文章编号:1004-0609(2015)06-1579-11
多孔基体负载型钯膜制备质量控制和缺陷修补的研究进展
魏 磊1,俞 健2,胡小娟2,黄 彦2
(1. 中国船舶重工集团公司 第七一八研究所,邯郸 056027;
2. 南京工业大学 化学化工学院 材料化学工程国家重点实验室,南京 210009)
摘 要:
钯膜对氢气有着优异的渗透性和选择性,在氢分离和膜催化反应领域备受关注。将钯膜直接沉积于多孔基体表面形成的钯复合膜,具有厚度薄、力学强度高、氢渗透率高、成本低等优点,但制膜难度较高。在各种制膜方法中,化学镀法最为常用。在化学镀制膜过程中,对钯膜的质量控制和缺陷修补工艺进行了综述,以期进一步推动高性能钯复合膜的发展及应用。
关键词:
中图分类号:TB34 文献标志码:A
Research progress of fabrication quality control and defects repairing of palladium membranes supported on porous substrate
WEI Lei1, YU Jian2, HU Xiao-juan2, HUANG Yan2
(1. The 718th Research Institute, China Shipbuilding Industry Corporation, Handan 056027, China;
2. State Key Laboratory of Materials-oriented Chemical Engineering, College of Chemistry and Chemical Engineering,
Nanjing Tech University, Nanjing 210009, China)
Abstract: Owing to the excellent permeability and permselectivity toward hydrogen, palladium membranes have attracted much attention in the fields of hydrogen separation and membrane catalytic reaction. Palladium membranes supported on porous substrate (i.e., composite palladium membranes) have many advantages, such as thin membrane thickness, robust mechanical strength, high H2-permeance and low cost, but the membrane preparation is challenging. Among various preparation methods, electroless plating is the most popular. Based on such process, the membrane quality control and defects repairing are summarized to push forward the development and application of composite palladium membranes.
Key words: porous substrate; composite palladium membrane; quality control; defects repairing; electroless plating
钯膜(包括钯合金膜)对氢及其同位素气体有着优异的渗透性和选择性,除氢气以外的其他任何气体均无法透过钯膜,因此,可用于氢气的纯化。早在1866年,GRAHAM[1]就发现了金属钯的这一特性,在20世纪60年代,Johnson Matthey公司将钯银合金膜成功地用于纯氢的生产[2]。另外,钯膜还作为催化反应器用于烃类或醇类水蒸气重整、水煤气变换和催化脱氢等反应,从而将氢分离和催化功能相耦合,并打破热力学平衡的限制[3-7]。
传统的钯膜是通过逐级冷拔和冷轧技术而制备的钯合金管或片,在轧制过程中需要进行多次退火处理。钯膜以管式居多,因为其密封和安装使用更为方便,俗称“钯管”。直接拉拔和轧制获得的钯管为无缝管,将钯合金片经扩散焊加工后也可制得钯管,但焊接之前要将钯合金片裹在管式透气性支撑材料上[8]。由于轧制技术水平的限制和膜力学强度的要求,传统钯膜的厚度一般在50 μm以上。由于轧制工艺复杂,设备投资大且贵金属消耗量大,所以传统钯膜的成本居高不下。由于钯膜的透氢率与膜厚成反比,膜层越厚则氢气渗透率越低[9]。
鉴于传统钯膜的显著缺点,复合膜(Composite membrane)的概念应运而生,亦即将钯膜沉积在多孔基体材料上。复合膜的钯层厚度可降至仅几个微米,氢渗透率可相应提高一个数量级,而且贵金属的消耗量也大幅下降。同时,由于多孔基体的支撑作用,钯膜的机械强度也得到了增强,从而更易于安装和密封。
目前,钯膜的基体材料主要为多孔陶瓷和多孔不锈钢[10-11],通常它们分别采用挤出成型法和粉末冶金法制得,以管式和片式为主,但中空纤维型[12]、多通道型[13]和齿轮型[14]基体也有报道。在多孔基体表面制备钯膜的方法主要有化学镀、磁控溅射、CVD、光催化沉积、电镀等[15-18],一些综述性论文[10-11, 19]已进行了总结,此处不再赘述。在众多制膜方法中,化学镀法是一种公认的较为理想的制膜方法,能在各种材质上沉积优质膜层,设备简单且操作简便。制备钯合金膜时,可采用共沉积或分步沉积的方式引入一种或多种其他金属,然后进行高温合金化处理[20-21]。由于纯钯膜元素组成单一,且制膜基础性强,故本文作者仅对纯钯复合膜的制备进行综述。
对钯复合膜而言,最重要的是要厚度薄(以获得高的氢渗透率)且致密无缺陷(以实现高的氢气纯度)。为此,在化学镀膜时必须对镀层的沉积过程加以控制,以获得均匀度高、缺陷少的镀层;镀膜完成后,如果膜缺陷较多,则还需对其进行修补,以保证成品率并降低制膜成本。鉴于此,本文作者将对现有的技术和方法进行总结,以期进一步推动高性能钯复合膜的制备及应用。
1 化学镀制备钯复合膜
1.1 化学镀
化学镀,也称无电镀(Electroless plating),它无需外加电源,而是利用自催化反应实现金属离子的还原和自组装沉积。化学镀前,基体表面需进行除油、除尘等清洁处理。对于导电性良好的金属基体材料可在表面清洁后直接施镀。而陶瓷、玻璃、塑料等非金属工件,还需进行表面活化。
