文章编号:1004-0609(2014)02-0448-13
车用动力电池回收技术进展
余海军1,谢英豪1,张铜柱2
(1. 广东邦普循环科技有限公司,佛山 528244;
2. 中国汽车技术研究中心,天津 300300)
摘 要:
近年来,作为高能汽车动力电池的镍氢电池和锂离子电池凭借其能量密度高、充放电速度快、循环寿命长以及无污染等优点得到快速发展。但经过数百至上千次的循环充放电后其容量下降并最终报废,从保护环境、节约自然资源角度看,回收电池再利用成为必然。作者总结了近年来国内外回收利用锂离子电池和镍氢电池的方法,包括湿法工艺、火法工艺和联合工艺等,并对各工艺作出了评价;概述了研究现状中存在的二次污染、安全性问题与解决方法和回收制备产物的种类,为中国未来动力电池回收利用奠定基础。
关键词:
中图分类号:X705;TQ150.9 文献标志码:A
Technical progress on power batteries recovery for electric vehicle
YU Hai-jun1, XIE Ying-hao1, ZHANG Tong-zhu2
(1. Guangdong Brunp Recycling Technology Co., Ltd., Foshan 528244, China;
2. China Automotive Technology & Research Center, Tianjin 300300, China)
Abstract: Recently, Ni-MH battery and lithium ion battery as high-energy vehicle power batteries have been developed rapidly for some advantages, such as high energy density, fast process of charge and discharge, long cycle life, non-pollution. However, the battery capacity decreases after hundreds of charge-discharge cycles, which finally leads to the battery scrap. From the view of environmental protection, natural resources conservation and lower the cost, the battery recycling is necessary. The authors summarized the methods of Ni-MH battery and lithium ion battery recycling in domestic and foreign researches, including the hydrometallurgical processing method, pyrometallurgical processing method and combined processing method, and each method was evaluated. And then an overview was given about secondary pollution, security problems and solutions in the existing methods, which lays a foundation for future recycling of traction battery in China.
Key words: power battery; recycling; separation; hydrometallurgy; pyrometallurgy
在能源危机加深和环保意识增强的背景下,动力汽车得到了迅猛的发展。电动汽车是提高汽车产业竞争力、保障能源安全和发展低碳经济的重要途径。我国电动汽车科技发展“十二五”专项规划中要求加快推动电动汽车科技发展、持续支持电动汽车科技创新。混合动力汽车技术逐步成熟,已进入产品市场竞争期,率先实现产业化;纯电动汽车电池技术进步迅速,整车产品更加接近消费者的需求。预计在2020年,电动汽车的市场占有额将达到5%。作为高能绿色动力电池,镍氢电池和锂离子电池凭借其能量密度高、充放电速度快、循环寿命长以及无污染等优点,近年来得到迅速发展。镍氢电池和锂离子电池的技术不断成熟,其回收利用技术也得到不断发展。我国《节能与新能源汽车产业发展规划(2012-2020年)》电动汽车累计销售到2015年达50 万辆,2020年达500万辆。 等[1]预计在未来电动汽车广泛应用时,将有可能出现Li紧缺的情况,届时将不得不寻找新的电池技术。欧盟第2006/66/EC号电池指令要求到2012年9月回收率最低为25%,2016年达到45%,根据电池种类的不同,循环再利用率应达到50%~75%[2]。本工作分析了近年来国内外镍氢电池和锂离子电池回收利用的方法,为动力电池的低成本回收提供方向性的指导。
等[1]预计在未来电动汽车广泛应用时,将有可能出现Li紧缺的情况,届时将不得不寻找新的电池技术。欧盟第2006/66/EC号电池指令要求到2012年9月回收率最低为25%,2016年达到45%,根据电池种类的不同,循环再利用率应达到50%~75%[2]。本工作分析了近年来国内外镍氢电池和锂离子电池回收利用的方法,为动力电池的低成本回收提供方向性的指导。
1 锂离子电池和镍氢电池组成
不同的厂家生产出来的电池成分不尽相同,因此所回收有价组分也各有差异,这将会影响后续处理的回收效率和回收成本。
由于锂离子动力电池能量高,但材料稳定性差,容易出现安全问题,虽然钴酸锂电池具有质量轻、体积小等优点,但它的热稳定性差、原料价格高、污染严重,不适合应用于电动车。目前国内外己商品化的锂离子动力电池正极材料普遍采用磷酸铁锂、锰酸锂和三元材料。国外在继续研发现有材料的基础上还重点研发磷酸钒锂正极材料。同时,改进钴酸锂、锰酸锂材料性能的研究也从未停歇。
1.1 锂离子电池组成
图1所示为锂离子电池的组成[3]。正极制备时通常以粘合剂聚偏氟乙烯(PVDF)将正极材料颗粒固定在电极上制得。负极一般采用石墨结构的碳素材料,如碳/石墨插入材料,由碳素材料、乙炔黑、粘合剂按一定比例混合涂覆在铜箔上制得。具有极好的循环性能和可快充快放高功率特性的钛酸锂(Li4Ti5O12)有望成为新一代锂离子动力电池的主要负极材料[4]。隔膜主要由聚丙烯、聚乙烯微孔薄膜或二者双层组成,其厚度约10 μm;电解液主要是含锂盐的有机溶剂,其中锂盐通常是LiPF6,也会用LiClO4或LiBF4[5-7],三者各有其局限性,LiPF6高温时稳定性差,LiBF4常温时离子电导率低,LiClO4具有强氧化性[8];有机溶剂通常为碳酸酯类(碳酸二甲酯、碳酸乙烯酯、碳酸甲乙酯、碳酸二乙酯等);外壳为不锈钢、镀镍钢或铝壳等。
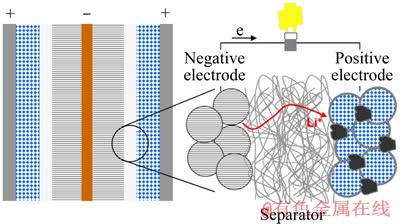
图1 锂离子电池的组成[3]
Fig. 1 Cell chemistry in lithium-ion battery[3]
1.2 镍氢电池组成
镍氢电池正极是在镍基材(泡沫镍或冲孔镀镍钢带)上涂覆活性物质NiOOH制成;负极是储氢合金的氢化物电极,通常由AB5储氢合金(B为Ni、Co、Mn和Al混合物,A为稀土金属La、Pr、Nd、Sm和Ce混合物)添加少量抗腐蚀剂Y2O3或Yb2O3后,将粉末涂覆在铁片或者铜片上制得[9-10];在正、负极之间有隔膜,隔膜通常采用多孔的尼龙、聚丙烯和维尼纶无纺布等;由于电极材料中的电解质没有完全固态化,仍普遍采用一定量的电解液,通常是添加有少量LiOH的KOH水溶液。
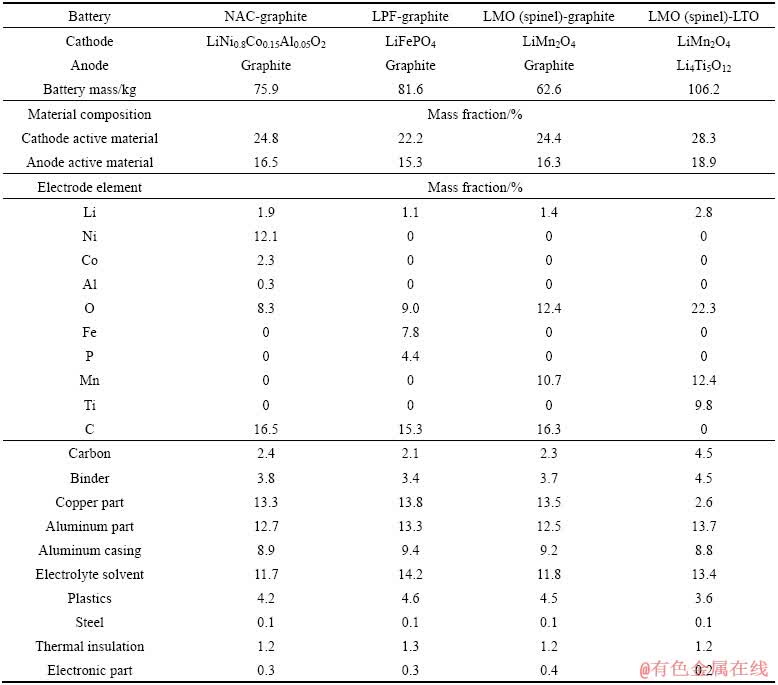
