J. Cent. South Univ. Technol. (2010) 17: 218-222
DOI: 10.1007/s11771-010-0033-3 ![]()
Photogenerated carrier transfer mechanism and photocatalysis properties of TiO2 sensitized by Zn(Ⅱ) phthalocyanine
LI Li(李丽)1, 2, XIN Bai-fu(辛柏福)1, 2
1. Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education,
Heilongjiang University, Harbin 150080, China
2. School of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, China? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract:
The Zn(Ⅱ) phthalocyanine sensitized TiO2 (ZnPc-TiO2) nanoparticles were prepared by hydrothermal method via impregnation with ZnPc. The as-prepared photocatalysts were characterized by X-ray diffractometry (XRD) and diffuse reflectance spectroscopy (DRS), and the surface photovoltage spectroscopy (SPS) and photocatalytic degradation of rhodamine B (RhB) were studied under illuminating. The experimental results indicate that TiO2 sensitized by ZnPc extends its absorption band into the visible region effectively, and the sensitized TiO2 has higher activity than TiO2 (Degussa P-25) under the simulated solar light and the visible light. Based on the DRS and SPS results, the mechanism about the photogenerated carrier transfer between TiO2 and ZnPc is proposed. At a lower ZnPc content (≤0.20 ?mol/g), ZnPc monomer acts as the electron donor, which provides the photoinduced electrons to the conduction band of TiO2. These photoinduced electrons can transfer to molecular oxygen (O2), leading to the formation of active species, such as superoxide/hydroxide radicals and singlet oxygen, which is beneficial to the photocatalytic reaction. While at a higher ZnPc content (>0.20 ?mol/g), the formation of ZnPc dimer results in the decrease of photocatalytic activities of ZnPc-TiO2 photocatalyst.
Key words:
1 Introduction
The utilization of TiO2 as a catalyst for photodegradation of organic pollutants in water is a relevant topic in view of a possible application in economically advantageous and environmental friendly process [1-2]. However, these photocatalysts are generally materials with a wide band gap, thus they can only absorb a small fraction of visible light. Coupled semiconductor systems, such as organic functional dye/TiO2, which can extend its absorption band into the visible region, are used to overcome this defect [3-5]. Phthalocyanine was found to have many applications. This group of compounds have a strong absorption in the visible/near-IR region [6], a good photostability and a low probability of desorption from the semiconductor surface once adsorbed, which make them interesting candidates [7-8].
Some photocatalysts were reported, such as iron, copper, cobalt and metal-free phthalocyanine with carboxylic acid or sulfonic acid groups and without any functional group, which were efficient in photocatalytic reactions used for the degradation of organic pollutants in water [9-11]. In these research reports, the mechanisms of TiO2 photocatalysis reactions are almost the same, ILIEV and TOMOVA [11] thought that the high photocatalytic activity of the sample is explained by the realization of an electron transfer from the conduction band of the excited phthalocyanine particle to the conduction band of TiO2 support. The increase of the quantum yield of the redox process results from the additional formation of superoxide radical on the TiO2 conduction band [11].
These reports, however, mainly focus on the investigation of reaction mechanism via detection of intermediate active species, which lack the sufficient evidences in the photogenerated charge carrier transfer. In this work, the mechanism about the photogenerated carrier transfer between TiO2 and ZnPc was studied using diffuse reflectance spectroscopy (DRS) and surface photovoltage spectroscopy (SPS).
2 Experimental
2.1 Reagents
Zinc (Ⅱ) phthalocyanine was purchased from Acros Organics (USA) with a purity of 98%. Titanium dioxide (TiO2, Degussa P-25) was used as a comparison photocatalyst. Degussa P-25 consisted of 75% anatase and 25% rutile with a specific BET-surface area of 50 m2/g and a primary particle size of 20 nm. Other reagents were all analytical grade compounds and were used without any further purification.
