文章编号:1004-0609(2009)03-0459-05
电解液组成对ZAlSi12Cu2Mg1微弧氧化陶瓷膜的影响
刘彩文,刘向东,乌 迪,吕 凯
(内蒙古工业大学 材料科学与工程学院,呼和浩特 010051)
摘 要:
采用电解液成分逐渐加入法,在6种电解液中对ZAlSi12Cu2Mg1试样进行微弧氧化处理,研究电解液组成对微弧氧化陶瓷膜形成的影响,寻找合适的电解液组成。结果表明:电解液组成对陶瓷膜层的厚度、粗糙度、硬度、耐磨性、膜层微观形貌及相组成的影响很大,通过调节电解液成分,可获得性能优良的陶瓷膜。适宜的电解液组成为:8 g/L NaSiO3,1 g/L NaOH,2 g/L Na2WO4,0.5 g/L Na2EDTA及10 mL/L丙三醇。在此种电解液组成下,获得的陶瓷膜厚156 μm,面粗糙度为259 nm,显微硬度达HV 891。在干摩擦条件下,经30 min磨损后,其磨损仅为基体的13.29%。观察膜层微观形貌,膜层均匀致密。XRD分析表明:氧化层中含有Al、莫来石、SiO2、α-Al2O3、γ-Al2O3 和WO3相。
关键词:
中图分类号:TG 174.4 文献标识码:A
Effects of electrolyte constitutes on ceramic coatings of ZAlSi12Cu2Mg1 by micro-arc oxidation
LIU Cai-wen, LIU Xiang-dong, WU Di, L? Kai
(School of Materials Science and Engineering, Inner Mongolia University of Technology, Hohhot 010051, China)
Abstract: ZAlSi12Cu2Mg1 samples were treated by microarc oxidation (MAO) in six different electrolytes. To obtain appropriate electrolyte constitutes, the effects of electrolyte constitutes on ceramic coatings of ZAlSi12Cu2Mg1 MAO were investigated. The results indicate that the electrolyte composition has great influence on thickness, roughness, microhardness, wear resistance, morphology and phase compositions of ceramic coating. The fine ceramic coating is obtained by changing electrolyte. The appropriate electrolyte constitutes are 8 g/L Na2SiO3, 1 g/L NaOH, 2 g/L Na2WO4, 0.5 g/L Na2EDTA and 10 mL/L glycerin. In this electrolyte, it is feasible to obtain a 156 μm thick coating, the roughness decreases to 259 nm and the microhardness increases to HV 891. The wear mass loss of coating after 30 min is only 13.29% of the matrix, which is relative to ZAlSi12Cu2Mg1 under the condition of dry friction. The morphologies of coatings are continuity and density. The XRD analysis indicates that coatings are composed of mullite, SiO2, α-Al2O3, γ-Al2O3 and WO3.