图2所示为动力电池的模块结构[3]。混合电动汽车所用电池组质量为30~100 kg,纯电动小轿车的动力电池质量为300~400 kg,至于电动大巴或电动大货车的动力电池质量达1500~2000 kg。目前,我国汽车行业标准规定锂离子动力电池[11]和镍氢动力电池[12]循环寿命最低为500次,最好的动力电池(包括锂离子电池和镍氢电池)循环寿命不超过3000 次[13],根据国内现有纯电动公交车的运行情况来看,动力电池的寿命为3~5年。每年的报废电池在100 万t以上,届时将对环境造成巨大的压力。由表1可知,以上废旧镍氢动力电池含有大量Ni、Mn及轻稀土金属[14];由表2可知,废旧锂离子动力电池含有大量Li、Ni、Mn、Fe等金属元素[15]。DEWULF等[16]分析结果认为,回收锂离子电池可节约51.3%的自然资源,包括减少45.3%的矿石消耗和57.2%的化石能源。DUNN[17]计算出物理法直接回收工艺制备LiCoO2所消耗的总能量是正常生产工艺的6%。因此,从降低成本、环境保护和提高资源利用率方面考虑,回收利用废旧动力电池中的有价金属具有重要意义。
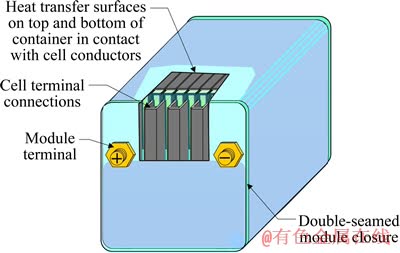
图2 动力电池模块结构[3]
Fig. 2 Module structure of power battery[3]
表1 镍氢电池破碎后物质组成[14]
Table 1 Composition of Ni-MH-cell scraps[14]

2 动力电池回收技术
表2 混合动力汽车中锂离子电池的物质组成[15]
Table 2 Material composition of selected Li-ion battery systems for PHEV[15]
动力电池回收方法多种多样,通常可以分为两大类:物理方法和化学方法。物理方法包括:重力法、磁力法和静电吸引法;化学方法包括:湿法工艺、火法工艺和联合工艺。在工业化生产中,常通过物理方法初步分离提纯物料,然后再根据物料成分的特点利用不同的化学方法进行回收。
2.1 湿法工艺
湿法工艺是将废弃电池破碎后溶解,然后选择性分离浸出液中的金属元素。对此方法的研究较多,是目前主要处理废旧镍氢电池和锂离子电池的技术。将废弃的锂离子电池或镍氢电池在高温炉中焙烧,碳和有机物将被高温燃烧去除,燃烧时产生的还原性气氛对金属元素起到一定的保护作用。筛分产生含有金属和金属氧化物的细粉体,将粉体经过酸溶和过滤,调节滤液pH值将Fe、Al及稀土金属沉淀后以氢氧化物形式回收。滤液再经过萃取和反萃取工艺,分别得到含Co和Ni的水溶液,最后以电解的手段提炼出高纯金属Co和Ni。Li则通过添加碳酸盐以碳酸盐沉淀析出[18]。图3所示为湿法工艺回收镍氢电池过程流程图[19]。
2.1.1 物理分离
物理分离回收动力电池是指将电极活性物、集流体和电池外壳等电池组分经破碎、过筛、磁选分离、精细粉碎和分类后得到高含量的物质然后再进行下一步回收的过程[20]。镍氢电池或锂离子电池经切割或者冲击粉碎,由于粉碎后有机聚合物的颗粒较大而电极材料颗粒较小,因此可通过筛选将电极材料和有机聚合物分离。水洗可改善二者的分离效率,同时还可去除固体中的可溶性盐和电解质。磁选可选择性分离钢铁,但该过程会夹带金属形式的镍粉和少量细小的电极材料颗粒[21]。其他一些颗粒较大的组分是锂离子电池电极上铜箔和铝箔破碎后的粉末。600 ℃真空热解30 min可将电极材料从铜箔或铝箔上剥离,使铜箔和铝箔的回收变得简单方便[22]。ITO等[23]针对火法工艺回收镍氢动力电池中Ni与Co和稀土难分离的问题,研究分离阳极材料和阴极材料的方法,发现在弱磁场(0.1 T)下,棱柱型电池阳极活性物集中在磁场一侧,此时可有效分离阳极材料和阴极材料。HUANG等[24]破碎电池至粒径为0.5~2 mm,重力分离可将金属含量提高75%~90%,分离效率超过90%,外加磁场时回收率超过95%。张涛等[25]发现锂离子电池经湿法冲击破碎后,碳素材料在粒度为0.125~0.250 mm和小于0.075 mm这两种破碎产物中富集,其质量分数分别为73.39%和72.65%;塑料外壳、隔离膜、铜箔、铝箔等物质主要富集在粒度大于0.250 mm的破碎产物中。卢毅屏等[26]研究发现高温焙烧与物理擦洗法都不能完全使集流体分离出来,而通过稀酸溶解-搅拌擦洗联合作用分离效果良好,铝箔和铜箔可直接作为产品回收。物理分离一般在浸出前进行,这可以提高目标金属元素的回收率和简化后续的提纯工艺[27]。
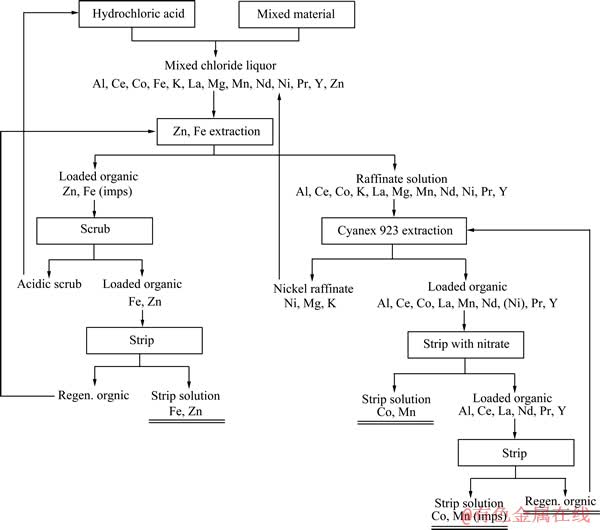
图3 湿法工艺回收镍氢电池过程流程图[19]
Fig. 3 Flowchart of generic hydrometallurgical recycling process of Ni-MH batteries[19]
2.1.2 浸出
溶解浸出过程通常有一步浸出法和两步浸出法。一步浸出法直接用酸溶解所有金属后,再采用不同的方法回收。两步浸出法先用碱浸出并回收铝,再用酸浸出其他金属后采用一步浸出法相同的分离技术回收其他金属[28]。常用于溶解浸出的酸有盐酸、硝酸和硫酸。使用盐酸时,Co(Ⅲ)可将盐酸氧化成Cl2等污染物而导致工作条件恶化,为此普遍采用硫酸中加入还原剂H2O2[22, 29]、Na2S2O3[30-31]或抗坏血酸[32]等作为浸出液,Co浸出率分别为99%、99.5%和94.8%。抗坏血酸既可作为酸又可作为还原剂,用抗坏血酸浸出时,采用固液比为25 g/L,抗坏血酸浓度为1.25 mol/L的条件,在70 ℃浸出20 min后,Co和Li的浸出率分别为94.8%和98.5%[32]。LARSSON等[10]研究镍氢动力电池电极活性物质在酸性条件下的溶解规律,发现当利用非氧化性酸(如盐酸和稀硫酸)在贫氧环境下溶解时,酸的用量可节省37%,因为此时酸可充分溶解阴极活性物质而不会溶解阴极上的金属镍。溶解的最佳条件为pH=1,反应时间不少于333 min。
传统的浸出方法能耗大,成本高并且存在严重的二次污染问题。利用无机化能营养、嗜酸硫氧化菌和嗜酸氧化亚铁菌从废锂离子电池中溶解金属的生物浸出法是一种新颖的、有发展潜力的浸出方法[30]。生物法溶解Li原理主要是细菌以硫元素和Fe(Ⅱ)为能量源,代谢产生硫酸和Fe(Ⅲ),将废旧电池电极材料溶解,该方法中Li浸出率达80%[33]。
2.1.3 回收浸出液中金属元素
浸出液中含有Ni、Co、Mn、Fe、Li、Al和Cu等多种元素,其中Ni、Co、Mn、Li为主要回收的金属元素。Al、Fe(Ⅲ)金属氢氧化物的pKsp分别为32.9和37.4,远远大于其他金属氢氧化物所对应的值,因此,通过调节pH将Al和Fe选择性沉淀出后,再对浸出液中的Ni、Co、Mn和Li等元素进行下一步的处理回收。常用的回收方法有化学沉淀法、盐析法、离子交换法、萃取法和电沉积法。NOGUEIRA等[21]研究发现最优的萃取顺序为稀土、Mn、Co,这样可以以最低的成本得到最高的分离效率。
1) 回收稀土金属
在回收废旧电池中稀土研究方面,PIETRELLI等[34]研究了AB2和AB5储氢合金中稀土回收的方法,采用H2SO4浸出金属元素后,以NaOH调节pH<1.5,对溶液进行加热,稀土元素则以难溶物NaRe(SO4)2·H2O(Re=La, Ce, Pr, Nd, Pm, Sm, Eu, Sc)形式沉淀析出。而通常Fe(OH)3在pH为2.5~3时才沉淀,因此,Fe的存在不会对稀土沉淀造成太大影响。经计算,1 t废旧镍氢电池可回收37.5 kg纯度约80%的稀土元素。ZHANG等[35]调节溶液pH≈1.2,加入25%的二(2-乙基己基)磷酸(D2EHPA)煤油溶液作为萃取液,沉淀物用酸溶解后再以草酸进一步沉淀提纯,可将混合稀土氧化物纯度提高至99%,产率达98%。FERNANDES等[36]萃取除去浸出液中Zn(Ⅱ)、Fe(Ⅲ)、Co(Ⅱ)、Ni(Ⅱ)后以2-乙基己基膦酸-2-乙基己基单酯(PC-88A)为萃取剂在pH=1萃取镧系金属,回收率超过99.9%。INNOCENZI等[37]将镍氢电池破碎后的粉末(<500 μm)依次采用2 mol/L H2SO4 80 ℃浸出3 h和1 mol/L H2SO4 25 ℃浸出1 h得到浸出液,不经过萃取除杂直接以NaOH调节pH=1.6,稀土金属以硫酸盐沉淀析出,回收率为99%,其中含64%镧系金属硫酸盐和28%铈硫酸盐。GASSER等[38]合成出一种吸附剂(Mg-Fe-LDH-A),对浸出液中的La和Nd进行选择性吸附分离。在5 g/L La(Ⅲ)、5 g/L Nd(Ⅲ)、pH=1的溶液里,进行2 h吸附,分离因子SLa/Nd可达23.2,La(Ⅲ)和Nd(Ⅲ)的吸附容量分别为481 mg/g和192 mg/g。