2.2 Preparation of ZnPc-TiO2 photocatalyst
The synthesis of TiO2 particles followed the procedure reported by HAO et al [12] and was slightly modified. A mixture of 40 mL tetrabutyl titanate and 10 mL isopropanol was slowly added into 250 mL deionized H2O containing 2 mL nitric acid solution at a rate of 1-2 drop/s under cooling and stirring. After refluxing at 80 ℃ for 4 h, the reactor was cooled to room temperature, and the pH value of the suspension was adjusted to 10 using NH3×H2O. The suspension was then transferred into a stainless steel Teflon-lined autoclave of 50 mL capacity and heated at 180 ℃ for 8 h without shaking or stirring during the heating. After the autoclave was naturally cooled to room temperature, the obtained sample was sequentially washed with deionized water and absolute ethanol several times. Then, anatase phase TiO2 nanoparticles with white color were obtained.
As-prepared TiO2 nanoparticles were impregnated with various amounts of ZnPc (0, 0.05, 0.15, 0.20, 0.30, and 0.40 ?mol/g TiO2) dissolved in N-methyl-2- pyrrolidone for 24 h in the dark, dried at 100 ℃, and ground to obtain ZnPc-TiO2 nanoparticles.
2.3 Characterization of samples
The surface photovoltage spectroscopy (SPS) instrument was assembled at Jilin University, China, and monochromatic light was obtained by passing light from a 500 W xenon lamp (CHF-XQ500W, China) through a double prism monochromator (SBP300, China). The slit widths of entrance and exit were 2 and 1 mm, respectively. A lock-in amplifier (SR 830, USA), synchronized with a light chopper (SR 540, USA), was employed to amplify the photovoltage signal. The powder sample was sandwiched between two ITO glass electrodes. The DRS patterns were obtained in the wavelength range of 300-700 nm using a Shimadzu UV-2550 UV-Vis spectrophotometer equipped with the integrating sphere accessory for diffuse reflectance spectra. BaSO4 was used as a reference.
2.4 Evaluation of photocatalytic activity of ZnPc-TiO2
The photocatalytic degradation of rhodamine B (RhB) over ZnPc-TiO2 was carried out in a home-built reactor. A 160 W high-pressure mercury lamp with and without a 410 nm cutoff filter was used as the visible light source and the simulated solar light source, respectively. In each run, 0.1 g ZnPc-TiO2 catalyst was added into 20 mL RhB solution of 10 mg/L. After the mixture was premixed in the dark for 20 min, the light was turned on to initiate the reaction for 30 min. A Shimadzu UV-2550 UV-Vis spectrophotometer was used to determine the concentration of RhB solution before and after photocatalytic degradation.
3 Results and discussion
3.1 DRS analysis
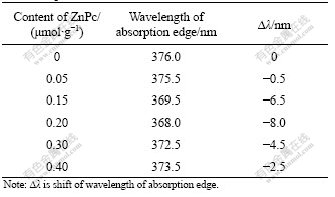
Fig.1 shows DRS patterns of pure TiO2 and ZnPc-TiO2 particles adsorbing different ZnPc contents. The absorption edge can be calculated by inflection point of UV spectra. Table 1 lists the shift values of UV spectra absorption edge. From Fig.1 and Table 1, a novel blue shift of TiO2 absorption edge can be found, which gradually increases with the increase in the content of ZnPc from 0 up to 0.20 ?mol/g (vs TiO2), and then drops down.
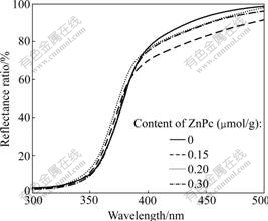
Fig.1 DRS patterns of ZnPc-TiO2 photocatalysts
Table 1 Shift values of UV spectrum absorption edge of ZnPc-TiO2 with different contents of sensitizer
3.2 SPS measurement
The surface photovoltage (SPV) method is a well- established contactless technique for the characterization of semiconductors. It can offer important information about semiconductor surface, interface, and bulk properties, mainly reflecting the carrier separation and transfer behavior with the aid of light [13].
The SPS principle can be explained by the top left corner of Fig.2. The absorbed photons induce the formation of free carriers by creating electron-hole pairs via band-to-band transitions. The photoinduced electrons may be transferred from the surface to the bulk, and the photogenerated holes can be moved to the surface under the built-in electric field. Thus, the surface potential is changed. The variational value of surface potential (ΔV) is the signal of SPS.