就化学镀制备钯膜而言,为保证镀层的均匀性,无论何种基体材料一般都要进行活化,亦即在其表面先沉积一层纳米级的钯金属晶种作为引发化学镀反应的催化剂[22]。目前,SnCl2/PdCl2活化法可谓最为普遍[10]。由于钯的沉积开始于钯晶种上,因此,钯晶种的数量和分散度将直接影响后续钯膜的生长和形成。如果活化不足、晶种偏少,则化学镀反应的引发和膜层的生长都会减慢。但是,活化过多也会增加活化时间和劳动强度,还会影响钯膜的附着力。最为常用的钯镀液一般由PdCl2、氨水和EDTA构成,其中PdCl2提供钯源,氨水为络合剂,EDTA为稳定剂。常用的还原剂为N2H4溶液,所发生的化学反应为
2Pd(NH3)42++N2H4+4OH- →2Pd↓+8NH3+N2↑+4H2O(1)
由于钯为重金属,氨水易挥发且有刺激和腐蚀性,N2H4有毒,所以镀液对环境和人体均有危害。因此,镀膜过程中应通风良好,操作者应做好防护措施,废液也应收集处理。
镀液组成和反应条件在不同程度上影响着钯膜的制备。CHENG等[23]对比还原剂N2H4和Na2H2PO2对化学镀钯膜的影响,由于H2PO22-与Pd2+反应时放出氢气,易造成钯层氢脆破裂,故Na2H2PO2不可用作还原剂。常使用的镀钯温度为20~60 ℃,温度过低时,反应缓慢;温度过高时,易导致反应失控、镀液生成沉淀。还原剂N2H4的加入量也直接影响着钯的沉积速率和成膜质量[24]。RYI等[25]考察了镀液中EDTA含量对化学镀钯膜形貌的影响,并提出一种无EDTA的制膜工艺。由于缺少稳定剂对反应的控制作用,这种无EDTA的制膜工艺需采用较低的操作温度和较慢的N2H4加入速率。
1.2 钯膜的形成
在化学镀过程中,由于膜层是直接沉积在多孔基体表面,所以膜层形貌与成膜质量极易受基体表面性能(如粗糙度、孔径分布等)的影响。化学镀初期,镀液易向基体孔内渗透,导致金属钯在孔内沉积;随着钯沉积量的增加,基体表面孔逐渐被覆盖并基本形成连续膜层;当膜厚达到一定程度时,膜缺陷将得到有效控制。当然,增加膜厚还有利于提高膜的稳定性。KITIWAN等[26]认为随着镀膜时间的延长,膜层依次经历了“不连续—连续—致密化”的过程。
为提高钯膜的氢渗透率,钯膜应尽可能薄,但膜层过薄又难以控制膜缺陷和保证产氢纯度。MA等[27]认为影响钯膜完整度的因素主要是基体表面的大孔缺陷,如划痕、凹坑、破损等。MARDILOVICH等[28]考察钯膜完整度与多孔不锈钢基体孔径的关系,认为致密钯膜所能实现的最小厚度约为基体最大孔径的3倍。不过,这一量化规律尚未得到其他研究者的印证。例如,SHI等[29]在化学镀钯膜时设法使钯层在多孔不锈钢基体表面的孔口处“架桥”,从而降低了钯膜所需的厚度。
鉴于基体孔径分布情况对钯膜的制备有着至关重要的影响,为提高制膜成功率,有必要对基体材料进行孔径分析从而加以筛选。其中,最常用的分析方法是泡点法(又称毛细流动法)。但是,该法所检测的实际上是孔道最狭窄处的直径(即“孔喉”),而钯膜却是沉积在基体的表面(即“孔口”),且孔口一般会明显大于孔喉。因此,泡点法所给出的结果有明显的局限性。为解决这一问题,本文作者研究组在传统泡点法的基础上发明了一种新方法[30-31],可用于测量多孔材料表面孔口直径的分布情况。另外,本文作者研究组还与南京高谦功能材料科技有限公司合作,成功开发了PSDA系列仪器,既能测量孔喉又能测量孔口,为多孔基体材料的分析和筛选提供了便利和依据。
2 钯膜的质量控制
为获得厚度薄、缺陷少、膜层均匀、附着力强的钯膜,研究者们主要从改善多孔基体表面状况和改进化学镀工艺两方面对钯膜镀层的质量加以控制。
2.1 基体表面预处理
2.1.1 陶瓷修饰层
一般情况下,基体材料(主要是多孔陶瓷和多孔不锈钢)表面孔径越小、越平整,则制膜越容易,也就是说膜层可以在更薄的厚度时即具有较高的完整度。从材料结构上划分,市面上的基体材料有均质型和梯度型(根据英文symmetric和asymmetric直译为对称型和非对称型的称谓也越来越流行)两大类。前者价格相对低廉,但小孔径的均质基体材料往往透气阻力过大;梯度型基体材料透气阻力小,但局部缺陷难以控制。因此在镀膜之前,孔径较大或存在局部大孔的基体都需要进行例行的表面修饰,以消除表面缺陷、缩小表面孔、降低粗糙度。最常见的表面修饰材料为陶瓷类(如Al2O3、SiO2、YSZ、沸石分子筛等)。对多孔不锈钢基材而言,陶瓷修饰层还能够消除钯膜与不锈钢基体间的金属相互扩散效应。
基体表面修饰方法很多,俞健等[32]在2008年针对多孔不锈钢材料的表面修饰进行了总结。HUANG等[33]分别采用等离子喷涂、磁控溅射和湿粉喷涂法在多孔不锈钢基体表面沉积YSZ、ZrO2和TiO2修饰层,发现YSZ涂层表面粗糙,渗透阻力大;由于磁控溅射过程中的遮蔽效应,ZrO2涂层修饰效果较差;而TiO2涂层最有利于钯膜的制备。溶胶-凝胶法是制备微孔陶瓷层的常见手段,但该法对基体表面的局部大孔修饰效果较差。
对孔径特别大的基体,可涂覆含有陶瓷颗粒的悬浮液然后进行烧结处理。CHI等[34]分别考察了平均粒度为10 μm和1 μm的α-Al2O3颗粒对多孔不锈钢基体表面的修饰效果,发现大陶瓷颗粒的修饰效果欠佳,小陶瓷颗粒的修饰效果较好,但增大了基体的透气阻力。WANG等[35]以ZrO2粒子填充多孔不锈钢基体的表面孔,也发现基体的透气阻力显著增加,降低了钯膜的透氢率。LI等[36]先对多孔不锈钢基体表面进行打磨+酸蚀处理以降低其表面粗糙度,之后通过抽吸的方式在基体表面依次沉积并烧结粒度为2.5 μm和0.3 μm的α-Al2O3颗粒,获得了较好的修饰层。XU等[37]发现,重复使用0.3 μm的α-Al2O3颗粒进行修饰可进一步消除修饰层的缺陷,从而有利于致密钯膜的制备。CHI等[38]则认为,陶瓷颗粒粒度的选择十分重要,应根据基体的孔径分布选择不同粒度的陶瓷颗粒按序分步处理,这样可以避免修饰后的基体阻力过大而影响钯膜透氢。但是,分步、多次地进行涂层和烧结操作,大大增加了劳动量和成本。
为了能够一步获得满足镀膜要求的微孔层,GUO等[39]通过水热法在大孔Al2O3基体表面制备了纳米孔Sil-1分子筛层,并在Sil-1层引入了Pd催化剂,所制备的钯膜中有部分钯金属呈“腿状”伸入分子筛层,这虽提高了钯膜附着力,但造成了膜氢渗透通量的下降。WANG等[40]和BOSKO等[41]分别以TS-1和NaA分子筛修饰了大孔Al2O3和不锈钢基体表面,并采用化学镀法成功制备了不同厚度的钯膜。由于水热法成本较高、技术难度大,可能不利于钯膜的工业化制备和应用。
与分子筛修饰法相比,本文作者所在课题组[42-45]开发的石墨铅笔涂层法操作更为简便,采用普通的市售铅笔芯在多孔基体表面涂划即可形成连续、牢固的修饰层。普通石墨铅笔芯成分为石墨和粘土,它们都具有优良的化学和热稳定性,对钯膜完全不会造成污染。石墨和粘土粒度小、可塑性强,可直接用于修饰大孔基体,对局部大孔的修补效果尤其出色,所得修饰层平整度高、透气性好,因此,有利于制备高质量的钯膜。该法可以选用低成本的大孔基体材料,且本身成本低廉,从而可以有效降低钯膜的成本。另外,该石墨铅笔涂层法还可用于回收废旧陶瓷基体材料,在此基础上制备的新钯膜依然具有优异的透氢性能[44]。