2) 回收Ni、Co等金属
有机磷萃取剂常用于萃取浸出液中Ni、Co和Cu等金属元素,常用的有二(2,4,4-三甲基戊基)次膦酸(Cyanex 272)、D2EHPA、三烷基氧膦混合物(Cyanex 923)、2-乙基己基膦酸单2-乙基己基酯(P507)和一些非磷萃取剂三辛胺(TOA)、Acorga M5640[39-40]等。这些萃取剂能与Co结合形成稳定的配合物,分离水相和有机相后将Co和其他金属离子分离,调节pH值或加入沉淀剂得到相应的Co盐。
Cyanex 272常用作分离Ni和Co的萃取剂[41-43]。当Mn存在时Cyanex 272失去对Co的选择性萃取能力,因此GRANATA等[44]采用多种萃取剂分步萃取废旧锂离子电池和镍氢电池混合浸出液中的Mn、Co和Ni。先以D2EHPA在pH=4,n(D2EHPA)/n(Mn)=2时萃取Mn,再用Cyanex 272在pH值为5~6、n(Cyanex)/ n(Co)=4时萃取Co,剩余溶液提纯回收Ni,总金属回收率超过50%。ZHANG等[35]采用25%TOA(三辛胺)煤油溶液几乎可完全萃取回收Co,剩余溶液主要含Ni,再通过添加草酸铵得到相应草酸盐沉淀;Co的纯度高达99%,Co和Ni的回收率分别为98%和96%。INNOCENZI等[45]对比D2EHPA 和Cyanex 272萃取镍氢电池浸出液中的Mn和Zn,发现D2EHPA比Cyanex 272具有更高的萃取率。SUZUKI等[40]分步萃取浸出液中的Al、Cu、Co和Li,先用Acorga M5640在pH值为1.5~2.0时萃取Cu,水相溶液用PC-88A在pH值为 2.5~3.0时萃取Al,后用PC-88A/TOA混合萃取剂在pH=5.4时萃取Co,分离效率βCo,90%/Li=1170。ZHANG等[46]还研究了用0.90 mol/L PC-88A的煤油溶液在pH≈6.7时萃取Co,有机相中w(Li)/w(Co)<5×10-5。分离Co和Li时,PC-88A比D2EHPA具有更高的选择性并可得到更纯的产品。近年来,有研究者[19, 47]以Cyanex 923萃取废旧电池中金属元素。镍氢电池电极浸出液经预萃取剂(8%Cyanex 923, 10%TBP, 82%煤油)萃取后,有机相含有Fe和Zn,经3 mol/L的HCl清洗后再进行洗脱得到Fe和Zn溶液,有机相分离循环使用;无机相中含有Al、Co、K、Mg、Mn、Ni和Y,加入萃取剂(70%Cyanex 923, 10%TBP, 10%煤油, 10%1-癸醇)后,所得无机相含Ni、Mg和K,有机相含Al、Co、Mn、Y(可能含有少量Ni);用硝酸洗涤有机相,洗出液含Co、Mn(可能含有少量Ni),有机相含Al和Y;用1 mol/L盐酸洗涤有机相,洗出液含Al和Y,有机相可循环使用。如果浸出液含有Ce、La、Nd、Pr等其他的稀土元素则回收时这些元素可与Y同时被回收。CHEN等[48]以P507作萃取剂时,Co(Ⅱ)萃取率和沉淀得到的草酸钴纯度分别为93%和99.9%。陈亮等[49]采用H2SO4+H2O2为浸出剂对锂离子电池活性物质进行浸出,然后采用黄钠铁矾法去除浸出液中的Fe,除Fe率为99.9%;再采用N902萃取分离Cu,Cu回收率为99.9%;调节pH=4.3,通过水解沉淀法除Al,最后采用碳酸盐共沉淀法制备镍钴锰碳酸盐前驱体,该方法能回收废旧锂离子电池中95%的Ni、Co和Mn。也有研究者开发绿色萃取剂,如LI等[50]利用柠檬酸、苹果酸和天冬氨酸在浸出液中回收Co和Li,发现柠檬酸和苹果酸萃取Li的回收率将近100%,Co的回收率超过90%,使用天冬氨酸效率稍低。
在利用电沉积法回收Ni、Co、Cu等金属元素方面,FREITAS等[51]将锂离子电池按不同的部分拆解分离正极、负极和外壳等,用水洗去正极的电解液后,用HCl和H2O2的混合溶液浸出Co;在pH=5.4、电荷密度为10.0 C/cm2时,电沉积得到100%金属Co,电荷效率为96.9%。FREITAS等[52]对拆解出的负极溶解后,加热至120 ℃保温24 h使电解液挥发,过滤除去石墨后电沉积Co,发现在pH=2.7时Co为瞬时成核,此时Co的还原伴随吸附氢的形成[53];当pH=5.4时Co为连续成核,溶液中的Cu和Co可发生共沉积。SANTOS等[54]在浸出液中直接电解得到电沉积物含有Ni、Co、CoO、Co(OH)2和Mn3O4,电势为-1.1 V (vs Ag/AgCl),电荷密度为-90 C/cm2,电荷效率达83.7%。电沉积法简单、易行,但能耗较高,对浸出液直接电沉积通常得到的是镍钴合金,萃取分离后再进行电沉积可得到纯度较高的金属Ni和Co[55],但会使得工艺复杂,成本增加。
除了常用的萃取法和电沉积法还有一些其他的方法,如李长东等[56]提出镍氢电池正极废料中提取制备超细金属镍粉,用硫酸和双氧水浸出后,经反萃取和萃取,再以水合肼作为还原剂制得粒径约0.4 μm镍粉,回收率达98.5%,纯度高于99.7%。李丽等[57]以柠檬酸、苹果酸、琥珀酸或天冬氨酸等有机酸和双氧水溶解废旧锂离子电池正极材料得到Li+、Co2+的有机酸盐溶液,过滤后加入锂盐或钴盐使n(Li+)/n(Co2+)为 0.95~1.6,加热后滴加氨水后干燥制得干凝胶,煅烧后得到电池材料LiCoO2,该方法成本较低、工艺简单、适宜工业化。
3) 回收Li
早期回收Li是通过以LiOH作为碱,调节浸出液的pH值将锂盐沉淀,过滤后用H2SO4清洗,在洗出液中通入CO2或加入饱和Na2CO3,将Li以Li2CO3沉淀形式回收。TEDJR等[58]通过将废旧电池破碎、磁选分离制得超细粉体(粒径小于3 mm),该粉体在溶液中可发生剧烈的水解,然后加入CO2饱和溶液得到Li2CO3沉淀。赵存良等[59]采用火湿法联合工艺回收废旧锂离子电池中的Li:将电池放电后进行碱浸处理,在75 ℃的NaOH溶液中搅拌1.5 h,然后与硫酸盐一起焙烧,使电池中的Li转化成可溶于水的硫酸盐,而其他金属化合物基本难溶于水;经水洗得到含锂离子的滤液,滤液沉淀后回收Li,回收率达90%。马伟等[60]采用浸没式超滤膜和经酸洗后的LiMg0.5Mn1.5O4形成离子筛,作为吸附剂,对浸出液中的Li+选择性吸附,用盐酸可完全洗脱锂离子,得到含锂离子的溶液。谭群英等[61]通过对废旧锂离子电池或极片破碎、焙烧、碱洗、酸洗后加入铁粉除去Cu2+,调节pH值到4.0~5.0,将大部分Fe和Al去除,然后加入氟盐得到LiF沉淀。经多次碱洗、酸洗、除杂和沉淀可得到纯度高达98%的LiF,回收率为75%~92%。
湿法工艺比火法工艺制得的产品具有更高的纯度,反应过程更容易控制,对环境影响较小,但是再生有机溶剂和水使得成本较高,工艺复杂,对原材料成分敏感,原料成分差异对产率和纯度影响较大。
2.2 火法工艺
将废旧锂离子电池或镍氢电池破碎并通过振动筛和磁选分离塑料包装、电池金属部分和电极材料部分,将电极材料部分放入电弧炉中加热,加入适当的熔剂使金属形成合金[62],对加入的熔剂要求一方面是对Co和Mn有较小的溶解度,另一方面是对Li有较大的溶解度。钴锰合金可通过真空感应炉进一步提纯制备钴基超级合金。高温反应容器的材料通常是石墨,虽然长期使用石墨会有损耗,但MgO和Al2O3材料高温下可与稀土发生如下反应[63],更不适宜采用。
 (1)
(1)
式中:RE为稀土元素;Me为金属元素。
图4 所示为火法工艺回收镍氢电池过程流程图[64]。不同的熔渣对火法工艺影响很大,所选用的熔剂要满足以下6点[64]:
1) 熔剂组分蒸汽压低;
2) 黏度尽可能小;
3) 熔剂与金属的密度差大(至少为2 g/cm3);
4) 对稀土氧化物有较强的溶解能力(溶解度至少可达20%);
5) 对Si和其他杂质有较强的溶解能力;
6) 对Ni和Co溶解度小(最大不超过1%)。
