effect of EPS on adhesion of Acidithiobacillus ferrooxidans on chalcopyrite and pyrite mineral surfaces [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 407-412.
[29] LI Hong-mei, KE Jia-jun. Effect of Cu2+ on the growth and activity of thiobacillus ferrooxidans [J]. Gold, 2000, 21: 27-29. (in Chinese)
[30] YU Run-lan, ZHONG Dai-li, MIAO Lei, WU Fa-deng, QIU Guan-zhou, GU Guo-hua. Relationship and effect of redox potential, jarosites and extracellular polymeric substances in bioleaching chalcopyrite by acidithiobacillus ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(7): 1634-1640.
嗜酸氧化亚铁硫杆菌(ATCC 23270)浸出黄铜矿过程中的EPS、Cu2+和Fe3+的相互作用机制
余润兰, 刘 晶,陈 安, 钟代立, 李 乾, 覃文庆,邱冠周, 顾帼华
中南大学 资源加工与生物工程学院,生物冶金教育部重点实验室,长沙 410083
摘 要:采用超声-离心方法提取嗜酸氧化亚铁硫杆菌(ATCC 23270)胞外多聚物 (EPS)、EPS中的Cu2+、Fe3+离子,研究生物浸出黄铜矿过程中 Cu2+、Fe3+ 和EPS 的相互作用机制。结果表明:与Fe3+离子相比,Cu2+ 离子可刺激细菌产生更多的EPS;当Cu2+离子浓度从 0.01 mol/L 增加到 0.04 mol/L时,EPS中 Fe3+/Cu2+ 质量比从4:1降低到2:1;从1%黄铜矿的无铁9K介质中提取的EPS中铜铁含量是从含0.04 mol/L Cu2+ 离子的9K介质中提取的量的2倍。在生物浸出黄铜矿过程中,黄铜矿表面结合黄铁钾钒的EPS层减弱了Cu2+、Fe3+ 离子的迁移,逐渐成为离子扩散壁垒。
关键词:胞外多聚物;铁离子;铜离子;生物浸出;黄铜矿
(Edited by Sai-qian YUAN)
Foundation item: Project (50621063) supported by the National Natural Science Foundation of China; Project (2010CB630903) supported by the National Basic Research Program of China
Corresponding author: Run-lan YU; Tel: +86-731-88877472; E-mail: yrl715@sina.com
DOI: 10.1016/S1003-6326(13)62450-4
Abstract: The extracellular polymeric substances (EPS) of Acidithiobacillus ferrooxidans ATCC 23270, and iron and copper enclosed in EPS were extracted by ultrasonication and centrifugation methods to determine the interaction mechanism of Cu2+, Fe3+ and EPS during bioleaching chalcopyrite. Generally, Cu2+ ions can stimulate bacteria to produce more EPS than Fe3+ ions. The mass ratio of Fe3+/Cu2+ enclosed in EPS decreased gradually from about 4:1 to about 2:1 when the concentration of Cu2+ ions increased from 0.01 to 0.04 mol/L. The amount of iron and copper bound together by EPS in ferrous-free 9K medium containing 1% chalcopyrite was about 2 times of that in 9K medium containing 0.04 mol/L Cu2+ ions. It was inferred that the EPS with jarosites on the surface of chalcopyrite gradually acted as a weak diffusion barrier for Cu2+, Fe3+ ions transference during bioleaching chalcopyrite.