表1所列为几种钯膜的透氢性能进行了对比,其中以石墨铅笔修饰陶瓷为基体的钯膜(Pd/Pencil/Al2O3膜)具有更高的氢渗透通量和选择性,这应归功于石墨铅笔修饰法对基体表面大孔的修饰效果更好且渗透阻力更小。在化学镀钯膜之前,经石墨修饰后的多孔不锈钢基体可直接浸于PdCl2/HCl溶液进行活化,利用原电池反应(石墨为阴极,不锈钢为阳极)在石墨涂层表面沉积一层钯催化剂[45]。该活化法与传统PdCl2/SnCl2法相比,具有操作简便、废液少的特点。利用这种原电池反应成功地活化了银/不锈钢基体,并制备了钯膜[46]。
2.1.2 凝胶修饰层
多孔基体表面孔径越小、越平整,就越容易制得薄且致密的钯膜。鉴于此,可在多孔基体表面预先修饰不透气的无孔过渡层,待镀钯膜完成后再对该过渡层进行热处理以恢复其透气性。显然,在致密基体表面制备钯膜就比较容易,在同样膜厚的情况下,其均匀度和完整性也会更好。虽然这种方法巧妙地把在多孔表面上制膜转化为在致密表面制膜,但是在过渡层热分解的过程中,如何避免钯膜的脱落和损坏也将成为新的问题。
邓超等[47]开发了一种操作十分简便的凝胶修饰法(如图1所示),即预先在多孔陶瓷基体表面涂覆一层γ-AlOOH溶胶,干燥后形成不透气的凝胶层,直接在该凝胶层上化学镀钯后再通过热处理的方式分解凝胶,最后制得了厚度约3 μm的超薄钯膜。这种凝胶修饰技术有效阻挡了金属钯进入基体孔道中,该膜的氢渗透率是未经修饰的同样厚度钯膜的3倍。但是,破坏性耐压测试结果表明,膜层的附着力较弱,在使用时不能对钯膜反向施压。
由于普通凝胶层的含水量高,在热分解后体积收缩较大,因此,钯膜容易成为“空中楼阁”而出现附着力问题。可采用与胶体相似的悬浮液或悬浊液来代替溶胶修饰液,其固含量应适当提高,固体粒子的粒径也要足够小。WANG等[48]发现凝胶修饰法对制备碳分子筛膜十分有效,且不存在附着力问题。HUANG等[49]考察了各种可能用来测试钯膜附着力的方法,如十字划格法、拉力法、气体压力法、热震法、氢脆法等,其中热震法基本无效,其他多种方法并用可以提供更全面的信息,而单一的测试方法给出的结果可能失之偏颇或不可靠。
2.1.3 堵孔法
该法的原理是将固体或胶体粒子(如氧化铝、氧化硅、氧化钛)填入基体表面孔口,化学镀钯膜之后再进行热处理。堵孔操作可通过抽吸来实现,类似于过滤过程中的滤材孔道堵塞。虽然堵孔处理后基体表面的透气性较差,但在热处理之后,基体孔道中的固体粒子会烧结收缩,从而会提高透气性。
TONG等[50]采用硝酸铝与碳酸铵制备了铝溶胶,通过超声震荡并抽吸的方式来堵塞基体孔道(所用基体材料是表面孔为20~100 μm孔径的多孔不锈钢),然后通过化学镀的方法制备了厚度约为6 μm的钯膜,最后将钯膜进行500 ℃的热处理。TONG等[51]还尝试在修饰大孔不锈钢基体的堵孔剂中引入钯催化剂,从而将堵孔和基体活化同步完成。徐恒泳等[52]则以含有氢氧化物或碳酸盐的沉淀或胶体作为堵孔剂。
表1 基于沸石分子筛和石墨铅笔修饰的多孔Al2O3基体的钯膜透氢性能的对比
Table 1 Comparison among H2-permeation performances of palladium membranes based on zeolite molecular sieve- and pencil- coated porous Al2O3 substrate
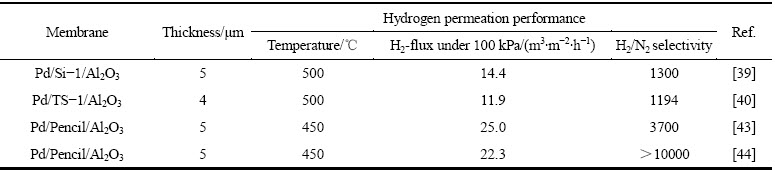
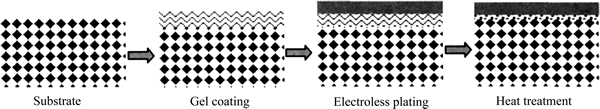
图1 基体表面的凝胶修饰及钯膜的制备原理[47]
Fig. 1 Mechanism of gel coating on porous substrate for preparation of palladium membrane[47]
堵孔法和凝胶修饰法都有一个共同优点,即可以在化学镀过程中有效避免钯金属进入基体孔道,减小了气体扩散阻力,从而可以获得更高的透氢率。但是,这也意味着钯膜的附着力得到削弱,正如凝胶修饰法中所发现的那样。如果在堵孔法的操作过程中,能够避免基体表面形成额外的修饰层,亦即避免堵孔粒子遮盖基体的表面骨架,则钯膜附着力弱的问题应该可以得到改善。如图2所示。
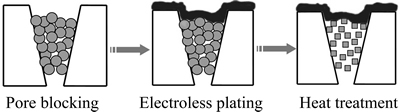
图2 堵孔法修饰多孔基体及钯膜的制备
Fig. 2 Pore blocking treatment of substrate for preparation of palladium membrane
2.2 化学镀过程强化
由于基体表面的孔径不可能是均一的,而是有一定的分布范围,那么在化学镀过程中,小孔表面上的钯膜将会更早形成,而要在大孔表面形成钯膜则需要继续进行化学镀,亦即增加膜厚。因此,如果能够通过化学镀的过程强化来加速大孔表面的化学镀反应,则可以促进钯膜的尽早形成并有效降低钯膜的平均厚度,从而达到降低成本、提高透氢率的效果。
有必要指出的是,这里所说的过程强化不是指化学镀本身工艺条件的优化,如镀液配方、反应条件、操作细节等,而是指采取额外的手段来提高化学镀效果和膜层质量。但是归根结底,化学镀本身的工艺优化永远都是首要的。
2.2.1 高浓度溶液渗透强化法
高浓度溶液渗透强化的化学镀工艺由VARMA 等[53-57]最早提出,其原理是以高浓度溶液(如蔗糖溶液)作为渗透剂在多孔基体背侧循环流动,利用渗透压原理来摄取镀液中的水分并促进镀液向渗透剂扩散;一旦钯膜形成,则该处的渗透效应将自动停止,而钯膜尚有缺陷之处则继续起到强化作用。他们认为渗透强化法的优点是:1) 削弱了粗糙表面对钯沉积的“遮蔽效应”,促使钯在凹陷处均匀沉积,如图3所示;2) 钯在孔内沉积可提高钯膜附着力和热稳定性。SOULEIMANOVA等[56]甚至还发现,反渗透技术不仅能强化化学镀效果,而且还可以用来增进化学镀之前的SnCl2/PdCl2预活化过程,亦即有利于细化钯晶种和提高膜附着力。但是,有些他们在实验中所使用的基体孔径很小,甚至达到纳米级。一般而言,如果使用了孔径极小的基体材料,即使不采用任何强化技术也应该很容易制得薄而无缺陷的钯膜,除非原始的化学镀工艺尚未达到优化状态。
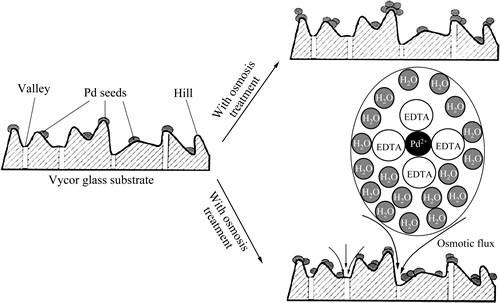
图3 渗透对化学镀钯膜的强化作用示意图[55]
