
Electrochemical behavior of Co-Cr and Ni-Cr dental cast alloys
Viswanathan S. SAJI, Han-Cheol CHOE
Department of Dental Materials, College of Dentistry, Chosun University, Gwangju 501-759, Korea
Received 18 June 2008; accepted 10 March 2009
Abstract:
The cast structures influencing the electrochemical corrosion behavior of Co-Cr and Ni-Cr dental alloys were studied using potentiodynamic polarization and AC impedance in 0.9% (mass fraction) NaCl solution at (37±1) ℃. The phase and microstructure of the alloys that were fabricated using two different casting methods viz. centrifugal casting and high frequency induction casting, were examined using X-ray diffraction analysis, scanning electron microscopy and energy dispersive spectroscopy. The roles of alloying elements and the passive film homogeneity on the corrosion resistance of Co-Cr-Mo and Ni-Cr-Mo dental cast alloys were reviewed. The results of electrochemical study show that the dependence of corrosion resistance on the microstructure associated with the casting methods is marginal. The Co-Cr alloy exhibits more desirable corrosion resistance properties than the Ni-Cr alloy. There is severe preferential dissolution of Ni-rich, Cr and Mo depleted zones in the Ni-Cr alloy.
Key words:
Co-Cr-Mo; Ni-Cr-Mo; dental cast alloy; corrosion; electrochemical impedance spectroscopy;
1 Introduction
In dental restorations, the most decisive property of a cast alloy in biocompatibility is corrosion[1]. Co and Ni based alloys were widely used in dental skeletal structures and orthopedic implants such as screws, pins and plates. And recently they have been applied for making stents[1-2]. The advantages of these alloys include low cost of casting, matching thermal expansion coefficient with the ceramics of metal-ceramic restorations, and acceptable mechanical and tribological properties in vivo[3-5]. However, the possible release of toxic metal ions due to corrosion remains a major concern[3-4]. In vivo studies showed that corrosion products of Ni-Cr alloys decreased cellular proliferation[6-7].
Casting is one of the main methods of producing shaped metals and alloys. Electrochemical corrosion behavior of Co-Cr and Ni-Cr dental cast alloys depends primarily on the Cr and Mo levels in the alloy[8]. In commercial alloys, the compositions of Cr and Mo range from 11% to 25% and from 0 to 10% (mass fraction), respectively[9]. Both the microstructure and the casting defect have pronounced effect on the ion release in actual practice. The defects of dental cast alloys include mainly shrinkage porosity, inclusion, micro-crack and dendritic structure [10]. Only few reported works were available on the influence of casting procedures on the corrosion resistance of dental alloys[11-14].
In this work, Co-Cr-Mo and Ni-Cr-Mo alloys were fabricated by two different casting methods, centrifugal casting and high frequency induction casting. The corrosion behavior of the alloys was studied using potentiodynamic polarization method and impedance spectroscopy in de-aerated 0.9% NaCl solution at (37±1) ℃. The effects of the processing induced structure and passive film homogeneity on the electrochemical behavior of the alloys were discussed.
2 Experimental
The Co-Cr and Ni-Cr alloys were prepared for fabricating partial denture frameworks using centrifugal casting (Kerr, USA) and high frequency induction casting (Jelenko Eagle, USA). The casting temperatures used were 1 380 and 1 420 ℃, respectively. The samples for this study were selected from the runner area of the denture framework. Table 1 represents the chemical compositions of the alloys. The samples were chemically etched and the microstructures were analyzed using scanning electron microscope (FE-SEM, H-4700, Hitachi, Japan). The phase structure was identified by X-ray diffractometer using a Cu Kα radiation (XRD, X’pert Pro, Philips, Netherlands).
Table 1 Chemical compositions of alloys in mass fraction (%)
Corrosion behavior of the alloys was studied using a potentiostat/galvanostat (EG&G, 263A) and electro- chemical impedance spectroscope (EIS, EG&G, 1025). A conventional three-electrode system with high density graphite as counter electrode and saturated calomel electrode(SCE) as reference was used. The sample was mounted on a cold mount epoxy resin. The sample edges were carefully covered with the epoxy to avoid the possible crevice attack. The electrolyte was de-aerated with high purity Ar gas for 30 min before starting the experiment. De-aeration was continued at a uniform rate during the experiment and the solution was subjected to mild stirring using a magnetic stirrer. The scan rate used for potentiodynamic polarization was 1.667 mV/s. A similar three-electrode set-up was used for electro- chemical impedance spectroscopy(EIS) studies. The frequency range used for EIS was 10-1-105 Hz. The amplitude of AC signal was 10 mV and 5 points per decade was used.