常用的熔剂有SiO2、SiC、Al2O3、MgO、CaO和CaF等,含Cl的熔剂不适合使用,因为温度超过1 500 ℃时,氯化钙的高蒸汽压会引起其蒸发[65]。
王常春等将锂离子电池[66]或镍氢电池[67]放电、破碎、筛分,对筛上物进行磁选分离后进行热处理,然后在真空熔炼炉坩埚中加入熔剂(w(CaO)≥50%、w(MgO)≤20%、w(SiO2)≤20%、w(Al2O3)≤10%、w(B2O3)≤10%)和还原焦炭进行熔炼,筛上钢壳在 1 500~2 500 ℃熔炼后得到铁镍基合金,筛下物在1 800~2 800 ℃熔炼后分别得到钴基合金和镍基合金,金属元素回收率达99%。CHERET等[68]在SiO2-CaO熔剂中加入少量Al2O3、Pb、Fe,将熔炼温度降低至1 200~1 450 ℃,Ni的回收率可达99.5%。BROUSSELY等[69]将电池破碎后直接加入到高温炉中,包装塑料、石墨和有机溶剂既可提供还原气氛又可作为燃料降低成本,但燃烧后会产生有毒废气,需要对其净化处理才能排放。
火法工艺通常具有更高的成本效益,对废旧电池原材料组成要求较低,但火法需要消耗大量的能量,回收的过程可能将金属氧化,并且焚烧含Al、Li和有机物的电极回收过程,环境控制难度较大。
2.3 联合工艺
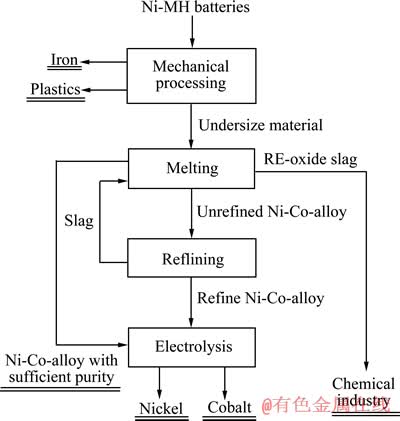
图4 火法工艺回收镍氢电池过程流程图[64]
Fig. 4 Flowchart of generic pyrometallurgical recycling process of Ni-MH batteries[64]
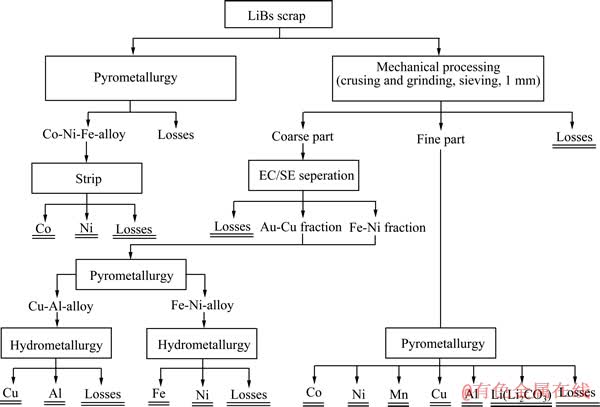
图5 物理分离、火法、湿法工艺回收锂离子电池过程[9]
Fig. 5 Li-ion batteries recycling by mechanical processing, pyrometallurgy and hydrometallurgy[9]
火法最大的缺点就是不能回收Li,因此火湿法联合工艺应运而生。图5所示为物理分离、火法、湿法工艺回收锂离子电池过程[9]。电池破碎后无需再经过任何预处理直接送到特制的熔炉里融化,炉渣中Cu、Fe、Mn则通过湿法工艺回收,而Al、Li等金属则因沸点较低难以得到回收[70]。少部分Li以炉渣的形式回收,将炉渣破碎研磨至粒径小于100 μm后用熔剂浸出回收Li;由于多次使用后熔剂对Co和Mn溶解饱和,因此后续使用时可减少Co和Mn成渣[70]。但大部分的Li是以烟道灰的形式形成了锂精矿,烟道灰的颗粒很小,不需要研磨可直接进行浸出。
HEEGN等[65]利用火湿法联合工艺对镍氢电池回收进行了研究。因MgO和SiO2作为熔渣体系时具有较大的黏度,不利于重力分离。而以15%CaO、85% CaF2作为熔剂体系可得到较低的共熔温度1360 ℃,稀土的氟化物和氯化物具有较高的沸点,不会气化蒸发而是以炉渣形式回收,而Ni、Co和Mn则以合金形式回收。炉渣中稀土元素通过湿法进行提纯,球磨至粒径小于0.1 mm后,用H2SO4溶解,过滤后往滤液添加NaOH调节pH值,稀土元素以难溶物(RE)2(SO4)3·Na2SO4·xH2O形式析出。但高氟的熔渣并不利于后续的浸出工艺[63]。
3 动力电池工业化回收技术
表3所列为国外电池回收公司的工艺[14, 28, 69-84]。由表3可知,AEA公司回收锂离子电池通过在低温下破碎后,分离出钢材后加入乙腈作为有机溶剂提取电解液,再以N-甲基吡咯烷酮(NMP)为溶剂提取粘合剂(PVDF),然后对固体进行分选,得到Cu、Al和塑料,在LiOH溶液中电沉积回收溶液中的Co,产物为CoO[85]。
Recupyl公司在惰性混合气体保护下对电池进行破碎,磁选分离得到纸、塑料、钢铁和铜,以LiOH溶液浸出部分金属离子,不溶物再用H2SO4浸出,加入Na2CO3得到Cu和其他金属的沉淀物,过滤后滤液溶液中加入NaClO氧化处理得到Co(OH)3沉淀和Li2SO4的溶液,将惰性气体中的CO2通入含Li的溶液中得到Li2CO3沉淀。
Umicore公司通过特制的熔炉回收锂离子电池和镍氢电池制得Co(OH)2/CoCl2和Ni(OH)2,石墨和有机溶剂则作为燃料放出能量,Cu、Zn、Mn和Fe则用湿法回收,CoCl2制备电极材料LiCoO2出售[86]。
Mitsubishi公司采用液氮将废旧电池冷冻后拆解,分选出塑料,破碎、磁选、水洗得到钢铁,振动分离,经分选筛水洗后得到铜箔,剩余的颗粒进行燃烧得到LiCoO2,排出的气体用Ca(OH)2吸收得到CaF2和Ca3(PO4)2。
IME公司回收锂离子电池通过分选电池外壳和电极材料后,将电极材料置于反应罐中加热至250 ℃使电解液挥发后冷凝回收,再对粉末进行破碎、筛选、磁选分离和锯齿形分类器将大颗粒(粒径大于200 μm,主要含有Fe和Ni)和小颗粒(粒径小于200 μm,主要含有Al和电极材料)分离。采用电弧炉熔解小颗粒部分,制得钴合金;采用湿法溶解烟道灰和炉渣制得Li2CO3。
低温可大大降低Li的化学反应活性,Toxco公司[41]在-198 ℃(74 K)下将电池破碎后加入固体NaOH,此时金属Li转化成LiOH,再加入CO32-使LiOH反应生成Li2CO3。球磨后,粉末在颠选板上洗涤,LiCoO2和Li2CO3等电极材料与塑料分离。LiCoO2和Li2CO3等电极材料可直接售往电池制造企业。有研究[87]报道,LiFePO4电极材料经低温处理后较简单的回收具有更大的容量(接近理论值170 mA·h/g)。低温球磨法具有工艺简单、环境友好、成本低等优点。
OnTo公司采用CO2超临界流体恢复锂离子电池的容量[88],将电池放在干燥的环境下,调节适当的压力和温度,液态的CO2溶解电池中的电解液转移到回收的容器后改变温度和压力使CO2气化,电解液析出。电解液被循环的超临界CO2携带出来,注入新的电解液后用环氧树脂封口,使电池恢复充放电能力。
表3 国外电池回收公司的工艺
Table 3 Methods for battery recycling used by abroad organizations

4 回收电池的安全问题
回收处理锂离子电池时需要注意两个问题。1) 如何处置有害废弃物;2) 如何避免机械处理过程引起锂离子电池发生爆炸。
火法工艺中磁选分离有机物隔膜和塑料可避免高温处理时电极隔膜和塑料外壳燃烧释放出有毒废气。湿法工艺中常见的酸浸和萃取会产生大量含有Ni、Co、Mn、Cu的废水,不经处理直接排放不仅浪费有价金属,还对环境造成二次污染。李长东等[89]对废水先进行分步混凝沉降后进行砂滤、炭滤和离子交换处理。分步混凝沉淀是将废水用碳酸钠饱和溶液调节pH值至8.5~9.8,加入絮凝剂聚合氯化铝和助凝剂聚丙烯酰胺使Ni和Co沉淀析出,再加入碱液和絮凝剂使Mn和Cu以沉淀析出。然后以硫酸调节pH值至8.1~8.5依次进行砂滤、炭滤和离子交换处理,离子交换单元采用弱酸性Na型离子树脂。出水水质达到了国家污水综合排放标准。
正常使用时锂离子电池发生爆炸的概率较低,但当电池在非正常条件下工作时,如充电回路失效或过充电,则有可能产生金属Li[90]。回收时拆解出的金属Li接触到水后将发生反应,放出大量的H2和热量引起爆炸。这个潜在的问题在工业规模回收时不容忽视,解决方法大致有以下5个途径:
1) 在惰性或保护气氛下进行机械处理;
2) 低温处理;
3) 火法处理;
4) 在锂盐溶液中进行机械处理;
5) 避免采用机械处理。
虽然低温处理可以避免爆炸,但是维持低温需要消耗大量的液氮,成本很高。SOHN等[91]将锂离子电池用0.5 mol/L的硫酸浸泡放电,电池中的金属Li大部分溶解在硫酸中,以此避免破碎时发生爆炸。火法工艺也有爆炸的可能,当有机溶剂在电池单体中被外壳困住,受热时突然挥发并瞬间释放出大量有机溶剂,引起温度骤升导致爆炸。采用分阶段缓慢升温可使有机溶剂蒸汽安全地从电池中逸出的方式避免爆炸[57]。
5 小结与展望
