DOI:10.19476/j.ysxb.1004.0609.2019.11.25
稀释剂对3-氧戊二酰胺类萃取剂萃取稀土的影响
马 晨1,徐源来1,马驰远1,周 芳1,王 芬1, 2,池汝安1
(1. 武汉工程大学 绿色化工过程教育部重点实验室 化工与制药学院,武汉430074;
2. 四川文理学院 化学化工学院,达州 635000)
摘 要:
为了从风化壳淋积型稀土矿铵盐浸出液中萃取分离出稀土离子,合成3种3-氧戊二酰胺类萃取剂N,N,N′,N′-四丁基-3-氧戊二酰胺、N,N,N′,N′-四己基-3-氧戊二酰胺和N,N,N′,N′-四辛基-3-氧戊二酰胺。考察在NH4Cl、(NH4)2SO4、NH4NO3这3种铵盐溶液中,萃取剂浓度和不同稀释剂类型对3种3-氧戊二酰胺类萃取剂萃取稀土离子的影响,并筛选出合适的稀释剂。结果表明:在NH4Cl溶液中,以正辛烷-正辛醇(体积比7:3)为稀释剂时,N,N,N′,N′-四丁基-3-氧戊二酰胺的萃取效果最好,对Y离子和Gd离子的单级萃取剂分别为为89%和91%,且萃取率随着萃取剂浓度的增大而增大,在相比为4:6时萃取率达到最大并几乎恒定。在(NH4)2SO4、NH4NO3溶液中,不同稀释剂对萃取效果的影响差别则较小。
关键词:
文章编号:1004-0609(2019)-11-2681-10 中图分类号:TD955 文献标志码:A
稀土元素特殊的f层电子结构使其具有独特的物理和化学性质,在光、电、磁等诸多新材料领域有非常广泛的应用[1-2]。我国是稀土资源大国,不仅储量丰富,而且品种齐全[3],稀土资源的开发利用一直备受重视。风化壳淋积型稀土矿[4]中的稀土元素主要以离子相形态存在于黏土矿物中[5],广泛分布于我国南方地区。其中稀土配分齐全,尤其中重稀土的配分占比高[6],是一种重要的战略资源。全球中重稀土资源紧缺的局势使得风化壳淋积型稀土矿的开发利用受到了国内外的广泛关注[7-8]。
目前,我国风化壳淋积型稀土矿的工业开采使用的是原地浸出工艺[9-10],得到的稀土浸出液中含有浸取剂、杂质离子和稀土离子,其中稀土离子浓度较低。工业上一般采用沉淀法提取其中的稀土,即采用碳酸氢铵对浸出液进行除杂、以草酸作为沉淀剂沉淀浸出液中的稀土,得到草酸稀土沉淀物[11-12]。该沉淀物经过高温焙烧后为稀土氧化物,用酸溶解,得到的稀土溶液再利用萃取分离生产单一稀土[13-14]。整个生产过程不仅需要消耗大量的沉淀剂和酸碱试剂,还需要消耗能源。因此,如何不经过沉淀和焙烧流程,直接从稀土浸出液中萃取分离稀土已成为一个热门的研究方向[15]。目前,溶剂萃取法[16]是稀土提取分离的重要方法之一,而溶剂萃取法中选择合适的萃取剂和稀释剂用于稀土浸出液中稀土的分离提纯则至关重要。目前工业上使用的铵盐浸出剂有氯化铵、硫酸铵和少量的硝酸铵,本课题组使用铵盐浸出剂进行了大量风化壳淋积型稀土矿的分离富集基础研究工作。