Fig. 3 Schematic diagram of enhancement of electroless plating for palladium membrane by osmosis treatment[55]
LI等[58]和蒋柏泉等[59]也分别以高浓度NaCl溶液和蔗糖溶液作为反渗透剂来强化化学镀过程,分别制备了钯膜和钯银合金膜,他们也报道了与VARMA等[53-57]类似的反渗透强化效果。
渗透强化化学镀的必要性只有在制膜困难或膜质量不高时才能体现出来,如果化学镀技术本身已经比较完善,则渗透强化的效果很可能根本体现不出来。本文作者所在课题组也进行了渗透强化化学镀的多方尝试,但并没有发现任何强化效果。虽然这不排除与实验水平有关,但认为渗透强化效果本身就是非常有限的,原因如下:1) 化学镀液本身的渗透压并不低,因为镀液一般含有大量的EDTA作为稳定剂,而且镀液中还含有高浓度的氨水。因此,即使基体背面采用饱和溶液,膜两侧渗透压差也不会太高;2) 化学镀液和渗透剂之间的渗透只能通过基体孔道和膜缺陷而且只有镀液和渗透剂直接接触才能进行,但是由于并不存在维持二者渗透压的接触界面,二者直接接触时只会相互溶解,因此渗透只能是相互的,而非只是镀液向渗透剂侧渗透;3) 镀液和渗透剂都是浓溶液,都有一定的黏度,如果再考虑基体孔道的扩散阻力,势必会得出结论,即镀液和渗透剂之间的渗透效应在动力学上将是极其缓慢的。
此外,渗透强化法还有着突出的缺点,即膜清洗困难并产生更多废液,加剧了人们因废液而对化学镀工艺环保问题的担忧。具体分析如下:1) 基体一侧已被致密钯膜遮盖,基体孔道中的杂质只能通过微小的孔道向基体背面缓慢扩散,难以彻底清洗;2) 以蔗糖等有机溶液为渗透剂时,一旦有机物有残留,则膜在高温下工作时势必会因有机物碳化结焦而受到污染;3) 由于渗透剂必须在浓度极高甚至达到饱和时才能起到作用,一旦发生溶质在孔道内结晶而堵塞孔道,则几乎无法清洗。
2.2.2 抽吸强化法
抽吸强化法即在化学镀过程中在基体背面进行抽吸,促进镀液向膜缺陷处流动,从而加速膜缺陷处的钯沉积反应。抽吸活化法和渗透法的原理基本相同,但优于渗透法。
CHEN等[60]在化学镀制备Pd/Al2O3膜时发现,抽吸强化法有利于减少膜缺陷,但过高的抽吸力则会导致钯沉积在基体孔道内,反而不利于钯膜的形成。ZHANG等[61]在基体两侧都进行抽吸,只不过基体背面的真空度更高,其中对镀液抽吸的主要目的是为了脱除联胺与钯离子反应过程中产生的氮气,以免这些小气泡附在膜面上影响钯的沉积。
抽吸强化法的不利因素如:1) 在操作过程中,被抽出的含贵金属镀液容易受到污染而难以继续使用;2) 钯镀液中一般含有高浓度的氨水,抽吸操作不仅会进一步加剧氨气的挥发,造成环境污染和镀液失效。为改进抽吸强化法,WEI等[45]提出了一种循环化学镀工艺,并申请了相关专利[62],即通过蠕动泵对基体背侧抽吸,同时能够使渗出的镀液及时回流至镀槽,不仅避免了氨气的挥发,还不会造成镀液的浪费与污染。
抽吸强化法不仅对化学镀有效,对电镀也有效。NAM等[63-64]在多孔不锈钢基体上制备Pd-Ni合金膜的过程中,将基体背侧用泵抽吸,并且随着钯的不断沉积而逐渐增大抽吸力,最终获得了良好效果。显然,无论何种抽吸强化工艺,对抽吸力(即基体背侧真空 度)的控制都很有必要。在镀膜初期,应减少抽吸力甚至是不抽吸;在镀膜后期,膜缺陷越来越小、越来越少,则应加大抽吸力,促进膜缺陷的尽快愈合。
2.2.3 还原剂渗透法
本文作者所在课题组[65]发明了一种还原剂渗透法。把作为化学镀还原剂的N2H4溶液放入基体背面,在渗透压或外力作用下N2H4经基体孔道向镀液渗透时,将会极大地促进钯在膜缺陷处的沉积并加速成膜。与渗透强化法和抽吸强化法一样,还原剂渗透法也可以起到膜缺陷的定点消除作用,一旦膜缺陷愈合则还原剂也将停止渗透。还原剂的渗透速率很重要,过慢则起不到强化作用,过快则有可能导致膜缺陷处反应失控。不过,这可以通过调节化学镀温度和还原剂浓度来解决。SANZ等[66]运用这种方法在多孔不锈钢基体表面制备钯膜,发现有助于消除膜缺陷和降低膜厚度。
这一方法对化学镀的强化不仅体现在膜缺陷的有效消除,而且还有利于膜的清洗。这是由于:1) N2H4本身易于分解且不残留任何杂质;2) N2H4溶液抑制了镀液向基体孔道内的不断扩散;3) N2H4溶液还具有清洗效果。当然,还原剂渗透法中的还原剂并不限定为N2H4,而且该法也可以用来制备其他金属膜。
3 膜缺陷修补
在钯膜的制备完成之后,如果发现膜缺陷太多则宣告制膜失败,造成人力、物力的浪费。为消除膜缺陷,最简单的方法是继续进行膜的沉积来加厚钯膜,但这样做的不利后果是透氢率下降且贵金属消耗量增加。能够在基本不增加或少增加膜厚的情况下将钯膜次品进行修补,显然是理想的解决方法。实际上,这也和化学镀过程强化的理念相吻合。因此,上述过程强化方法都可以用于膜的修补,只不过是将化学镀过程强化操作实施于次品或缺陷较多的钯膜而已。
3.1 渗透法
LI等[67]在Pd/Al2O3膜内管注入高浓度NaCl溶液,利用渗透压原理对膜缺陷进行修补,修补后钯膜H2/N2理想选择性由约10提升至970,但膜厚增加了2.7 μm,氢渗透通量降低约30%。
在本文作者专利[65]中,还原剂渗透法已被用于钯膜的后期修补。ZENG等[68]也报道类似工作,发现膜表面的裂缝和孔洞等缺陷都得到了修补,膜的H2/N2选择性提高了1-2个数量级,但修补前后膜的透氢率十分接近,说明膜层没有明显增厚,其工作原理示意图如图4所示。
3.2 填充法
膜的缺陷有大有小,其中危害最大、最需修补也最难修补的是其中较大的膜缺陷。不过,这可以从基体材料的表面修饰工艺中得到启发。对于较大膜缺陷的修补,填充法是行之有效的,其原理如图5所示,即以固体和胶体预先填充膜缺陷孔洞,然后补镀一定厚度的钯膜,直到膜缺陷被愈合。唐春华等[69]以0.1~1 μm粒度的γ-Al2O3颗粒为填充物,采用抽吸方式对厚度为0.87 μm的钯膜缺陷进行填充,补镀后膜层增至3.01 μm。尽管氢渗透通量降低了50%,但H2/N2选择性由123升至5007。在他们的另一项研究[70]中,以γ-Al2O3负载钯作为填充物,补镀后膜层由1.22 μm增至1.32 μm,H2/N2选择性由370升至13700,而膜的氢渗透通量只降低了约20%。
本文作者所在课题组发明了一种Pd(OH)2胶体填充法[71],即以Pd(OH)2胶体为填充物,经N2H4溶液还原后得到钯金属催化剂,不仅实现了钯颗粒对膜缺陷处的填充,而且这些钯颗粒还是高效的化学镀催化剂,因此,在补镀过程中膜缺陷处的钯沉积会更快,从而可以高效实现膜缺陷的修补。胡小娟等[72]采用Pd(OH)2胶体填充法修补钯膜后,膜层增厚约0.9 μm,H2/N2选择性由900升至4200,氢渗透通量只降低了约8%。
3.3 其他方法