Key words: ZAlSi12Cu2Mg1; electrolyte constitute; microarc oxidation; ceramic coatings
铝的微弧氧化是在常规阳极氧化成膜的基础上,通过高电压大电流的作用[1],产生微区局部高温[2?3],利用高温烧结作用[4?5],使初始生成的无定形铝常规陶瓷膜发生相转化生成α-Al2O3相陶瓷氧化物膜[6?7]。
ZAlSi12Cu2Mg1是制造低热膨胀系数的高速内燃机活塞等零件的主要材料[8?9],但由于它的抗摩擦性差,大大限制了使用寿命。在ZAlSi12Cu2Mg1材料表面通过微弧氧化形成一定厚度且均匀致密的陶瓷膜,可以显著提高其表面硬度及耐磨性等性能,大幅度延长其使用寿命,从而拓展了其应用领域。国内外研究者在工艺条件、电参数、陶瓷膜性能及陶瓷膜形成等多方面作了许多研究[10?15]。
本文作者采用6种电解液组成对试样进行微弧氧化,研究ZAlSi12Cu2Mg1合金陶瓷膜层厚度、粗糙度、硬度、耐磨性、陶瓷膜层微观形貌及膜层相组成,确定ZAlSi12Cu2Mg1微弧氧化适宜的电解液组成成分。
1 实验
实验材料选用ZAlSi12Cu2Mg1铸造铝合金,其化学成分(质量分数)为:(11.0%~13.0%)Si;(1.0%~3.0%) Cu;(0.4%~1.0%)Mg;(0.3%~0.9%)Mn;<0.7%Fe;余量为Al,尺寸为30 mm×20 mm×5 mm。
本实验采用电解液成分逐渐加入法,对试样进行微弧氧化(见表1)。采用WHD?30型多功能微弧氧化装置进行表面微弧氧化处理,电解槽外壁通循环水冷却,内部吹入空气搅拌。处理时间为50 min,起弧负向电压均设为60 V,频率100 Hz,正/负成膜电压取480/130 V,处理液温度低于35 ℃。
表1 6种电解液的成分
Table 1 Compositions of six different electrolytes
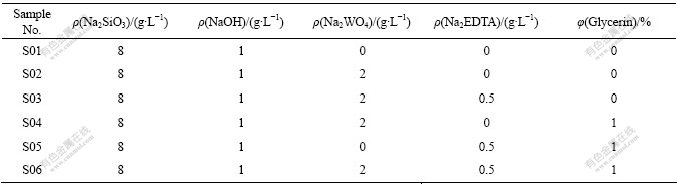
用HCC?25型电涡流测厚仪测量膜层厚度,选用CSPM4000扫描探针显微镜测试膜层面粗糙度Sa(Sa计算原理与Ra相同)。FM400型半自动数字显微硬度计施加载荷0.49 N在膜层截面处测其硬度,分别用QUANTA400型SEM和Neopht2光学显微镜观察陶瓷膜层表面与截面形貌。以360号水砂纸为研磨介质,施加40 N载荷,磨头转速200 r/min,在干摩擦条件下,对微弧氧化处理前后的试样,用MMW?1型立式万能摩擦磨损试验机进行30 min磨损试验,每磨5 min换一次砂纸,称量试样,测量磨损前后试样的质量损失。
2 结果与讨论
电解液只加入吸附组分Na2SiO3和调整组分NaOH,试验时电解液混浊,氧化反应剧烈,响声大,微弧氧化7 min息弧,获得的陶瓷膜厚34 μm;当再加入调整组分Na2WO4处理50 min后,氧化过程极不稳定,响声巨大,陶瓷膜厚尽管达133 μm,但膜层非常粗糙,有颗粒状物突起,面粗糙度超过扫描探针显微镜测试上限值2 400 nm,不能进行检测(表2)。正常局部脱落如图1(b)所示,很明显此电解液组成不适宜ZAlSi12Cu2Mg1微弧氧化陶瓷膜的形成;继续加入稳定组分Na2EDTA后,电解液变得清澈,氧化也不稳定,陶瓷膜厚65 μm且粗糙,面粗糙度超过扫描探针显微镜测试上限值2 400 nm,也不能进行检测。