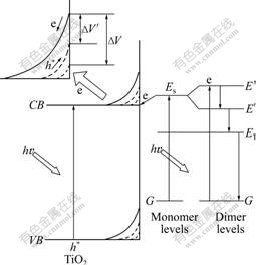
Fig.2 Schematic diagram of photogenerated carrier transfer mechanism between TiO2 and ZnPc (CB and VB are energy levels of conduction band and valence band, respectively; G is energy level of ground state; Es is singlet energy level; ET is triplet energy level; E′ and E″ are singlet band split energy levels, respectively)
Fig.3 shows the SPS spectra of pure TiO2 and ZnPc-TiO2 with various ZnPc contents. It can be found that the SPS spectrum of pure TiO2 is different from that of sensitized TiO2. The SPS intensity decreases with the increase in sensitizer ZnPc content from 0 up to 0.20 ?mol/g, and then increases.
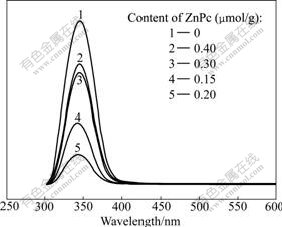
Fig.3 SPS spectra of pure TiO2 and ZnPc-TiO2
3.3 Mechanism of photoinduced carrier transfer
The above experimental results indicate that the shift value of UV spectrum absorption edge and the intensity of SPS of ZnPc-TiO2 vary with the increase in the amounts of ZnPc adsorbed on the surface of TiO2 nanoparticles. The substrates are the same TiO2 nanoparticles. So, the fact derives from the variety of the amounts of ZnPc adsorbed on the surface of TiO2 nano- particles. ZnPc contains a planar configuration of π-conjugated Pc ring structure and tends to aggregate into dimer, so-called H-aggregate, which is commonly seen in many phthalocyanine systems [14]. In this work, the mechanism about the photogenerated carrier transfer between TiO2 and ZnPc is proposed based on the UV-Vis and SPS experimental results as well as the nature of ZnPc (shown in Fig.2). At a rather lower ZnPc content (≤0.20 ?mol/g), ZnPc molecules adsorbed on the surface of TiO2 may exist as the monomer form. Because Es, the energy level of the singlet of ZnPc, is located above ECB of TiO2, as shown in Fig.2. While the surface of ZnPc-TiO2 is irradiated by the light (hυ≥Eg), the photoexcited electrons of ZnPc can be easily transferred into the conduction band of TiO2 semiconductor, resulting in the increase of Eg of TiO2 and variational value of surface potential of TiO2 before and after the decrease of illumination, so the blue shift of absorption edge and descend of SPS intensity (i.e., ΔV′<ΔV) can be observed. At a higher ZnPc content (>0.20 ?mol/g), a fraction of ZnPc molecules adsorbed on the surface of TiO2 may aggregate to form ZnPc dimer [14]. The overlap of the intermolecular- conjugated electron orbital within ZnPc dimer leads to a band split. Because transitions from the ground state to the excited state E′ are forbidden while transitions from the ground state to the excited state E″ are allowed [14], electrons of the ground state can be directly excited to the excited state E″. Due to rapid internal conversion between singlet states, the excited electron in the excition state E″ transfers to the excition state E′ quickly. E′ is located under Ecb of TiO2 semiconductor, resulting in the fact that ZnPc dimer cannot provide photoinduced electrons to TiO2. Hence, the photoinduced electrons transferring from the excited state Es of ZnPc to the conductor band of TiO2 reduce, the blue shift may decrease and the intensity of SPS may increase with increasing ZnPc content.
3.4 Photocatalytic reactions
Fig.4 shows the photocatalytic degradation ratio curves of RhB vs content of ZnPc under simulated solar light. It can be found that the degradation ratio of RhB increases with the increase of the ZnPc content from 0 up to 0.20 ?mol/g, and then drops down. This fact can be explained by the mechanism of photoinduced carrier transfer between TiO2 and ZnPc mentioned above.