大规模推广电动汽车,可以降低汽车对石油的依赖,减少温室气体的排放。但是昂贵的造价不是每个消费者都能承受,降低电池的成本成为必然的趋势。目前,动力汽车电池的研究主要集中在锂离子电池上,预计将来的电极材料将采用成本较低的LiFePO4、 LiNi1/3Co1/3Mn1/3O2、LiNi0.8Co0.15Al0.05O2、LiMn2O4、LiTi4O7和LiSix等。昂贵的金属Co将不再使用,因此需要对现有的回收工艺作出适当调整;另一方面,回收的利润下降,未来应加强废电池回收综合利用技术的工业化研究,寻求更廉价、更环保高效的回收处理技术,如超临界流体技术。另外,处理过程中产生的污染和安全性问题也需要进行系统的研究。由于不同的厂家生产出来的锂离子电池成分不尽相同,回收的动力电池成分必然各有差异,并且动力电池更新换代较快,因此对原料成分要求低、适用范围广、低能耗、低污染的回收工艺成为今后研究的重点。
REFFERENCES
[1]  . Lithium availability and future production outlooks[J]. Applied Energy, 2013, 110(10): 252-266.
. Lithium availability and future production outlooks[J]. Applied Energy, 2013, 110(10): 252-266.
[2] MIEDEMA J H, MOLL H C. Lithium availability in the EU27 for battery-driven vehicles: The impact of recycling and substitution on the confrontation between supply and demand until 2050[J]. Resources Policy, 2013, 38(2): 204-211.
[3] NELSON P A, BLOOM K G, I DEES D W. Modeling the performance and cost of lithium-ion batteries for electric-drive vehicles (No. ANL-11/32)[R]. 2011. Argonne National Laboratory (ANL), Argonne, IL (United States). http://www.ipd. anl.gov/ anlpubs/2011/10/71302.pdf.
[4] 池永庆, 孙彦平. 锂离子动力电池负极材料研究进展[J]. 材料导报, 2012, 26(21): 20-24, 55.
CHI Yong-qing, Sun Yan-ping. Research progress in anode materials for power Li-ion batteries[J]. Materials Review, 2012, 26(21): 20-24, 55.
[5] BERNARDES A M, ESPINOSA D C R, 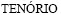 J A S. Recycling of batteries: A review of current processes and technologies[J]. Journal of Power Sources, 2004, 130(1): 291-298.
J A S. Recycling of batteries: A review of current processes and technologies[J]. Journal of Power Sources, 2004, 130(1): 291-298.
[6] ZAGHIB K, STRIEBEL K, GUERFI A, SHIM J, ANNAUD M, GAUTHIER M. LiFePO4/polymer/natural graphite: Low cost Li-ion batteries[J]. Electrochimica Acta, 2004, 50(2): 263-270.
[7] MAROM R, HAIK O, AURBACH D, HALALAY I C. Revisiting LiClO4 as an electrolyte for rechargeable lithium-ion batteries[J]. Journal of the Electrochemical Society, 2010, 157(8): A972-A983.
[8] 尹成果, 马玉林, 程新群, 尹鸽平. 锂离子电池高温电解液[J]. 化学进展, 2013, 25(1): 54-59.
YIN Cheng-guo, MA Yu-lin, CHENG Xin-qun, YIN Ge-ping. Elevated-temperature electrolytes for Li-ion batteries[J]. Progress in Chemistry, 2013, 25(1): 54-59.
[9] AL-THYABAT S, NAKAMURA T, SHIBATA E, IIZUKAB A. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review[J]. Minerals Engineering, 2013, 45: 4-17.
[10] LARSSON K, EKBERG C, 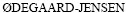 A. Dissolution and characterization of HEV NiMH batteries[J]. Waste Management, 2013, 33(3): 689-698.
A. Dissolution and characterization of HEV NiMH batteries[J]. Waste Management, 2013, 33(3): 689-698.
[11] QC/T 743—2006. 电动汽车用锂离子蓄电池[S].
QC/T 743—2006. Lithium-ion batteries for electric vehicles[S].
[12] QC/T 744—2006. 电动汽车用金属氢化物镍蓄电池[S].
QC/T 744—2006. Nickel-metal hydride batteries for electric vehicles[S].
[13] RIEGEL B, SAUER D U. Vehicle batteries in China and Germany[EB/OL]. 2009. http://m.dena.de/fileadmin/user_ upload/ Projekte/Verkehr/Dokumente/GCSFP-Study_-_Vehicle_Batteries_in_China_and_Germany.pdf.
[14] 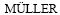 D I T. Development of a new metallurgical process for closed-loop recycling of discarded nickel-metalhydride- batteries[C]// Proceedings of EMC, 2003: 1. http://www. metallurgie. rwth-aachen.de/data/publications/development_of_a_new_id_5253.pdf.
D I T. Development of a new metallurgical process for closed-loop recycling of discarded nickel-metalhydride- batteries[C]// Proceedings of EMC, 2003: 1. http://www. metallurgie. rwth-aachen.de/data/publications/development_of_a_new_id_5253.pdf.