但膜层与基体结合差,脱落严重(图1(c)),同样也不能获得理想的陶瓷膜;电解液稳定组分不加Na2EDTA而是加入丙三醇,电解液仍混浊,氧化反应剧烈,响声大,陶瓷膜厚达121 μm,膜层均匀不脱落但稍粗糙,膜层面粗糙度为366 nm;电解液调整组分不加Na2WO4只加NaOH时,将稳定组分Na2EDTA和丙三醇同时加入,电解液清澈,氧化稳定,试件陶瓷膜均匀细密,陶瓷膜厚达162 μm,而显微硬度只有HV 374。电解液中加入吸附组分Na2SiO3、调整组分NaOH 和Na2WO4、稳定组分Na2EDTA和同时加入丙三醇,电解液清澈,氧化稳定,试件陶瓷膜均匀细密(图1(d)),膜层厚度为156 μm,膜层面粗糙度降低为259 nm,显微硬度达HV 891,显然此电解液组成适宜ZAlSi12Cu2Mg1微弧氧化陶瓷膜的形成。
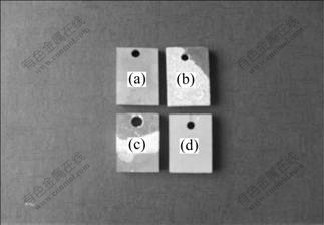
图1 不同试件的微弧氧化陶瓷膜
Fig.1 Microarc oxidation ceramic coatings of different samples: (a) ZAlSi12Cu2Mg1; (b) Sample S02; (c) Sample S03; (d) Sample S06
表2 不同试样陶瓷膜的粗糙度
Table 2 Roughness of ceramic coatings on different samples
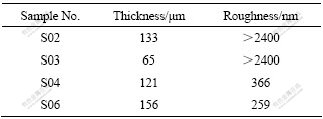
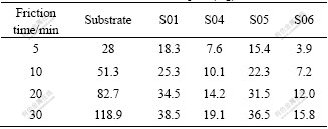
表3所列为不同试样的质量磨损测试结果,试件S02 和S03的陶瓷膜经5 min 磨损后膜层几乎全部脱落,耐磨性极差。试件S01 和S05的陶瓷膜经30 min磨损后,其质量磨损分别38.5和36.5 mg,约为基体的30%。试件S04的陶瓷膜经30 min磨损后,其质量磨损19.1 mg,为基体的16.17%。试件S06经不同转速磨损后,膜层磨损约为基体的13%~15%,经30 min磨损后,其质量磨损15.8 mg,仅为基体的13.29%。因此,丙三醇和Na2EDTA同时作用可细化膜层陶瓷颗粒,增加膜层与基体的结合力,提高膜层的耐磨 性[16],ZAlSi12Cu2Mg1在电解液6中更易得到性能优良的陶瓷膜。
表3 不同试样的质量磨损
Table 3 Mass loss of different samples (mg)
不同电解液组成不仅影响陶瓷膜性能和质量,其膜层表面形貌也不相同。图2(a)所示为试件S02的陶瓷膜表面SEM像,由图可以看到,微弧氧化陶瓷膜上出现了团状的熔融物,放电微孔的尺寸较大,陶瓷膜表面非常粗糙。图2(b)所示为试件S03的SEM像,陶瓷膜上微孔的周围有熔化的痕迹,孔边外翻,有大量的裂纹出现,裂纹很深很宽;裂纹的出现是因为喷出熔融物遇电解液急冷凝固时应力不均,这些裂纹会造成膜层与基体结合能力差,膜层大片脱落。图2(c)所示为试件S04的SEM像,陶瓷膜表面出现陶瓷颗粒,孔与孔相互融合,孔径大而孔边外翻,陶瓷膜表面较粗糙。图2(d)所示为试件S06的SEM像,由于氧化时间长,膜层表面形成典型的球状陶瓷颗粒,颗粒细小而均匀,放电微孔孔径减少,试件陶瓷膜均匀细密。
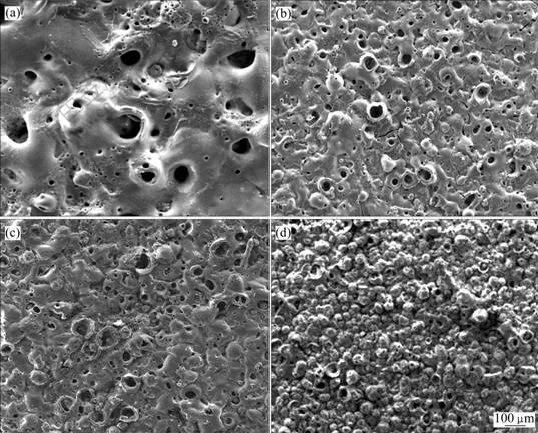
图2 不同试件微弧氧化陶瓷膜的表面形貌