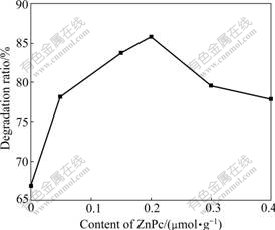
Fig.4 Photocatalytic degradation ratio of RhB vs content of ZnPc
At a lower ZnPc content (≤0.20 ?mol/g TiO2), ZnPc monomer acts as the electron donor, which provides the photoinduced electrons to the conduction band of TiO2. These photoinduced electrons can transfer to molecular oxygen (O2), leading to the formation of active specials, such as superoxide/hydroxide radicals and singlet oxygen in the system. These active elements can degrade the pollution [15]. The number of photoinduced electrons increases with the increase in ZnPc content, so the photocatalytic activity of photocatalysts increases with the increase of ZnPc content.
While at a higher ZnPc content (>0.20 ?mol/g), the formation of ZnPc dimer on the surface of TiO2 leads to the decrease of photoinduced electrons transferring from the excited state Es of ZnPc to the conductor band of TiO2, so photocatalytic activity is reduced. The photocatalysis experimental results are consistent with the conclusion of SPS and DRS test.
To examine the stability of as-prepared photocatalysts, the repetition experiments were carried out at the 0.2 μmol/g ZnPc-TiO2 under the same condition mentioned above. Fig.5 shows that the degradation ratio of RhB is stable during circulating photodegradation test.
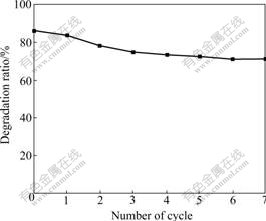
Fig.5 Stability of ZnPc-TiO2 photocatalyst
Fig.6 shows the photodegradation ratio of RhB to P-25 and 0.2 μmol/g ZnPc-TiO2 powders under different light sources. From Fig.6, it can be found that the sensitized TiO2 has a higher activity than P-25 under the simulated solar light and the visible light. Herein, ZnPc plays an important role in the system. The experimental results indicate that TiO2 sensitized by ZnPc extends its absorption band into the visible region effectively and has an excellent activity.
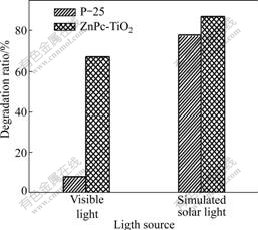
Fig.6 Comparison of photocatalytic activity of P-25 and 0.2 μmol/g ZnPc-TiO2
4 Conclusions
(1) ZnPc-TiO2 nanoparticles are prepared by the impregnation method. The absorption band of TiO2 extends into the visible region effectively. The photocatalytic activity is the highest when the ZnPc content on the surface of TiO2 is 0.20 ?mol/g, and the photocatalytic activity is higher than that of P-25 under the simulated solar light and the visible light.
(2) A novel blue shift of absorption edge and the SPS intensity varieties of TiO2 sensitized by ZnPc are observed. The mechanism about the photogenerated carrier transfer between TiO2 and ZnPc is proposed.
References
[1] Carlos A, Martínez H, Enric B. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review [J]. Applied Catalysis B: Environmental, 2009, 87(3/4): 105-145.
[2] Witte K D, Busuioc A M, Meynen V, Mertens M, Bilba N, Tendeloo V G, Cool P, Vansant E F. Influence of the synthesis parameters of TiO2-SBA-15 materials on the adsorption and photodegradation of rhodamine-6G [J]. Microporous and Mesoporous Materials, 2008, 110(1): 100-110.
[3] Han F, Kambala V S R, Srinivasan M, Rajarathnam D, Naidu R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review [J]. Applied Catalysis A: General, 2009, 359(1/2): 25-40.
[4] Butler M J, George W M, Schoonover R J, Sazanovich I V, Towrie M, Weinstein J A. Application of transient infrared and near infrared spectroscopy to transition metal complex excited states and intermediates [J]. Coordination Chemistry Reviews, 2007, 251(3/4): 492-514.
[5] Vinu R, Madras G. Synthesis and photoactivity of Pd substituted nano-TiO2 [J]. Journal of Molecular Catalysis A: Chemical, 2008, 291(1/2): 5-11.
[6] Xiao Qi, Si Zhi-chun, Yu Zhi-ming, Qiu Guan-zhou. Sol-gel auto-combustion synthesis of samarium-doped TiO2 nanoparticles and their photocatalytic activity under visible light irradiation [J]. Materials Science and Engineering B, 2007, 37(1/3): 189-194.