随着高新技术产业对稀土金属的需求量和纯度要求的不断提高,新型高效萃取剂的研发一直备受关注。近年来,已开发出多种新型萃取剂,如二烷基次磷酸[17]、含磷套索冠醚[18]、离子液体[19]和酰胺类萃取剂[20-21]等。其中二烷基次磷酸在稀土金属萃取分离中有重要的应用,其pKa较大,萃取时不需要较高的酸度,可通过调节烷基结构得到相应的空间效应,从而对特定稀土离子有很好的选择性[22-23]。酰胺类萃取剂因其合成简单、性质稳定、产物无污染等优点备受关注。酰胺类萃取剂包括单酰胺类、双酰胺类、酰亚胺类、3-氧戊二酰胺类[24]。其中3-氧戊二酰胺类萃取剂的萃取性能大大优于其他类型萃取剂的,这是因为3-氧戊二酰胺类萃取剂结构中的两个羰基和醚氧键能够同时参加配位,而N—C=O结构增加了配合物的稳定性[25]。但是以往关于3-氧戊二酰胺类萃取剂对稀土元素的萃取研究大多针对盐酸或硝酸溶液[26],而在铵盐浸取剂中使用的研究报道较少。因此,为了从铵盐溶液中直接分离提纯稀土,尤其是提纯化壳淋积型稀土矿中含量较高的中重稀土,本文合成了三种3-氧戊二酰胺类萃取剂,研究了不同稀释剂种类对三种3-氧戊二酰胺类萃取剂对La、Y、Gd离子的萃取性能影响,得到了最优的稀释剂类型和配比,为后续的萃取行为研究提供基本的工艺参数。
1 实验
1.1 仪器与试剂
本实验主要用的实验仪器有R-3旋转蒸发仪(BUCHI Operations India Private Limited)、400 MHz 核磁共振波谱仪(安捷伦公司)、Nicolet 6700 型傅里叶变换红外光谱仪(赛默飞世尔科技公司)、XH-C型旋涡混合器(常州越新仪器制作有限公司)、EL204型电子分析天平(梅特勒-托利多仪器(上海)有限公司)。
萃取剂N,N,N′,N′-四丁基-3-氧戊二酰胺、N,N,N′,N′-四己基-3-氧戊二酰胺和N,N,N′,N′-四辛基- 3-氧戊二酰胺由实验室合成。试剂硝酸钆六水合物、硝酸镧六水合物、硝酸钇六水合物、氯化铵、硫酸铵、硝酸铵、正辛烷、正辛醇、磺化煤油、乙酸乙酯、二氯甲烷、正十二烷、正庚烷均为分析纯。
1.2 萃取剂的合成
以2,2′-氧代二乙酰氯为原料,分别与二正丁胺,二正己胺和二正辛胺反应,合成3种萃取剂N,N,N′,N′-四丁基-3-氧戊二酰胺(C1)、N,N,N′,N′-四己基-3-氧戊二酰胺(C2)和N,N,N′,N′-四辛基-3-氧戊二酰胺(C3),具体的合成路线[27-28]分别如下。
N,N,N′,N′-四丁基-3-氧戊二酰胺的合成路线:
 (1)
(1)
N,N,N′,N′-四己基-3-氧戊二酰胺的合成路线:
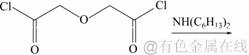
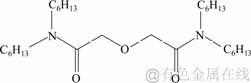 (2)
(2)
N,N,N′,N′-四辛基-3-氧戊二酰胺的合成路线:
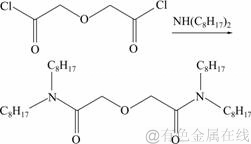 (3)
(3)
N,N,N′,N′-四己基-3-氧戊二酰胺的合成步骤如下:向装有磁力搅拌器、恒压滴液漏斗的三口烧瓶中加入二正己胺和10 mL吡啶,以二氯甲烷作溶剂,冰浴下磁力搅拌反应。将2,2′-氧代二乙酰氯通过恒压滴液漏斗缓慢滴加至烧瓶中,2 h内滴加完毕,保持烧瓶内温度在0~5 ℃。滴加完毕后,室温下搅拌反应6 h。先用10%(质量分数)的稀盐酸洗涤反应后的液体,除去未反应的二正己胺,再用二次水洗涤至中性,无水硫酸钠干燥5 h,旋蒸除去二氯甲烷后得到红褐色油状液体。通过柱层析法进行分离提纯(洗脱剂为体积比是3:1的石油醚和乙酸乙酯),得到的淡黄色粘稠油状物即目标产物,产率约为55%。