3 Results and discussion
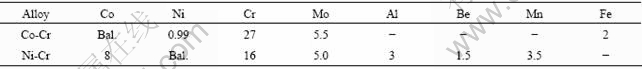
The microstructures of Co-Cr and Ni-Cr alloys after chemical etching are shown in Figs.1 and 2, respectively. The etched surface of the as-cast Co-Cr alloy (Fig.1) reveals two-phase dendritic structure. EDS analysis shows that the chemical compositions of the dendrite and interdendritic regions are close. However, the dendritic regions are slightly rich in Cr and poor in Co. An XRD analysis suggests that the dendrite and interdendritic regions correspond to hcp and fcc type crystal structures. Allotropic phase transformation of the elemental cobalt from high temperature fcc (α) phase to low temperature hcp (e) phase occurs at about 420 ℃ [15]. Alloying elements such as Fe and Ni can stabilize α phase; Cr and Mo tend to stabilize e structure[15].
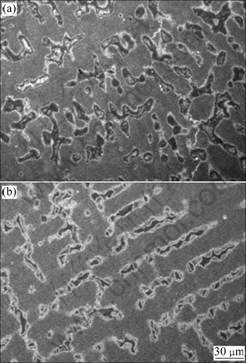
Fig.1 Representative FE-SEM images of cast structure of Co-Cr alloy: (a) Centrifugal casting; (b) High frequency induction casting
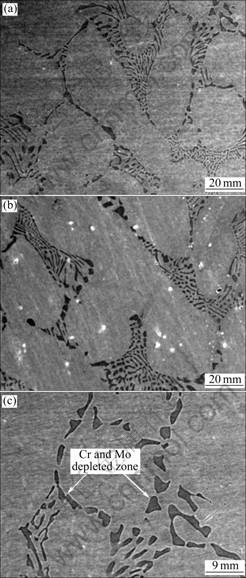
Fig.2 Representative FE-SEM images of cast structure of Ni-Cr alloy: (a) Centrifugal casting; (b) High frequency induction casting; (c) Higher magnification image of (a)
The corresponding SEM micrographs of the Ni-Cr alloys are shown in Fig.2. The microstructure exhibits two distinct regions in elemental composition: matrix and Ni-rich zones. The darker regions are rich in Ni and poor in Cr and Mo. EDS spectral analysis reveals that the compositions of the matrix and the Ni-rich zones are approximately 68Ni-20Cr-5Mo and 92Ni-4Cr-1Mo respectively. Preferential segregation of Ni rich phases occurs during solidification to a larger extent.
Nucleation and growth are two major mechanisms leading to the final structure of a solidifying metal. The final grain structure depends on the ratio of the temperature gradient in the liquid to the rate of advancement of the solid/liquid interface. For most alloys it is impracticable to avoid constitutional undercooling, and so, dendritic solidification occurs during the production of almost all alloys[16].
Fig.3 represents the potentiodynamic polarization plots recorded for the Co-Cr and Ni-Cr alloys. Corrosion current density(Jcorr) was evaluated by Tafel extrapolation method based on software based approximation. Table 2 represents the corresponding corrosion parameters. The Co-Cr alloy exhibits nobler Jcorr values than the Ni-Cr alloy, irrespective of the casting method followed. The passive region of the Co-Cr alloy is extended over a wide potential range. The breakdown potential(φpit), at which the current density increases abruptly, is nobler for the Co-Cr alloy. These observations indicate that the Co-Cr alloy possesses more desirable corrosion resistance property than the Ni-Cr alloy. Between two casting methods, the centrifugal casted alloys show comparatively lower Jcorr than the high frequency induction casted alloys.
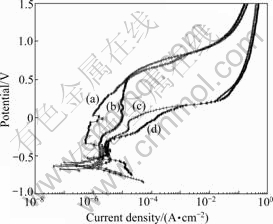
Fig.3 Potentiodynamic polarization plots: (a) Co-Cr alloy by centrifugal casting; (b) Co-Cr alloy by high frequency induction casting; (c) Ni-Cr alloy by centrifugal casting; (d) Ni-Cr alloy by high frequency induction casting
Table 2 Corrosion parameters

The surfaces of the alloys after the potentiodynamic polarization experiment were examined using SEM to identify the sites of corrosion attack (Figs.4 and 5). There is thick corrosion product layer on the Co-Cr alloy. Such a layer is visible to the naked eye. The SEM image after removing the corrosion product layer (Fig.4(c)) shows that the sample has undergone uniform corrosion (when polarized above the breakdown potential). EDS line analysis of the corroded areas indicates severe depletion of cobalt from the matrix. In the case of the Ni-Cr alloy, the surface remains the same without any visible change. However, the SEM images (Fig.5) reveal severely localized dissolution. The Cr and Mo depleted zones are locally removed from the surface.