[7] Li Bin, Wang Li-duo, Kang Bo-nan, Wang Peng, Qiu Yong. Review of recent progress in solid-state dye-sensitized solar cells [J]. Solar Energy Materials and Solar Cells, 2006, 90(5): 549-573.
[8] Kiselev A, Mattson A, Andersson M, Palmqvist A E C, ?sterlund L. Adsorption and photocatalytic degradation of diisopropyl fluorophosphate and dimethyl methylphosphonate over dry and wet rutile TiO2 [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2006, 184(1/2): 125-134.
[9] Lhomme L, Brosillon S, Wolbert D. Photocatalytic degradation of pesticides in pure water and a commercial agricultural solution on TiO2 coated media [J]. Chemosphere, 2008, 70(3): 381-386.
[10] Evgenidou E, Fytianos K, Poulios I. Semiconductor- sensitized photodegradation of dichlorvos in water using TiO2 and ZnO as catalysts [J]. Applied Catalysis B: Environmental, 2005, 59(1/2): 81-89.
[11] Iliev V, Tomova D. Photocatalytic oxidation of sulfide ion catalyzed by phthalocyanine modified titania [J]. Catalysis Communications, 2002, 3(7): 287-292.
[12] Hao Y Z, Yang M Z, Yu C, Cai S M, Zhou G D. Photoelectrochemical studies on acid-doped polyaniline as sensitizer for TiO2 nanoporous film [J]. Solar Energy Materials and Solar Cells, 1998, 56 (1): 75-84.
[13] Xin B F, Jing L Q, Ren Z Y, wang b q, fu h g. Effects of simultaneously doped and deposited Ag on the photocatalytic activity and surface states of TiO2 [J]. Journal of Physical Chemistry B, 2005, 109: 2805-2809.
[14] Deng H H, Mao H F, Liang B J, Li J m, Xu H j. Cosensitization of a nanostructured TiO2 electrode with tetrasulfonated gallium phthalocyanine and tetrasulfonated zinc porphyrin [J]. Journal of Photochemistry and Photobiology A: Chemistry, 1997, 110(1): 47-52.
[15] DERosa M C, Crutchley R J. Photosensitized singlet oxygen and its applications [J]. Coordination Chemistry Reviews, 2002, 233/234: 351-371.
Foundation item: Project(20431030) supported by the National Natural Science Foundation of China; Project(2006RFQXS096) supported by the Foundation for Science and Technology Innovation Talents of Harbin, China; Project(1152Z002) supported by the Key Projects of Educational Department of Heilongjiang Province, China; Project(LBH-Q07111) supported by Heilongjiang Postdoctoral Funds for Scientific Research Initiation
Received date: 2009-05-16; Accepted date: 2009-12-20
Corresponding author: XIN Bai-fu, PhD, Professor; Tel: +86-451-86608781; E-mail: xinbf@263.net
(Edited by CHEN Wei-ping)
Abstract: The Zn(Ⅱ) phthalocyanine sensitized TiO2 (ZnPc-TiO2) nanoparticles were prepared by hydrothermal method via impregnation with ZnPc. The as-prepared photocatalysts were characterized by X-ray diffractometry (XRD) and diffuse reflectance spectroscopy (DRS), and the surface photovoltage spectroscopy (SPS) and photocatalytic degradation of rhodamine B (RhB) were studied under illuminating. The experimental results indicate that TiO2 sensitized by ZnPc extends its absorption band into the visible region effectively, and the sensitized TiO2 has higher activity than TiO2 (Degussa P-25) under the simulated solar light and the visible light. Based on the DRS and SPS results, the mechanism about the photogenerated carrier transfer between TiO2 and ZnPc is proposed. At a lower ZnPc content (≤0.20 ?mol/g), ZnPc monomer acts as the electron donor, which provides the photoinduced electrons to the conduction band of TiO2. These photoinduced electrons can transfer to molecular oxygen (O2), leading to the formation of active species, such as superoxide/hydroxide radicals and singlet oxygen, which is beneficial to the photocatalytic reaction. While at a higher ZnPc content (>0.20 ?mol/g), the formation of ZnPc dimer results in the decrease of photocatalytic activities of ZnPc-TiO2 photocatalyst.