N,N,N′,N′-四丁基-3-氧戊二酰胺和N,N,N′,N′-四辛基-3-氧戊二酰胺的合成方法与上述相同,只是将反应物二正己胺换为二正丁胺和二正辛胺,产率分别为56 %和52 %。使用1H NMR和红外分光光度计对产物进行表征。
1.3 萃取实验
本文考察的稀释剂有正辛烷-正辛醇(体积比7:3)、磺化煤油、正辛烷、乙酸乙酯、二氯甲烷、正十二烷和正庚烷。
有机相按萃取剂和稀释剂的质量比1:9准确称量萃取剂和稀释剂混合而成。
准确称量定量的硝酸钆六水合物和氯化铵药品,用适量二次水溶解,待温度恢复至常温后准确定容至100 mL容量瓶,得到Gd(Ⅲ)-NH4Cl水相溶液。与以上方法类似,配制得到La(Ⅲ)-NH4Cl、Y(Ⅲ)-NH4Cl、La(Ⅲ)-(NH4)2SO4、Y(Ⅲ)-(NH4)2SO4、Gd(Ⅲ)-(NH4)2SO4、La(Ⅲ)-NH4NO3、Y(Ⅲ)-NH4NO3、Gd(Ⅲ)-NH4NO3水相溶液。
移取有机相和水相(Vo:Vw=2:8)于试管中,在振荡器上振荡20 min使两相充分接触,静置分层后,取萃余水相,以电感耦合等离子体质谱(ICP-MS)和EDTA容量法滴定分析萃取前后水相中稀土离子浓度,实验均在室温下完成。使用差减法计算负载有机相的稀土离子浓度,依次按下列公式计算萃取率:
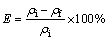 (4)
(4)
式中: 为水相中的稀土离子初始浓度,mg/L;
为水相中的稀土离子初始浓度,mg/L; 为萃取后水相中的稀土离子浓度,mg/L。
为萃取后水相中的稀土离子浓度,mg/L。
2 结果与讨论
2.1 萃取剂的表征
图1(a)所示为萃取剂的结构示意图,3种萃取剂分别用C1、C2、C3表示:N,N,N′,N′-四辛基-3-氧戊二酰胺(C1);N,N,N′,N′-四己基-3-氧戊二酰胺(C2);N,N,N′,N′-四丁基-3-氧戊二酰胺(C3)。图1(b)所示为3种萃取剂的红外吸收光谱,3种萃取剂结构类似,如图所示,2955 cm-1和2930 cm-1处对应的是甲基和亚甲不对称伸缩振动吸收峰,1647 cm-1处对应的是酰胺上羰基伸缩振动吸收峰,1437 cm-1处对应的是甲基变形振动吸收峰,1122 cm-1处对应的是醚氧键上C—O—C的伸缩振动吸收峰,722 cm-1对应的是4个以上的亚甲基相连的吸收峰与产物相符合。
图2所示为3种萃取剂的核磁共振氢谱(400 MHz, CDCl3),N,N,N′,N′-四丁基-3-氧戊二酰胺的谱图解析为δ=0.83×10-6~0.88×10-6 (12H, t, —CH3),δ= 1.20× 10-6~1.26×10-6 (8H, m, —CH2—),δ=1.43×10-6~ 1.46×10-6 (8H, m, —N—C—CH2—),δ=3.22×10-6~ 3.26×10-6 (8H, tt, —N—CH2—),δ=4.25×10-6 (4H, s, —CH2—O—CH2—)。