钯膜的其他修补方法也有报道。TONG等[73]以六氟乙酰丙酮合钯为前驱体,经3次CVD处理后,膜层厚度由6增至6.4 μm,H2/Ar选择性由14增至565。袁立祥等[74]在钯镀液中加入2-巯基苯并噻唑,利用巯基与金属钯间的强吸附作用,使钯的沉积限制在膜缺陷处。RYI等[75]以平均粒度为0.9 μm的Al2O3悬浮液为抛光液,对溅射法制得的钯膜表面进行抛光处理,由于钯金属具有良好的延展性,处理后膜缺陷得到了愈合,H2/He选择性由52越升至40000以上。
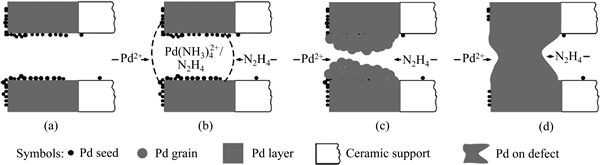
图4 还原剂渗透强化的化学镀法修补钯膜缺陷示意图[68]
Fig. 4 Schematic diagram of repairing of palladium membrane defects through reductant osmosis during electroless plating[68]
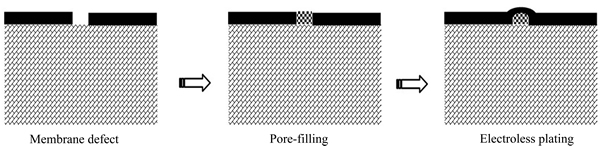
图5 填充法修补膜缺陷的工作原理示意图[72]
Fig. 5 Schematic diagram of repairing of palladium membrane defects through pore-filling and electroless plating[72]
4 小结与展望
与传统的轧制钯膜相比,负载于多孔基体的钯膜更具优势,但制膜工艺仍需进一步提高,以降低成本、提高透氢性能、延长膜的使用寿命。就目前而言,化学镀法仍然是制备负载型钯膜的行之有效的方法,但其每一个步骤都应充分优化以实现最佳的镀层质量,即厚度薄且均匀、附着力强、缺陷少。除化学镀外,热喷涂、PVD、电镀等方法也有各自优点,而且工艺和设备也在不断发展,如将两种或多种方法相结合则可能相得益彰。
不管是采用何种制备方法,基体材料的表面性能都起着十分关键的作用,因此基体表面性能的检测,特别孔径分布的检测对控制钯膜质量是十分必要的。制备高质量钯膜的最大难点在于膜缺陷的消除,这可以通过化学镀过程强化和膜修补技术来解决。在钯膜的使用过程中,随着膜缺陷的逐渐出现和扩大,产氢纯度会逐渐下降,达到一定程度后,钯膜的使用寿命就结束了。将钯膜从氢分离器中取出来再进行修补的做法是很不方便的,如果能够找到一种原位修补钯膜的方法,则钯膜的使用寿命大大延长。
纯金属钯膜存在氢脆问题,使用过程中要完全避免其在300 ℃以下接触氢气。虽然一些钯合金(如Pd-Cu、Pd-Ag)膜不存在氢脆问题,但对负载型钯合金膜而言,一般采取分步沉积的方式进行,其制备难度要比纯钯膜更大。一方面,其他金属膜的沉积同样是质量要求高、技术难度大;另一方面,钯合金的形成离不开高温合金化处理,而这一过程极易导致膜的损坏。一旦合金化不充分,则不仅依然存在氢脆问题,而且膜的氢渗透率也大受影响。
基于钯膜的氢分离器体积小、操作简单,特别适合于氢能领域的应用。近年来,氢能技术和产业的日益发展,为钯膜(特别是负载型钯膜)开辟了广阔的市场前景。因此,相关科学研究也应更侧重于负载型钯膜的实用性和产业化。
REFERENCES
[1] GRAHAM T. On the absorption and dialytic separation of gases by colloid septa[J]. Philosophical Transactions of the Royal Society, 1866, 156: 399-439.
[2] SHU J, GRANDJEAN B P A, VAN NESTE A, KALIAGUINE S. Catalytic palladium-based membrane reactors: A review[J]. Canadian Journal of Chemical Engineering, 1991, 69(5): 1036-1060.
[3] 雷敏志, 陈森凤. 钯膜与钯膜反应器应用研究进展[J]. 材料导报, 2004, 18(5): 41-44.
LEI Min-zhi, CHEN Sen-feng. Progress in application of palladium membranes and palladium membrane reactor[J]. Materials Review, 2004, 18(5): 41-44.
[4] YU C Y, LEE D W, PARK S J, LEE K Y, LEE K H. Study on a catalytic membrane reactor for hydrogen production from ethanol steam reforming[J]. International Journal of Hydrogen Energy, 2009, 34(7): 2947-2954.
[5] LIN Y M, REI M H. Study in the hydrogen production from methanol steam reforming in supported palladium membrane reactor[J]. Catalysis Today, 2001, 67(1/3): 77-84.
[6] LI H, GOLDBACH A, LI W, XU H. On CH4 decomposition during separation from H2 mixture with thin Pd membranes[J]. Journal of Membrane Science, 2008, 324(1/2): 95-101.
[7] TOSTI S, BASILE A, BETTINALI L, BORGOGNONI F, GALLUCCI F, RIZZELLO C. Design and process study of Pd membrane reactors[J]. International Journal of Hydrogen Energy, 2008, 33(19): 5098-5105.
[8] TOSTI S, BETTINALI L. Diffusion bonding of Pd-Ag rolled membranes[J]. Journal of Material Science, 2004, 39(9): 3041-3046.
[9] PAGLIERI S N, WAY J D. Innovations in palladium membrane research[J]. Separation & Purification Methods, 2002, 31(1): 1-169.