Fig.2 Surface morphologies of microarc oxidation coatings of different samples: (a) Sample S02; (b) Sample S03; (c) Sample S04; (d) Sample S06
从图3中可以看出,基体中有片状的初晶硅和粗大针状的共晶硅存在,陶瓷膜与基体齿状交错。图3(a)所示为试件S01氧化膜截面的形貌,氧化时间短,陶瓷膜很薄。图3(b)所示为试件S06氧化膜截面的形貌,可以看出陶瓷膜可分为两层结构,靠近基体的部分比较致密,为致密层,与基体结合良好;靠近表面的部分疏松,孔洞很多,为疏松层;致密层与疏松层之间没有明显的界限,而是逐渐过渡的。
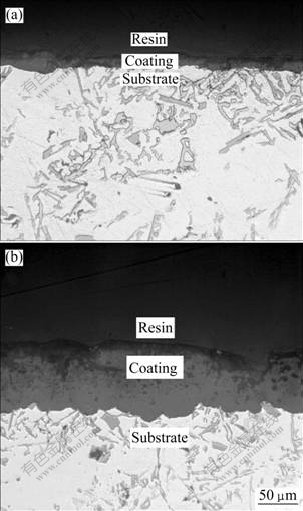
图3 不同试件的微弧陶瓷膜截面形貌
Fig.3 Cross-section morphologies of microarc oxidation coatings of different samples: (a) Sample S01; (b) Sample S06
图4所示为试件S01和S06微弧陶瓷膜的XRD 谱。从图4(a)中可看到Al和Si的衍射峰,这是由于试件S01陶瓷膜太薄且陶瓷膜中存在大量的孔洞,孔洞的直径远远大于X射线的波长,所以X射线能够直接照射到基体上。除Al和Si外还存在少量的晶态Al2O3即γ-Al2O3相。由图4(b)可知, 试件S06陶瓷膜的相组成非常复杂,包括Al、莫来石、α-Al2O3相、γ-Al2O3、SiO2 相和钨的氧化物相。膜中WO3 和SiO2 的存在, 说明界面溶液中的电解质也参与了微弧氧化的成膜过程,Na2WO4的加入使陶瓷膜中出现WO3,可提高膜层硬度。
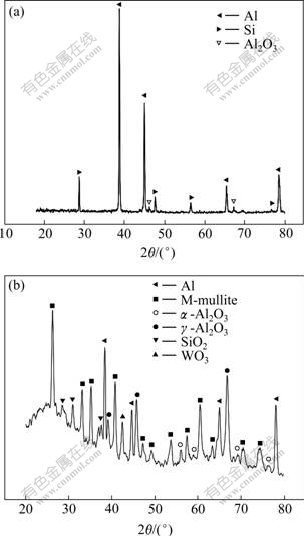
图4 不同试件微弧陶瓷膜的相组成
Fig.4 XRD patterns of microarc oxidation coatings of different samples: (a) Sample S01; (b) Sample S06
3 结论
1) 电解液组成对ZAlSi12Cu2Mg1微弧氧化陶瓷膜的厚度、硬度、耐磨性、陶瓷膜层微观形貌及膜层相组成均有不同程度的影响,试件S01~S05分别在电解液1~5中处理,均未获得理想的陶瓷膜。
2) 确定ZAlSi12Cu2Mg1微弧氧化所适宜的电解液组成为Na2SiO3、NaOH、Na2WO4、Na2EDTAg/L和 丙三醇,其中Na2SiO3是电解液的主要成分,NaOH可调节电解液pH值在11~13呈碱性,Na2WO4可提高膜层硬度,Na2EDTA可使氧化稳定,丙三醇同时作用可细化膜层陶瓷颗粒、改善膜层的耐磨性。
3) 通过调节电解液组成成分,ZAlSi12Cu2Mg1经过微弧氧化处理可获得硬度高、耐磨性好及均匀致密的陶瓷膜。
[1] KOLOMEICHENKO A V. Reconditioning technology by argon-arc surfacing and hardening by microarc oxidation of components made of aluminium alloys[J]. Welding International, 2004, 18(6): 494?497.