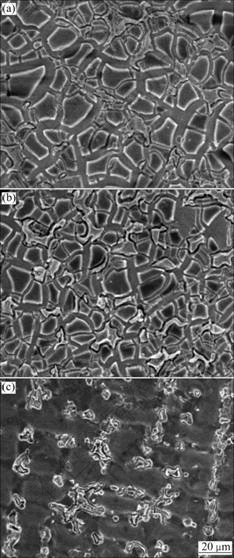
Fig.4 SEM images of Co-Cr alloy after polarization test: (a) Centrifugal casting; (b) High frequency induction casting; (c) After removing corrosion product layer of (b)
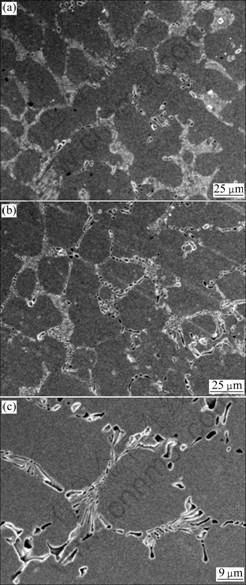
Fig.5 SEM images of Ni-Cr alloy after polarization test: (a) Centrifugal casting; (b) High frequency induction casting; (c) High magnification image of (b)
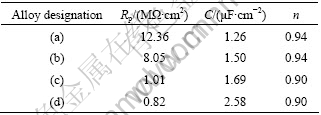
The non-destructive impedance studies support the polarization results. The EIS plots measured at open circuit potentials are given in Bode format in Fig.6. All the alloys exhibit high impedance values of the order of 106, indicating high corrosion resistances. The Bode phase shift plot gives a clearer picture. The Co-Cr alloy exhibits phase angles close to 90? at medium and low frequencies, suggesting the formation of compact passive film at the interface. However, the phase angles of the Ni-Cr alloys are dropped to about 50? at lower frequencies. Such a behavior indicates that the passive film formed on the Ni-Cr alloy is defective in nature. A simple Randles equivalent circuit model consists of a constant phase element(CPE), which represents oxide film capacitance parallel with oxide film resistor(Rp); Both in series with the solution resistance is used to describe the experimental results. Chi-square values of the order of 10-3 indicate excellent agreement between the experimental and model values. The simulated values are listed in Table 3. A high value of Rp implies higher corrosion resistance.
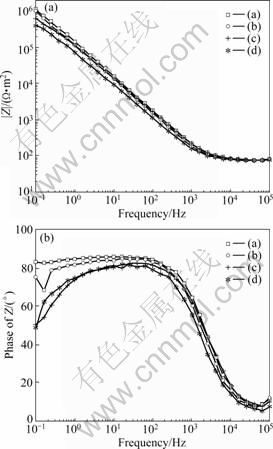
Fig.6 EIS Bode plots: (a) Co-Cr alloy by centrifugal casting; (b) Co-Cr alloy by high frequency induction casting; (c) Ni-Cr alloy by centrifugal casting; (d) Ni-Cr alloy by high frequency induction casting
Table 3 EIS simulation results
The results of electrochemical experiments of the present study indicate that the dependence of corrosion resistance of Ni-Cr and Co-Cr alloys on the microstructural variation associated with the casting methods is marginal. MULDERS et al[11] reported that casting processes had no significant effect on the corrosion resistance of palladium based alloys. Analogous to the present result, few reported works showed that commercial Co-Cr alloys possess more desirable corrosion resistance properties than Ni-Cr counterparts. According to results of KHAMIS et al[17], Wirolloy corroded 26 times faster than Wironit alloy. Their study suggested that increasing the times of successive remelting and recasting of Wirolloy enhanced the pitting potential and improved the corrosion resistance. However, recasting had no significant effect on the corrosion resistance of the Wironit alloy. The ion release study on commercially available Ni-Cr and Co-Cr casting alloys in lactated sodium chloride solution has shown that Ni-Cr alloys were more susceptible to corrosion[18]. Similar to the present result, few studies reported that corrosion of Ni-Cr alloys occurred through selective dissolution of Ni-rich regions formed during solidification[9, 19].