[10] 黄 彦, 李 雪, 范益群, 徐南平. 透氢钯复合膜的原理、制备及表征[J]. 化学进展, 2006, 18(2/3): 230-238.
HUANG Yan, LI Xue, FAN Yi-quan, XU Nan-ping. Palladium-based composite membranes: Principle, preparation and characterization[J]. Progress in Chemistry, 2006, 18(2/3): 230-238.
[11] YUN S, OYAMA S. Correlations in palladium membranes for hydrogen separation: A review[J]. Journal of Membrane Science, 2011, 375(1/2): 28-45.
[12] PAN X, XIONG G, SHENG S, STROH N, BRUNNER H. Thin dense Pd membranes supported on α-Al2O3 hollow fibers[J]. Chemical Communication, 2001, 24: 2536-2537.
[13] HU X, HUANG Y, SHU S, FAN Y, XU N. Toward effective membranes for hydrogen separation: Multichannel composite palladium membranes[J]. Journal of Power Sources, 2008, 181(1): 135-139.
[14] 黄 彦, 王红志, 俞 健, 胡小娟. 一种齿轮型透氢钯或钯合金膜及氢气分离器: 中国, 201010578052.9[P]. 2010-12-08.
HUANG Yan, WANG Hong-zhi, YU Jian, HU Xiao-juan. Gear-typed H2-permeable palladium and palladium alloy membranes and hydrogen separator: China, 201010578052.9[P]. 2010-12-08.
[15] JAYARAMAN V, LIN Y S, PAKALA M, LIN R Y. Fabrication of ultrathin metallic membranes on ceramic supports by sputter deposition[J]. Journal of Membrane Science, 1995, 99(1): 89-100.
[16] ITOH N, ALIHA T, SATO T. Preparation of thin palladium composite membrane tube by a CVD technique and its hydrogen permselectivity[J]. Catalysis Today, 2005, 104(2/4): 231- 237.
[17] WU L, XU N, SHI J. Novel method for preparing palladium membranes by photocatalytic deposition[J]. AIChE Journal, 2000, 46(5): 1075-1083.
[18] CHEN S C, TU G C, HUNG C C Y, HUANG C A, REI M H. Preparation of palladium membranes by electroplating on AISI 316L porous stainless steel supports and its use for methanol steam reformer[J]. Journal of Membrane Science, 2008, 314(1/2): 5- 14.
[19] 刘 伟, 张宝泉, 刘秀凤. 钯复合膜的研究进展[J]. 化学进展, 2006, 18(11): 1468-1481.
LIU Wei, ZHANG Bao-quan, LIU Xiu-feng. Progress in palladium composite membranes[J]. Progress in Chemistry, 2006, 18(11): 1468-1481.
[20] LIN W, CHANG H. Characterizations of Pd-Ag membrane prepared by sequential electroless deposition[J]. Surface and Coating Technology, 2005, 194(1): 157-166.
[21] HUANG T, WEI M, CHEN H. Preparation of hydrogen- permselective palladium-silver alloy composite membranes by electroless co-deposition[J]. Separation and Purification Technology, 2003, 32(1/3): 239-245.
[22] 李 宁. 化学镀实用技术[M]. 北京: 化学工业出版社, 2004.
LI Ning. Practical technology of electroless plating [M]. Beijing: Chemical Industry Press, 2004.
[23] CHENG Y S, YEUNG K L. Effects of electroless plating chemistry on the synthesis of palladium membranes[J]. Journal of Membrane Science, 2001, 182(1/2): 195- 203.
[24] NAIR B K R, CHOI J, HAROLD M P. Electroless plating and permeation features of Pd and Pd/Ag hollow fiber composite membranes[J]. Journal of Membrane Science, 2007, 288(1/2): 67- 84.
[25] RYI S K, XU N, LI A W, LIM C J, GRACE J R. Electroless Pd membrane deposition on alumina modified porous Hastelloy substrate with EDTA-free bath[J]. International Journal of Hydrogen Energy, 2010, 35(6): 2328-2335.
[26] KITIWAN M, ATONG D. Effects of porous alumina support and plating time on electroless plating of palladium membrane[J]. Journal of Materials Science and Technology, 2010, 26(12): 1148-1152.
[27] MA Y H, MARDILOVICH I P, ENGWALL E E. Thin composite palladium and palladium/alloy membranes for hydrogen separation[J]. Annals of the New York Academy of Sciences, 2003, 984(1): 346-360.
[28] MARDILOVICH I P, ENGWALL E, MA Y H. Dependence of hydrogen flux on the pore size and plating surface topology of asymmetric Pd-porous stainless steel membranes[J]. Desalination, 2002, 144(1/3): 85-89.
[29] SHI Z L, WU S Q, SZPUNAR J A, ROSHD M. An observation of palladium membrane formation on a porous stainless steel substrate by electroless deposition[J]. Journal of Membrane Science, 2006, 280(1/2): 705-711.
[30] HUANG Y, YU J. Method of determining surface pore mouth diameter distribution. US patent, 8528384[P]. 2013.
[31] YU J, HU X, HUANG Y. A modification of the bubble-point method to determine the pore-mouth size distribution of porous materials[J]. Separation and Purification Technology, 2010, 70(3): 314-319.
[32] 俞 健, 胡小娟, 黄 彦. 多孔不锈钢表面的陶瓷修饰及所负载的透氢钯膜[J]. 化学进展, 2008, 20(7/8): 1208-1215.
YU Jian, HU Xiao-juan, HUANG Yan. Ceramic modification of the porous stainless-steel surface toward the supported palladium membranes for hydrogen separation[J]. Progress in Chemistry, 2008, 20(7/8): 1208-1215.
[33] HUANG Y, DITTMEYER R. Preparation and characterization of composite palladium membranes on sinter-metal supports with a ceramic barrier against intermetallic diffusion[J]. Journal of Membrane Science, 2006, 282(1/2): 296-310.
[34] CHI Y H, HSU W F, HUANG T W, YANG C C, LIN Y L, JENG M S. Thin Pd membrane on a modified porous stainless steel tube: Al2O3 particle size selection strategy[J]. Journal of Chinese Institute of Engineers, 2011, 34(1): 49-55.
[35] WANG D, TONG J, XU H, MATSUMURA Y. Preparation of palladium membrane over porous stainless steel tube modified with zirconium oxide[J]. Catalysis Today, 2004, 93/95: 689-693.
[36] LI A W, R GRACE J, LIM C J. Preparation of thin Pd-based composite membrane on planar metallic substrate: Part I Pre-treatment of porous stainless steel substrate[J]. Journal of Membrane Science, 2007, 298(1/2): 175-181.
[37] XU N, RYI S, LI A W, GRACE J R, LIM J, BOYD T. Improved pre-treatment of porous stainless steel substrate for preparation of Pd-based composite membrane[J]. The Canadian Journal of Chemical Engineering, 2013, 91(10): 1695-1701.
[38] CHI Y H, YEN P S, JENG M S, KO S T, LEE T C. Preparation of thin Pd membrane on porous stainless steel tubes modified by a two-step method[J]. International Journal of Hydrogen Energy, 2010, 35(12): 6303-6310.