[2] HAN Jian-min, DING Yi. Study on the growth process of the microarc oxidation ceramic coating on aluminum alloy[J]. Plating and Finishing, 2005, 27(1): 8?1.
[3] CHIGRINOVA N M, CHIGRINOVA V E, KUKHAREV A A. Formation of coatings by anodic microarc oxidation and their operation in thermally-stressed assemblies[J]. Powder Metallurgy and Metal Ceramics, 2001, 40(5/6): 213?217.
[4] ZHONG Tao-sheng, JIANG Bai-ling, LI Jun-ming. Characteristics, application and research direction of micro-arc oxidation technology[J]. Electroplating and Finishing, 2005, 24(6): 47?50.
[5] KRISHTAL L R, SOMARAJU K R C, SUNDARARAJAN G. The tribological performance of ultra-hard ceramic coatings obtained through microarc oxidation[J]. Surface and Coating Technology, 2003, 163/164: 484?490.
[6] SUNDARARAJAN G, KRISHNA L R. Mechanisms underlying the form, action of thick alumina coatings through the MAO coating technology[J]. Surface and Coating Technology, 2003, 167: 269?273.
[7] WEI Tong-bo, YAN Feng-yuan, TIAN Jun. Characterization and wear- and corrosion-resistance of microarc oxidation ceramic coatings on aluminum alloy[J]. Journal of Alloys and Compounds, 2005, 389: 169?176.
[8] CHIGRINOVA N M. Use of ceramic coatings as thermal barriers in heat cycling[J]. Journal of Engineering Physics and Thermophysics, 2001, 7(6): 1582?1592.
[9] KRISHTAL M M. Effect of structure of aluminum-silicon alloys on the process of formation and characteristics of oxide layer in microarc oxidizing[J]. Metal Science and Heat Treatment, 2004, 46(9): 337?339.
[10] BOGUTA D L, RUDNEV V S, GORDIENKO P S. Current mode effect on the composition and characteristics of anodic-spark coatings[J]. Protection of Metals, 2004, 40(30): 275?279.
[11] XUE Wen-bin, WANG Chao, LI Yong-liang, CHEN Ru-yi, ZHANG Tong-he. Analyses of microarc oxidation coatings formed on Si containing cast aluminum alloy in silicate solution[J]. ISIJ International, 2002, 42(11): 1273?1275.
[12] LU Li-hong, SHEN De-jiu, WANG Yu-lin. Influence of technical parameters on the properties of micro-arc oxidation film on cast aluminum-silicon alloy[J]. Plating and Finishing, 2001, 23(1): 32?40.
[13] HE Zi-kai, TANG Song-pei. Effects of current density on thickness and hardness of microarc oxide film[J]. Surface Technology, 2003, 32(3): 21?23.
[14] PUSHKAREV O I, SUKHONOS S I. Composite wear-resistant material based on aluminum oxide[J]. Refractories and Industrial Ceramics, 2001, 42(1/2): 15?18.
[15] XIN Shi-gang, SONG Li-xin, ZHAO Rong-gen. Influence of cathodic current on composition, structure and properties of Al2O3 coatings on aluminium alloy prepared by microarc oxidation process[J]. Thin Solid Films, 2006, 515(1): 326?332.
[16] LIU Cai-wen, LIU Xiang-dong, L? Kai, WU Di. Function of electrolyte compositions on ceramic coating formed of ZAlSi12Cu2Mg1 alloy by MAO[J]. Special Casting and Nonferrous Alloys, 2008, 28(9): 725?727.
基金项目:内蒙古科技攻关资助项目(20040202)
收稿日期:2008-06-25;修订日期:2008-11-25
通讯作者:刘彩文,讲师,在读博士研究生;电话:0471-6552870, 013848112060;E-mail: liucaiwen@yahoo.cn
(编辑 李向群)