[15] GAINES L, SULLIVAN J, BURNHAM A,
[16] DEWULF J, van der VORST G, DENTURCK K, van LANGENHOVE H, GHYOOT W, TYTGAT J, VANDEPUTTE K. Recycling rechargeable lithium ion batteries: Critical analysis of natural resource savings[J]. Resources, Conservation and Recycling, 2010, 54(4): 229-234.
[17] DUNN J B, GAINES L, SULLIVAN J, WANG M Q. Impact of recycling on cradle-to-gate energy consumption and greenhouse gas emissions of automotive lithium-ion batteries[J]. Environmental Science & Technology, 2012, 46(22): 12704-12710.
[18] 林俊仁, 张怡隆, 江玉琳, 许哲源. 从废二次电池回收有价金属的方法. 中国, ZL 200310103584.7[P]. 2003-11-11.
LIN Jun-ren, ZHANG Yi-long, JIANG Yu-lin, XU Zhe-yuan. Method for recovering valuable metal from waste secondary cell: CN, 200310103584.7[P]. 2003-11-11.
[19] LARSSON K, EKBERG C, 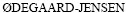 A. Using Cyanex 923 for selective extraction in a high concentration chloride medium on nickel metal hydride battery waste[J]. Hydrometallurgy, 2012, 129/130: 35-42.
A. Using Cyanex 923 for selective extraction in a high concentration chloride medium on nickel metal hydride battery waste[J]. Hydrometallurgy, 2012, 129/130: 35-42.
[20] KOSARAJU S. Investigation of HEV Li-ion batteries for lithium recovery[EB/OL]. 2012. http://publications.lib.chalmers.se/records/ fulltext/163605.pdf.
[21] NOGUEIRA C A, MARGARIDO F. Battery recycling by hydrometallurgy: Evaluation of simultaneous treatment of several cell systems[R]. Energy Technology 2012: Carbon Dioxide Management and Other Technologies, 2012: 227-234.
[22] SUN L, QIU K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries[J]. Journal of hazardous materials, 2011, 194(30): 378-384.
[23] ITO M, KASHIWAYA K, SUMIYA N, HIROYOSHI N, TSUNEKAWA M. Anode activating agent recovery by magnetic separation from the <0.075 mm fraction of crushed nickel metal hydride batteries from hybrid vehicles[J]. Separation and Purification Technology, 2009, 69(2): 149-152.
[24] HUANG K, LI J, XU Z. Enhancement of the recycling of waste Ni-Cd and Ni-MH batteries by mechanical treatment[J]. Waste Management, 2011, 31(6): 1292-1299.
[25] 张 涛, 吴彩斌, 王成彦, 何亚群. 废弃手机锂离子电池机械破碎的基础研究[J]. 中南大学学报: 自然科学版, 2012, 43(9): 3355-3362.
ZHANG Tao, WU Cai-bin, WANG Cheng-yan, HE Ya-qun. Mechanical crushing properties of spent cell phone lithium-ion batteries[J]. Journal of Central South University: Science and Technology, 2012, 43(9): 3355-3362.
[26] 卢毅屏, 夏自发, 冯其明, 龙 涛, 欧乐明, 张国范. 废锂离子电池中集流体与活性物质的分离[J]. 中国有色金属学报, 2007, 17(6): 997-1001.
LU Yi-ping, XIA Zi-fa, FENG Qi-ming, LONG Tao, OU Le-ming, ZHANG Guo-fan. Separation of current collectors and active materials from spent lithium-ion secondary batteries[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(6): 997-1001.
[27] YU H J, ZHANG T Z, YUAN J, LI C D, LI J M. Trial study on EV battery recycling standardization development[J]. Advanced Materials Research, 2013, 610: 2170-2173.
[28] 吴 越, 裴 锋, 贾路路, 刘晓磊, 张文华, 刘 平. 废旧锂离子电池中有价金属的回收技术进展[J]. 稀有金属, 2013, 37(2): 320-329.
WU Yue, PEI Feng, JIA Lu-lu, LIU Xiao-lei, ZHANG Wen-hua, LIU Ping. Overview of recovery technique of valuable metals from spent lithium ion batteries[J]. Chinese Journal of Rare Metals, 2013, 37(2): 320-329.
[29] FERREIRA D A, PRADOS L M Z, MAJUSTE D, MANSUR M B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries[J]. Journal of Power Sources, 2009, 187(1): 238-246.
[30] 谢光炎, 凌 云, 钟 胜. 废旧锂离子电池回收处理技术研究进展[J]. 环境科学与技术, 2009, 32(4): 97-101.
XIE Guang-yan, LING Yun, ZHONG Sheng. Overview of recovery techniques of spent lithium-ion batteries[J]. Environmental Science & Technology, 2009, 32(4): 97-101.
[31] WANG J, CHEN M, CHEN H, LUO T, XU Z. Leaching study of spent Li-ion batteries[J]. Procedia Environmental Sciences, 2012, 16: 443-450.
[32] LI L, LU J, REN Y, ZHANG X X, CHEN R J, WU F, AMINE K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries[J]. Journal of Power Sources, 2012, 218(15): 21-27.
[33] XIN B, ZHANG D, ZHANG X, XIA Y, WU F, CHEN S, LI L. Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria[J]. Bioresource Technology, 2009, 100(24): 6163-6169.
[34] PIETRELLI L, BELLOMO B, FONTANA D. Rare earths recovery from NiMH spent batteries[J]. Hydrometallurgy, 2002, 66(1): 135-139.
[35] ZHANG P, YOKOYAMA T, ITABASHI O. Hydrometallurgical process for recovery of metal values from spent nickel-metal hydride secondary batteries[J]. Hydrometallurgy, 1998, 50(1): 61-75.
[36] FERNANDES A, AFONSO J C, DUTRA A J B. Separation of nickel (Ⅱ), cobalt (Ⅱ) and lanthanides from spent Ni-MH batteries by hydrochloric acid leaching, solvent extraction and precipitation[J]. Hydrometallurgy, 2013, 133: 37-43.
[37] INNOCENZI V,  F. Recovery of rare earths and base metals from spent nickel-metal hydride batteries by sequential sulphuric acid leaching and selective precipitations[J]. Journal of Power Sources, 2012, 211(1): 184-191.
F. Recovery of rare earths and base metals from spent nickel-metal hydride batteries by sequential sulphuric acid leaching and selective precipitations[J]. Journal of Power Sources, 2012, 211(1): 184-191.
[38] GASSER M S, ALY M I. Separation and recovery of rare earth elements from spent nickel-metal-hydride batteries using synthetic adsorbent[J]. International Journal of Mineral Processing, 2013, 121(10): 31-38.
[39] NAN J, HAN D, ZUO X J. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction[J]. Power Sources, 2005, 152(1): 278-284.
[40] SUZUKI T, NAKAMURA T, INOUE Y, NIINAE M, SHIBATA J. A hydrometallurgical process for the separation of aluminum, cobalt, copper and lithium in acidic sulfate media[J]. Separation and Purification Technology, 2012, 98(19): 396-401.
[41] KANG J, SENANAYAKE G, SOHN J, SHIN S M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272[J]. Hydrometallurgy, 2010, 100(3): 168-171.
[42] PROVAZI K, CAMPOS B A, ESPINOSA D C R,  J A S. Metal separation from mixed types of batteries using selective precipitation and liquid-liquid extraction techniques[J]. Waste Management, 2011, 31(1): 59-64.
J A S. Metal separation from mixed types of batteries using selective precipitation and liquid-liquid extraction techniques[J]. Waste Management, 2011, 31(1): 59-64.
[43] JHA A K, JHA M K, KUMARI A, SAHU S K, KUMAR V, PANDEY B D. Selective separation and recovery of cobalt from leach liquor of discarded Li-ion batteries using thiophosphinic extractant[J]. Separation and Purification Technology, 2013, 104(5): 160-166.
[44] GRANATA G, PAGNANELLI F, MOSCARDINI E. Simultaneous recycling of nickel metal hydride, lithium ion and primary lithium batteries: Accomplishment of European guidelines by optimizing mechanical pre-treatment and solvent extraction operations[J]. Journal of Power Sources, 2012, 212(15): 205-211.
[45] INNOCENZI V,  F. Separation of manganese, zinc and nickel from leaching solution of nickel-metal hydride spent batteries by solvent extraction[J]. Hydrometallurgy, 2012, 129/130: 50-58.
F. Separation of manganese, zinc and nickel from leaching solution of nickel-metal hydride spent batteries by solvent extraction[J]. Hydrometallurgy, 2012, 129/130: 50-58.
[46] ZHANG P, YOKOYAMA T, ITABASHI O, SUZUKI T M, INOUE K. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries[J]. Hydrometallurgy, 1998, 47(2): 259-271.