[39] GUO Y, ZHANG X F, DENG H, WANG X B, WANG Y, QIU J S, WANG J Q, YEUNG K L. A novel approach for the preparation of highly stable Pd membrane on macroporous α-Al2O3 tube[J]. Journal of Membrane Science, 2010, 362(1/2): 241-248.
[40] WANG X B, TAN X Y, MENG B, ZHANG X F, LIANG Q, PAN H, LIU S M. TS-1 zeolite as an effective diffusion barrier for highly stable Pd membrane supported on macroporous α-Al2O3 tube[J]. RSC Advances, 2013, 3(14): 4821-4834.
[41] BOSKO M L, OJEDA F, LOMBARDO E A, CORNAGLIA L M. NaA zeolite as an effective diffusion barrier in composite Pd/PSS membranes[J]. Journal of Membrane Science, 2009, 331(1/2): 57- 65.
[42] HUANG Y, HU X, CHEN W. Method for the fabrication of composite palladium and palladium-alloy membranes. US patent, 8445055[P]. 2013-05-21.
[43] HU X, CHEN W, HUANG Y. Fabrication of Pd/ceramic membranes for hydrogen separation based on low-cost macroporous ceramics with pencil coating[J]. International Journal of Hydrogen Energy, 2010, 35(15): 7803-7808.
[44] HU X, YU J, SONG J, WANG X, HUANG Y. Toward low-cost Pd/ceramic composite membranes for hydrogen separation: A case study on reuse of the recycled porous Al2O3 substrates in membrane fabrication[J]. International Journal of Hydrogen Energy, 2011, 36(24): 15794-15802.
[45] WEI L, YU J, HU X, HUANG Y. Fabrication of H2-permeable palladium membranes based on pencil-coated porous stainless steel substrate[J]. International Journal of Hydrogen Energy, 2012, 37(17): 13007-13012.
[46] WEI L, YU J, HUANG Y. Silver coating on porous stainless steel substrate and preparation of H2-permeable palladium membranes[J]. International Journal of Hydrogen Energy, 2013, 38(25): 10833-10838.
[47] 邓 超, 张小亮, 黄 彦. 多孔陶瓷基体表面的凝胶修饰及钯膜的制备[J]. 南京工业大学学报(自然科学版), 2010, 32(1): 92-97.
DENG Chao, ZHANG Xiao-liang, HUANG Yan. Fabrication of thin composite palladium membranes: modification of the porous ceramic substrate with gel[J]. Journal of Nanjing University of Technology (Natural Science Edition), 2010, 32(1): 92-97.
[48] WANG C, HU X, YU J, WEI L, HUANG Y. Intermediate gel coating on macroporous Al2O3 substrate for fabrication of thin carbon membranes[J]. Ceramics International, 2014, 40(7): 10367-10373.
[49] HUANG Y, SHU S, LU Z, FAN Y. Characterization of the adhesion of thin palladium membranes supported on tubular porous ceramics[J]. Thin Solid Films, 2007, 515(13): 5233-5240.
[50] TONG J, MATSUMURA Y. Thin Pd membrane prepared on macroporous stainless steel tube filter by an in-situ multi- dimensional plating mechanism[J]. Chemical Communication, 2004, 21: 2460-2461.
[51] TONG J, SUDA H, HARAYA K, MATSUMURA Y. A novel method for the preparation of thin dense Pd membrane on macroporous stainless steel tube filter[J]. Journal of Membrane Science, 2005, 260(1/2): 10-18.
[52] 徐恒泳, 侯守福, 李文钊, 江 魁, 袁立祥. 一种复合金属钯膜或合金钯膜及其制备方法: 中国, 200410021025.6[P]. 2004-01-09.
XU Heng-yong, HOU Shou-fu, LI Wen-zhao, JIANG Kui, YUAN Li-xiang. Composite Pd and Pd-alloy membranes and the preparation method: China, 200410021025.6[P]. 2004-01-09.
[53] YEUNG K L, SEBASTIAN J M, VARMA A. Novel preparation of Pd/Vycor composite membranes[J]. Catalysis Today, 1995, 25(3/4): 231-236.
[54] SOULEIMANOVA R S, MUKASYAN A S, VARMA A. Pd membranes formed by electroless plating with osmosis: H2 permeation studies[J]. AIChE Journal, 2002, 48(2): 262-268.
[55] SOULEIMANOVA R S, MUKASYAN A S, VARMA A. Pd-composite membranes prepared by electroless plating and osmosis: synthesis, characterization and properties[J]. Separation and Purification Technology, 2001, 25(1/3): 79- 86.
[56] SOULEIMANOVA R S, MUKASYAN A S, VARMA A. Effects of osmosis on microstructure of Pd-composite membranes synthesized by electroless plating technique[J]. Journal of Membrane Science, 2000, 166(2): 249-257.
[57] SOULEIMANOVA R S, MUKASYAN A S, VARMA A. Study of structure formation during electroless plating of thin metal-composite membranes[J]. Chemical Engineering Science, 1999, 54(15/16): 3369-3377.
[58] LI A W, LIANG W Q, HUGHES R. Characterization and preparation of palladium/stainless steel composite membrane[J]. Journal of Membrane Science, 1998, 149(2): 259-268.
[59] 蒋柏泉, 高建华, 顾 騋. 渗透压在化学镀共沉积钯银中的应用研究[J]. 南昌大学学报(工科版), 2002, 24(2): 43-46.
JIANG Bai-quan, GAO Jian-hua, GU Lai. Effects of osmosis on self-catalyzed simultaneous deposition of Pd-Ag by electroless plating[J]. Journal of Nanchang University (Engineering & Technology), 2002, 24(2): 43-46.
[60] CHEN H, CHU C, HUANG T. Comprehensive characterization and permeation analysis of thin Pd/Al2O3 composite membranes prepared by suction-assisted electroless deposition[J]. Separation Science and Technology, 2010, 39(7): 1461-1483.
[61] ZHANG X, XIONG G, YANG W. A modified electroless plating technique for thin dense palladium composite membranes with enhanced stability[J]. Journal of Membrane Science, 2008, 314(1/2): 226-237.
[62] 黄 彦, 魏磊, 俞 健, 宋 军. 一种制备钯或钯合金膜的循环化学镀工艺: 中国, 201110118605.7[P]. 2011-05-10.
HUANG Yan, WEI Lei, YU Jian, SONG Jun. Circulatory electroless plating for the preparation of Pd and Pd-alloy membranes: China, 201110118605.7[P]. 2011-05-10.
[63] NAM S E, LEE S H, LEE K H. Preparation of a palladium alloy composite membrane supported in a porous stainless steel by vacuum electrodeposition[J]. Journal of Membrane Science, 1999, 153(2): 163-173.
[64] NAM S E, LEE K H. A study on the palladium/nickel composite membrane by vacuum electrodeposition[J]. Journal of Membrane Science, 2000, 170(1): 91-99.
[65] 黄 彦, 舒世立, 范菁菁, 胡小娟. 一种管式多孔材料负载金属膜的化学镀方法: 中国, 200710021806.9[P]. 2007-04-29.
HUANG Yan, SHU Shi-li, FAN Jing-jing, HU Xiao-juan. Electroless plating method for the preparation of metallic membranes based on tubular porous materials: China, 200710021806.9[P]. 2007-04-29.