A small amount of Be is often added to Ni-Cr alloys to improve their castability. Be acts as a grain refiner and hardener and enhances adhesion to the veneering ceramics[20]. Earlier works reported that Be-containing Ni-Cr alloys were less resistant to accelerated corrosion [7, 20-21]. Preferential segregation of Be was found in Ni-Cr alloys in areas low in Cr and O and these Cr-depleted NiBe eutectic phases were suggested to be the reason for the decreased corrosion resistance[21]. BUMGARDNER and LUCAS[7] reported that a more homogeneous Cr-Mo oxide surface was formed on non-Be alloys. PAN et al[22] reported that increasing the Be content to larger than 0.6% (mass fraction) led to severely localized dissolution irrespective of the contents of Cr and Mo in the alloy. The small atomic radius of Be element facilitated early Be migration to the surface, which resulted in Cr and Mo depleted areas[23].
The corrosion resistance of Ni-Cr and Co-Cr alloys varies with their chemical compositions and the homogeneity of the passive film formed[20]. Difference in microstructure can influence the initial growth, the compactness and the compositional homogeneity of a passive film[24]. As well known, the main component of the passive oxide film is Cr (about 90% Cr oxides) [25-27]. The minor constituents of the passive layer are oxides of Co, Mo and Ni. In the passive region, Cr is present mainly as Cr(Ⅲ) oxide and in smaller amount as Cr(Ⅲ) hydroxide [25]. Mo is less important than Cr; however, alloy with less Mo was more susceptible to pitting[28]. XPS investigation in Co-Cr alloys shows that during the formation of passive film, the oxides of Cr mostly forms the layer, where as oxides of Co gets dissolved[25]. Ni-Cr alloys with higher level of Cr (about 25%) exhibited superior corrosion resistance due to the more uniform distribution of Cr in the microstructure of alloy [9]. A higher contents of Cr2O3 and MoO3 in the passive film could lead to higher resistance to metal ion transfer through the passive film. The homogeneous distribution of Cr is critical especially in low-Cr nickel-based alloys for better corrosion resistance. Compared with Cr2O3, the oxide of nickel is more porous and has less protective ability to corrosion. Hence, the zones of passive film rich in NiO will act as weak regions for localized corrosion, which causes localized dissolution of Ni-rich phases. There is a current trend of replacing Ni-Cr alloys, commonly used as dental ceramic, with Co-Cr alloys, because of its more expected biocompatibility.
4 Conclusions
1) Variation in casting morphologies by casting method has only marginal influence on the overall corrosion rate.
2) Ni-rich, Cr and Mo depleted zones in Ni-Cr alloys undergo severely localized dissolution.
3) The overall corrosion resistance property of the Co-Cr alloy is better than that of the Ni-Cr alloy.
References
[1] WATAHA J C. Biocompatibility of dental casting alloys—A review [J]. The Journal of Prosthetic Dentistry, 2000, 83: 223-234.
[2] HRYNIEWICZ T, ROKICKI R, ROKOSZ K. Co-Cr alloy corrosion behaviour after electropolishing and magnetoelectropolishing treatments [J]. Materials Letters, 2008, 62: 3073-3076.
[3] WATAHA J C, O’DELL N L, SINGH B B, GHAZI M, WHITFORD G M, LOCKWOOD P E. Relating nickel induced tissue inflammation to nickel release in vivo [J]. Journal of Biomedical Materials Research, 2001, 58: 537-544.
[4] SCHMALZ G, GARHAMMER P. Biological interactions of dental cast alloys with oral tissues [J]. Dental Materials, 2002, 18: 396-406.
[5] JOIAS R M, TANGO R N, de ARAUJO J E J, de ARAUJO M A J, SAAVEDRA G S F A, PAES-JUNIOR T J A, KIMPARA E T. Shear bond strength of a ceramic to Co-Cr alloys [J]. The Journal of Prosthetic Dentistry, 2008, 99: 55-59.
[6] BUMGARDNER J D, LUCAS L C. Cellular response to metallic ions released from Ni-Cr dental alloys [J]. Journal of Dental Research, 1995, 74: 1521-1527.
[7] BUMGARDNER J D, LUCAS L C. Corrosion and cell culture evaluations of Ni-Cr dental casting alloys [J]. Journal of Applied Biomaterials, 1994, 5: 203-213.
[8] ROACH M D, WOLAN J T, PARSELL D E, BUMGARDNER J D. Use of XPS and cyclic polarization to evaluate the corrosion behaviour of six Ni-Cr alloys before and after PFM firing [J]. The Journal of Prosthetic Dentistry, 2000, 84: 623-634.