[47] LARSSON K, EKBERG C,  A. Using Cyanex 923 for selective extraction in a high concentration chloride medium on nickel metal hydride battery waste. Part Ⅱ: Mixer-settler experiments[J]. Hydrometallurgy, 2013, 133: 168-175.
A. Using Cyanex 923 for selective extraction in a high concentration chloride medium on nickel metal hydride battery waste. Part Ⅱ: Mixer-settler experiments[J]. Hydrometallurgy, 2013, 133: 168-175.
[48] CHEN L, TANG X, ZHANG Y, LI L, ZENG Z, ZHANG Y. Process for the recovery of cobalt oxalate from spent lithium-ion batteries[J]. Hydrometallurgy, 2011, 108(1): 80-86.
[49] 陈 亮, 唐新村, 张 阳, 瞿 毅, 王志敏. 从废旧锂离子电池中分离回收钴镍锰[J]. 中国有色金属学报, 2011, 21(5): 1192-1198.
CHEN Lian, TANG Xin-cun, ZHANG Yan, QU Yi, WANG Zhi-min. Separation and recovery of Ni, Co and Mn from spent lithium-ion batteries[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(5): 1192-1198.
[50] LI L, DUNN J B, ZHANG X X, GAINES L, CHEN R J, WU F, AMINE K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment[J]. Journal of Power Sources, 2013, 233(1): 180-189.
[51] FREITAS M, GARCIA E M. Electrochemical recycling of cobalt from cathodes of spent lithium-ion batteries[J]. Journal of Power Sources, 2007, 171(2): 953-959.
[52] FREITAS M, CELANTE V G, PIETRE M K. Electrochemical recovery of cobalt and copper from spent Li-ion batteries as multilayer deposits[J]. Journal of Power Sources, 2010, 195(10): 3309-3315.
[53] GARCIA E M, SANTOS J S, PEREIRA E C, FREITAS M B J G. Electrodeposition of cobalt from spent Li-ion battery cathodes by the electrochemistry quartz crystal microbalance technique[J]. Journal of Power Sources, 2008, 185(1): 549-553.
[54] SANTOS V E O, CELANTE V G, LELIS M F F, FREITAS M B J G. Chemical and electrochemical recycling of the nickel, cobalt, zinc and manganese from the positives electrodes of spent Ni–MH batteries from mobile phones[J]. Journal of Power Sources, 2012, 218(15): 435-444.
[55] LUPI C, PASQUALI M. Electrolytic nickel recovery from lithium-ion batteries[J]. Minerals Engineering, 2003, 16(6): 537-542.
[56] 李长东, 黄国勇, 徐盛明. 从镍氢电池正极废料中回收、制备超细金属镍粉的方法: 中国, ZL 200710031418.9[P]. 2007. 2007-11-16.
LI Chang-dong, HUANG Guo-yong, XU Sheng-ming. Method for recycling and preparing superfine nickel powder from nickel-hydrogen cell: CN, 200710031418.9[P]. 2007-11-16.
[57] 李 丽, 吴 锋, 陈人杰, 葛 静, 陈 实, 吴伯荣. 利用废旧锂离子电池回收制备钴酸锂的方法: 中国, ZL 200910093727.8[P]. 2009-09-25.
LI Li, WU Feng, CHEN Ren-jie, GE Jing, CHEN Shi, WU Bo-rong. Method for recovering and preparing lithium cobalt oxide by using disused lithium battery: CN, 200910093727.8[P]. 2009-09-25.
[58] TEDJAR F, FOUDRAZ J. Method for the mixed recycling of lithium-based anode batteries and cells: US, 7820317 B2[P]. 2005-04-04.
[59] 赵存良, 孙玉壮, 孟志强, 欧阳赛兰. 一种从废旧电池中回收锂金属的方法: 中国, ZL 201110116172.1[P]. 2011-05-06.
ZHAO Cun-liang, SUN Yu-zhuang, MENG Zhi-qiang, OUYANG Sai-lan. Method for recovering lithium metal from used batteries: CN, 201110116172.1[P]. 2011-05-06.
[60] 马 伟, 王 刃, 田丽妍, 王 禄, 秦承欢, 程子洪, 杨 港. 一种从锂离子电池中分离回收锂和钴的方法: 中国, ZL 200910300759.0[P]. 2009-03-09.
MA Wei, WANG Ren, TIAN Li-yan, WANG Lu, QIN Cheng-huan, CHENG Zhi-gang, YANG Gang. Method for separating and recovering lithium and cobalt from lithium ion cell: CN, 200910300759.0[P]. 2009-03-09.
[61] 谭群英, 周汉章, 唐红辉, 王 皓, 蒋快良, 李长东. 一种从废旧电池中回收锂金属的方法: 中国, ZL 201010523257.7[P]. 2010-10-28.
TAN Qun-ying, ZHOU Han-zhang, TANG Hong-hui, WANG Hao, JIANG Kuai-liang, LI Chang-dong. Method for recovering lithium from waste lithium ion battery and waste pole piece: CN, 201010523257.7[P]. 2010-10-28.
[62] GEORGI D I T. Investigation of a slag system for a Li-ion battery recycling process in the EAF[C]// Proceedings of EMC. 2007: 1.
[63]  T, FRIEDRICH B. A new metallurgical process for recycling of discharged nickel-metalhydride-batteries[C]// TMS Fall 2002 EPD Meeting on Recycling and Waste Treatment in Mineral and Metal Processing, 2002. http://www.metallurgie. rwth-aachen.de/data/publications/2002_tms_paeper_id_4409.pdf.
T, FRIEDRICH B. A new metallurgical process for recycling of discharged nickel-metalhydride-batteries[C]// TMS Fall 2002 EPD Meeting on Recycling and Waste Treatment in Mineral and Metal Processing, 2002. http://www.metallurgie. rwth-aachen.de/data/publications/2002_tms_paeper_id_4409.pdf.
[64]  T, FRIEDRICH B. Development of a recycling process for nickel-metal hydride batteries[J]. Journal of Power Sources, 2006, 158(2): 1498-1509.
T, FRIEDRICH B. Development of a recycling process for nickel-metal hydride batteries[J]. Journal of Power Sources, 2006, 158(2): 1498-1509.
[65] HEEGN H P, FRIEDRICH B,  T, WEYHE R. Closed-loop reycling of nickel, cobalt and rare earth metals from spent nickel-metalhydride batteries[C]// Proc: XXII International Mineral Processing Congress, Cape Town, South Africa, 2003. http://www.metallurgie.rwth-aachen.de/data/publications/uvr_ heegn_paepe_id_4020.pdf.
T, WEYHE R. Closed-loop reycling of nickel, cobalt and rare earth metals from spent nickel-metalhydride batteries[C]// Proc: XXII International Mineral Processing Congress, Cape Town, South Africa, 2003. http://www.metallurgie.rwth-aachen.de/data/publications/uvr_ heegn_paepe_id_4020.pdf.
[66] 王常春, 郭靖洪, 姜 波, 沈 欣. 一种从废旧锂电池中回收金属的方法: 中国, ZL 201110192260.X[P]. 2011-07-08.
WANG Chang-chun, GUO Jing-hong, JIANG Bo, SHEN Xin. Method for recovering metal from waste lithium battery: CN, 201110192260.X[P]. 2011-07-08.
[67] 王常春, 郭靖洪, 姜 波, 沈 欣. 一种从废旧镍氢电池中回收金属的方法: 中国, ZL 201110173754.3[P]. 2011-06-25.
WANG Chang-chun, GUO Jing-hong, JIANG Bo, SHEN Xin. Method for recycling metals from waste nickel-hydrogen batteries: CN, 201110173754.3[P]. 2011-06-25
[68] CHERET D C,  S. Battery recycling: EP, 1589121 B1[P]. 2005-03-30.
S. Battery recycling: EP, 1589121 B1[P]. 2005-03-30.
[69] BROUSSELY M, PISTOIA G. Industrial applications of batteries from cars to aerospace and energy storage[M]. Oxford, UK: Elsevier, 2007: 691-736.
[70] GEORGI-MASCHLER T, FRIEDRICH B, WEYHE R, HEEGN H, RUTZ M. Development of a recycling process for Li-ion batteries[J]. Journal of Power Sources, 2012, 207(1): 173-182.
[71] LAIN M J. Recycling of galvanic cells: US, 6447669B1[P]. 1998-12-03.
[72] LAIN M J. Recycling of lithium ion cells and batteries[J]. Journal of Power Sources, 2001, 97: 736-738.
[73] BATRE C. Lithium battery recycling[EB/OL]. 2009-07-28. http://www.batrec.ch/files/downloads/8c91f7dd0ab6693ccfd78380164a346d/S.18_Flyer%20Lithium.pdf.