[66] SANZ R, CALLES J A, ALIQUE D, FURONES L. New synthesis method of Pd membranes over tubular PSS supports via “pore-plating” for hydrogen separation[J]. International Journal of Hydrogen Energy, 2012, 37(23): 18476-18485.
[67] LI A W, LIANG W Q, HUGHES R. Repair of a Pd/α-Al2O3 composite membrane containing defects[J]. Separation and Purification Technology, 1999, 15(2): 113-119.
[68] ZENG G, GOLDBACH A, XU H. Defect sealing in Pd membranes via point plating[J]. Journal of Membrane Science, 2009, 328(1/2): 6-10.
[69] 唐春华, 邵 炜, 徐恒泳. 超薄金属钯复合膜表面缺陷修饰的研究[J]. 天然气化工, 2009, 34(1): 10-16.
TANG Chun-hua, SHAO Wei, XU Heng-yong. Study on the modification of the surface defect of thin Pd/ceramic composite membrane[J]. Natural Gas Chemical Industry, 2009, 34(1): 10-16.
[70] 唐春华, 邵 炜, 徐恒泳. Pd/γ-Al2O3对超薄金属钯复合膜表面缺陷修复的研究[J]. 天然气化工, 2009, 34(2): 1-6.
TANG Chun-hua, SHAO Wei, XU Heng-yong. Surface modification of thin Pd/ceramic composite membrane with Pd/γ-Al2O3[J]. Natural Gas Chemical Industry, 2009, 34(2): 1-6.
[71] 黄 彦, 魏 娟, 胡小娟, 陈卫东. 钯或钯合金复合膜的化学镀修补法: 中国, 201010018273.0[P]. 2010-01-21.
HUANG Yan, WEI Juan, HU Xiao-juan, CHEN Wei-dong. Repair of Pd and Pd-alloy composite membranes by electroless plating: China, 201010018273.0[P]. 2010-01-21.
[72] 胡小娟, 魏 娟, 黄 彦. Pd(OH)2胶体预处理及化学镀法修补复合Pd膜[J]. 南京工业大学学报(自然科学版), 2011, 33(3): 43-48.
HU Xiao-juan, WEI Juan, HUANG Yan. Repair of composite palladium membrane by electroless plating after pretreatment with Pd(OH)2 colloid[J]. Journal of Nanjing University of Technology (Natural Science Edition), 2011, 33(3): 43-48.
[73] TONG J, SU C, KURAOKA K, SUDA H, MATSUMURA Y. Preparation of thin Pd membrane on CeO2-modified porous metal by a combined method of electroless plating and chemical vapor deposition[J]. Journal of Membrane Science, 2006, 269(1/2): 101-108.
[74] 袁立祥, 徐恒泳. “自下而上”法修补含缺陷的钯膜[J]. 膜科学与技术, 2009, 29(3): 48-51.
YUAN Li-xiang, XU Heng-yong. Repair of defects in Pd composite membranes by bottom-up electroless plating[J]. Membrane Science and Technology, 2009, 29(3): 48-51.
[75] RYI S K, PARK J S, HWANG K R, LEE C B, LEE S W. Repair of Pd-based composite membrane by polishing treatment[J]. International Journal of Hydrogen Energy, 2011, 36(21): 13776-13780.
(编辑 李艳红)
基金项目:国家高技术研究发展计划资助项目(2009AA05Z103);江苏省高校自然科学重大基础研究项目(09KJA530003);江苏省自然科学基金资助项目(BK20130916, BK20130940)
收稿日期:2014-11-17;修订日期:2015-04-01
通信作者:黄 彦,教授,博士;电话:025-83172253;E-mail: huangy@njtech.edu.cn
摘 要:钯膜对氢气有着优异的渗透性和选择性,在氢分离和膜催化反应领域备受关注。将钯膜直接沉积于多孔基体表面形成的钯复合膜,具有厚度薄、力学强度高、氢渗透率高、成本低等优点,但制膜难度较高。在各种制膜方法中,化学镀法最为常用。在化学镀制膜过程中,对钯膜的质量控制和缺陷修补工艺进行了综述,以期进一步推动高性能钯复合膜的发展及应用。
[3] 雷敏志, 陈森凤. 钯膜与钯膜反应器应用研究进展[J]. 材料导报, 2004, 18(5): 41-44.
[10] 黄 彦, 李 雪, 范益群, 徐南平. 透氢钯复合膜的原理、制备及表征[J]. 化学进展, 2006, 18(2/3): 230-238.
[14] 黄 彦, 王红志, 俞 健, 胡小娟. 一种齿轮型透氢钯或钯合金膜及氢气分离器: 中国, 201010578052.9[P]. 2010-12-08.
[19] 刘 伟, 张宝泉, 刘秀凤. 钯复合膜的研究进展[J]. 化学进展, 2006, 18(11): 1468-1481.
[22] 李 宁. 化学镀实用技术[M]. 北京: 化学工业出版社, 2004.
LI Ning. Practical technology of electroless plating [M]. Beijing: Chemical Industry Press, 2004.
[32] 俞 健, 胡小娟, 黄 彦. 多孔不锈钢表面的陶瓷修饰及所负载的透氢钯膜[J]. 化学进展, 2008, 20(7/8): 1208-1215.
[47] 邓 超, 张小亮, 黄 彦. 多孔陶瓷基体表面的凝胶修饰及钯膜的制备[J]. 南京工业大学学报(自然科学版), 2010, 32(1): 92-97.
[52] 徐恒泳, 侯守福, 李文钊, 江 魁, 袁立祥. 一种复合金属钯膜或合金钯膜及其制备方法: 中国, 200410021025.6[P]. 2004-01-09.
[59] 蒋柏泉, 高建华, 顾 騋. 渗透压在化学镀共沉积钯银中的应用研究[J]. 南昌大学学报(工科版), 2002, 24(2): 43-46.
[62] 黄 彦, 魏磊, 俞 健, 宋 军. 一种制备钯或钯合金膜的循环化学镀工艺: 中国, 201110118605.7[P]. 2011-05-10.
[65] 黄 彦, 舒世立, 范菁菁, 胡小娟. 一种管式多孔材料负载金属膜的化学镀方法: 中国, 200710021806.9[P]. 2007-04-29.
[69] 唐春华, 邵 炜, 徐恒泳. 超薄金属钯复合膜表面缺陷修饰的研究[J]. 天然气化工, 2009, 34(1): 10-16.
[70] 唐春华, 邵 炜, 徐恒泳. Pd/γ-Al2O3对超薄金属钯复合膜表面缺陷修复的研究[J]. 天然气化工, 2009, 34(2): 1-6.
[71] 黄 彦, 魏 娟, 胡小娟, 陈卫东. 钯或钯合金复合膜的化学镀修补法: 中国, 201010018273.0[P]. 2010-01-21.
[72] 胡小娟, 魏 娟, 黄 彦. Pd(OH)2胶体预处理及化学镀法修补复合Pd膜[J]. 南京工业大学学报(自然科学版), 2011, 33(3): 43-48.
[74] 袁立祥, 徐恒泳. “自下而上”法修补含缺陷的钯膜[J]. 膜科学与技术, 2009, 29(3): 48-51.