[9] WYLIE C M, SHELTON R M, FLEMING G J, DAVENPORT A J. Corrosion of nickel-based dental casting alloys [J]. Dental Materials, 2007, 23: 714-723.
[10] FOSSATI A, BORGIOLO F, GALVANETTO E, BACCI T. Corrosion resistance properties of plasma nitrided Ti-6Al-4V alloy in nitric acid solutions [J]. Corrosion Science, 2004, 46: 917-927.
[11] MULDERS C, DARWISH M, HOLZE R. The influence of alloy composition and casting procedure upon the corrosion behaviour of dental alloys: An in vitro study [J]. Journal of Oral Rehabilitation, 1996, 23: 825-831.
[12] VIENNOT S, LISSAC M, MALQUARTI G, DALARD F, GROSGOGEAT B. Influence of casting procedures on the corrosion resistance of clinical dental alloys containing palladium [J]. Acta Biomaterialia, 2006, 2: 321-330.
[13] COHEN S M, KAKAR A, VAIDYNATHAN T K, VISWANATHAN T. Castability optimization of palladium based alloys [J]. The Journal of Prosthetic Dentistry, 1996, 76: 125-131.
[14] KIM S J, KO Y M, CHOE H C. Pitting corrosion of TiN coated dental cast alloy with casting methods [J]. Advanced Materials Research, 2007, 15/17: 164-168.
[15] ASM International. ASM handbook [M]. 9 ed. Ohio: Materials Park, 2004: 762-774.
[16] DOHERTY R D, FEEST E A, HOLM K. Dendritic solidification of Cu-Ni alloys [J]. Metallurgical Transactions, 1973, 4: 115-118.
[17] KHAMIS E, SEDDIK M. Corrosion evaluation of recasting non-precious dental alloys [J]. International Dental Journal, 1995, 45: 209-217.
[18] GEIS-GERSTORFER J, SAUER K H, PASSLER K. Ion release from Ni-Cr-Mo and Co-Cr-Mo casting alloys [J]. International Journal of Prosthodontics, 1991, 4: 152-158.
[19] de MICHELI S M, RIESGO O. Electrochemical study of corrosion in Ni-Cr dental alloys [J]. Biomaterials, 1982, 3: 209-212.
[20] GOFF A H L, JOIRET S, ABOURAZZOUK D. Raman investigation of crevice corrosion in Ni-Cr dental alloys containing Be [J]. Electrochimica Acta, 1998, 43: 53-62.
[21] BUMGARDNER J D, LUCAS L C. Surface analysis of Ni-Cr dental alloys [J]. Dental Materials, 1993, 9: 252-259.
[22] PAN J, GEIS-GERSTORFER J, THIERRY D, LEYGRAF C. Electrochemical studies of the influence of beryllium on corrosion resistance of Ni-25Cr-10Mo cast alloys for dental applications [J]. Journal of Electrochemical Society, 1995, 142: 1454-1458.
[23] HUANG H H. Surface characterization of passive film on NiCr based dental casting alloys [J]. Biomaterials, 2003, 24: 1575-1582.
[24] LIU L, LI Y, WANG F. Influence of microstructure on corrosion behaviour of a Ni-based superalloy in 3.5wt.% NaCl [J]. Electrochimica Acta, 2007, 52: 7193-7202.
[25] HODGSON A W E, KURZ S, VIRTANEN S, FERVEL V, OLSSON C O A, MISCHLER S. Passive and transpassive behavior of CoCrMo in simulated biological solutions [J]. Electrochimica Acta, 2004, 49: 2167-2178.
[26] KOCIJAN A, MILOSEV I, PIHLAR B. Cobalt-based alloys for orthopedic applications studied by electrochemical and XPS analysis [J]. Journal of Materials Science Materials in Medicine, 2004, 15: 643-650.
[27] KELLY J R, ROSE T C. Non-precious alloys for use in fixed prosthodontics [J]. The Journal of Prosthetic Dentistry, 1983, 49: 363-367.
[28] GEIS-GERSTORFER J, WEBER H. In vitro corrosion behaviour of four Ni-Cr dental alloys in lactic acid and sodium chloride solutions [J]. Dental Materials, 1987, 3: 289-295.
Corresponding author: Han-Cheol CHOE; Tel: +82-62-230-6896; E-mail: hcchoe@chosun.ac.kr
DOI: 10.1016/S1003-6326(08)60350-7