[74] FISHER K,  E, LAENEN P P, COLLINS M. Battery waste management life cycle assessment[EB/OL]. 2006. http://www.lcm2007.org/paper/424.pdf
E, LAENEN P P, COLLINS M. Battery waste management life cycle assessment[EB/OL]. 2006. http://www.lcm2007.org/paper/424.pdf
[75] TANII T, TSUZUKI S, HONMURA S, KAMIMURA T, SASAKI K, YABUKI M, NISHIDA K. Method for crushing cell: US, 6524737[P]. 1999-09-27.
[76] ELLIS T W, MIRZA A H. Battery recycling: defining the market and identifying the technology required to keep high value materials in the economy and out of the waste dump[EB/OL]. 2011. http://www.nist.gov/tip/wp/pswp/upload/245_battery_recycling_ defining_the_market.pdf.
[77] SLOOP S E. System and method for removing an electrolyte from an energy storage and/or conversion device using a supercritical fluid: US, 7198865[P]. 2003-01-09.
[78] SLOOP S E, KERR J B, KINOSHITA K. The role of Li-ion battery electrolyte reactivity in performance decline and self-discharge[J]. Journal of Power Sources, 2003, 119/121(1): 330-337.
[79] SLOOP S E. Recycling methods for lithium-ion and other batteries[C]//13th International Battery Materials Recycling Seminar, Ft. Lauderdale, FL. 2009.
[80] TEDJAR F, FOUDRAZ J C. Method for the mixed recycling of lithium-based anode batteries and cells: WO, 2005101564[P]. 2005-10-27.
[81] MILLER D G, MCLAUGHLIN B. Recycling the lithium battery[J]. Industrial Chemistry Library, 2001, 10: 263-293.
[82] MCLAUGHLIN W, ADAMS T S. Li reclamation process: US, 5888463[P]. 1999-01-02.
[83] JUNGST R G. Recycling of advanced batteries for electric vehicles[C]// 11th international battery waste management seminar, Deerfield Beach, FL (US)[EB/OL]. http://www.osti. gov/energycitations/servlets/purl/14070-HUPGbA/web.1999.
[84] CHERET D, SANTEN S. Battery Recycling: US, 7169206[P]. 2005-04-18.
[85] VADENBO C O. Prospective environmental assessment of lithium recovery in battery recycling[EB/OL]. 2009. http://www.uns. ethz. ch/pub/publications/pdf/1717.pdf.
[86]  C, REFINING U P M, GREINERSTRAAT A. Recycling of electronic scrap at Umicore’s integrated metals smelter and refinery[C]// Proceedings of EMC, 2005: 307-323.
C, REFINING U P M, GREINERSTRAAT A. Recycling of electronic scrap at Umicore’s integrated metals smelter and refinery[C]// Proceedings of EMC, 2005: 307-323.
[87] KOTAICH K, SLOOP S E. Cobalt-free batteries, a new frontier for advanced battery recycling[C]// IEEE International Symposium on Sustainable Systems and Technology, 2009. ISSST'09, IEEE, 2009: 1.
[88] SLOOP S E. System and method for removing an electrolyte from an energy storage and/or conversion device using a supercritical fluid: US, 7858216[P]. 2007-03-29.
[89] 李长东, 黄国勇, 龙桂花. 废旧电池处理过程中产生的镍钴锰废水的处理方法: 中国, ZL 200910044152.0[P]. 2009-08-18.
LI Chang-dong, HUANG Guo-yong, LONG Gui-hua. Treatment method of nickel-cobalt-manganese wastewater generated in waste and old battery treatment process: CN, 200910044152.0[P]. 2009-08-18.
[90] BECKER-IRVIN C, HONDA M Y. Battery overtemperature control system and method: US, 6928381B2[P]. 2003-12-17.
[91] SOHN J S, SHIN S M, YANG D H, KANG J G, YOO K. Study of physical treatment of spent military use lithium primary batteries for recycling[J]. Geosystem Engineering, 2007, 10(2): 27-30.
(编辑 何学锋)
基金项目:广东省战略性新兴产业核心技术攻关项目(2011A032302001);广东省产学研结合项目(2011A090700002)
收稿日期:2013-06-17;修订日期:2013-09-23
通信作者:余海军,学士;电话:0757-85615818;E-mail: yuhaijun@brunp.com.cn
摘 要:近年来,作为高能汽车动力电池的镍氢电池和锂离子电池凭借其能量密度高、充放电速度快、循环寿命长以及无污染等优点得到快速发展。但经过数百至上千次的循环充放电后其容量下降并最终报废,从保护环境、节约自然资源角度看,回收电池再利用成为必然。作者总结了近年来国内外回收利用锂离子电池和镍氢电池的方法,包括湿法工艺、火法工艺和联合工艺等,并对各工艺作出了评价;概述了研究现状中存在的二次污染、安全性问题与解决方法和回收制备产物的种类,为中国未来动力电池回收利用奠定基础。
[4] 池永庆, 孙彦平. 锂离子动力电池负极材料研究进展[J]. 材料导报, 2012, 26(21): 20-24, 55.
[8] 尹成果, 马玉林, 程新群, 尹鸽平. 锂离子电池高温电解液[J]. 化学进展, 2013, 25(1): 54-59.
[11] QC/T 743—2006. 电动汽车用锂离子蓄电池[S].
[12] QC/T 744—2006. 电动汽车用金属氢化物镍蓄电池[S].
[18] 林俊仁, 张怡隆, 江玉琳, 许哲源. 从废二次电池回收有价金属的方法. 中国, ZL 200310103584.7[P]. 2003-11-11.
[25] 张 涛, 吴彩斌, 王成彦, 何亚群. 废弃手机锂离子电池机械破碎的基础研究[J]. 中南大学学报: 自然科学版, 2012, 43(9): 3355-3362.
[26] 卢毅屏, 夏自发, 冯其明, 龙 涛, 欧乐明, 张国范. 废锂离子电池中集流体与活性物质的分离[J]. 中国有色金属学报, 2007, 17(6): 997-1001.
[28] 吴 越, 裴 锋, 贾路路, 刘晓磊, 张文华, 刘 平. 废旧锂离子电池中有价金属的回收技术进展[J]. 稀有金属, 2013, 37(2): 320-329.
[30] 谢光炎, 凌 云, 钟 胜. 废旧锂离子电池回收处理技术研究进展[J]. 环境科学与技术, 2009, 32(4): 97-101.
[49] 陈 亮, 唐新村, 张 阳, 瞿 毅, 王志敏. 从废旧锂离子电池中分离回收钴镍锰[J]. 中国有色金属学报, 2011, 21(5): 1192-1198.
[56] 李长东, 黄国勇, 徐盛明. 从镍氢电池正极废料中回收、制备超细金属镍粉的方法: 中国, ZL 200710031418.9[P]. 2007. 2007-11-16.
[57] 李 丽, 吴 锋, 陈人杰, 葛 静, 陈 实, 吴伯荣. 利用废旧锂离子电池回收制备钴酸锂的方法: 中国, ZL 200910093727.8[P]. 2009-09-25.
[59] 赵存良, 孙玉壮, 孟志强, 欧阳赛兰. 一种从废旧电池中回收锂金属的方法: 中国, ZL 201110116172.1[P]. 2011-05-06.
[60] 马 伟, 王 刃, 田丽妍, 王 禄, 秦承欢, 程子洪, 杨 港. 一种从锂离子电池中分离回收锂和钴的方法: 中国, ZL 200910300759.0[P]. 2009-03-09.
[61] 谭群英, 周汉章, 唐红辉, 王 皓, 蒋快良, 李长东. 一种从废旧电池中回收锂金属的方法: 中国, ZL 201010523257.7[P]. 2010-10-28.
[66] 王常春, 郭靖洪, 姜 波, 沈 欣. 一种从废旧锂电池中回收金属的方法: 中国, ZL 201110192260.X[P]. 2011-07-08.
[67] 王常春, 郭靖洪, 姜 波, 沈 欣. 一种从废旧镍氢电池中回收金属的方法: 中国, ZL 201110173754.3[P]. 2011-06-25.
[68] CHERET D C, S. Battery recycling: EP, 1589121 B1[P]. 2005-03-30.
[71] LAIN M J. Recycling of galvanic cells: US, 6447669B1[P]. 1998-12-03.
[82] MCLAUGHLIN W, ADAMS T S. Li reclamation process: US, 5888463[P]. 1999-01-02.
[84] CHERET D, SANTEN S. Battery Recycling: US, 7169206[P]. 2005-04-18.
[89] 李长东, 黄国勇, 龙桂花. 废旧电池处理过程中产生的镍钴锰废水的处理方法: 中国, ZL 200910044152.0[P]. 2009-08-18.