N,N,N′,N′-四己基-3-氧戊二酰胺的谱图解析为δ=0.83×10-6~0.88×10-6 (12H, t, —CH3),δ=1.20×10-6~1.26×10-6 (8H, m, —CH2—),δ= 1.43×10-6~1.46×10-6 (8H, m, —N—C—CH2—),δ= 3.22×10-6~3.26×10-6 (8H, tt, —N—CH2—),δ= 4.25×10-6×10-6 (4H, s, —CH2—O—CH2—)。N,N,N′,N′-四辛基-3-氧戊二酰胺的谱图解析为δ= 0.83×10-6~0.88×10-6 (12H, t, —CH3),δ=1.20×10-6~ 1.26×10-6 (40H, m, —CH2—),δ=1.43×10-6~1.46×10-6 (8H, m, —N—C—CH2—),δ=3.22×10-6~ 3.26×10-6 (8H, tt, —N—CH2—),δ=4.25×10-6 (4H, s, —CH2— O—CH2—)。
2.2 不同稀释剂对萃取稀土离子的影响
有机萃取剂的黏度较大,在液液萃取过程中,不利于萃取剂与水相充分混合接触,一般需要加入合适的有机溶剂作为稀释剂来减小黏度,提高萃取效率[29]。同时稀释剂与萃取剂的相互作用会影响整个液液萃取体系的物理化学性质,从而影响萃取剂对金属离子的萃取效果,所以选用不同的稀释剂,对萃取效果的影响明显[30]。图3、4、5所示分别为7种稀释剂在NH4Cl、(NH4)2SO4、NH4NO3溶液中对3种萃取剂萃取稀土离子的影响,稀土离子选用La、Y、Gd离子为代表,稀释剂分别用X1~X7表示:X1为正辛烷-正辛醇(体积比7:3)、X2为磺化煤油、X3为正辛烷、X4为乙酸乙酯、X5为二氯甲烷、X6为正十二烷、X7为正庚烷,萃取剂分别为C1、C2和C3。
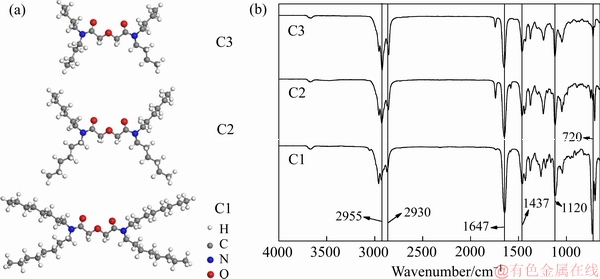
图1 萃取剂的分子结构示意图与红外光谱
Fig. 1 Chemical structures(a) and IR spectra(b) of extractants
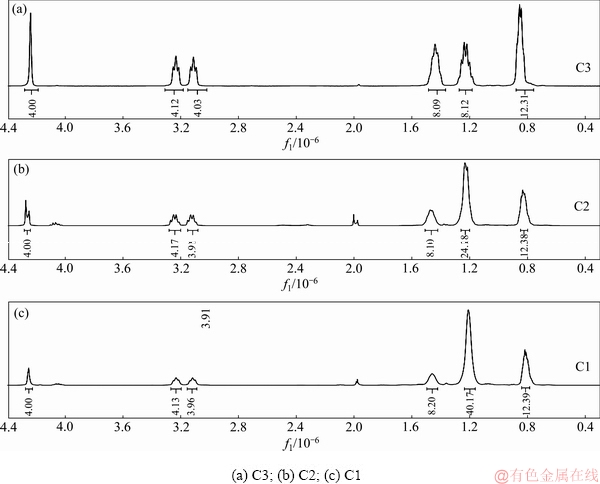
图2 萃取剂的核磁共振氢谱
Fig. 2 1H-NMR spectra of extractants
由图3可知,在NH4Cl溶液中,3种萃取剂对Y离子和Gd离子的萃取效果优于对La离子的萃取效果,且对La离子的萃取率均在20%以下;在不同稀释剂的条件下,以正辛烷-正辛醇(体积比7:3)为稀释剂时,3种萃取剂对Y离子和Gd离子的萃取效果最好,其中N,N,N′,N′-四丁基-3-氧戊二酰胺作为萃取剂时,萃取效果优于其他两种萃取剂的,Y离子的萃取率达到89%,Gd离子的萃取率达到91%。这可能与稀释剂的极性有关,稀释剂的极性会影响配合物的配位情况,相较于其他几种稀释剂混合稀释剂正辛烷-正辛醇(体积比7:3)的极性最大,有利于形成稀土配合物及进入极性的有机相。值得注意的是,以正辛烷-正辛醇(体积比7:3)为稀释剂比单独以正辛烷为稀释剂的萃取效果好。一方面,正辛醇的极性远大于正辛烷的极性,3种萃取剂的极性也很大,由“相似相容”理论可知,强极性的稀释剂有利于使配合物在有机相中更加稳定;另一方面,正辛醇可作为助溶剂,增大了萃取剂的溶解度,有利于萃合物更加容易进入有机相中,改善萃取效果[31]。
由图4可知,(NH4)2SO4溶液相较于NH4Cl和NH4NO3溶液,3种萃取剂对La、Y、Gd离子的萃取效果不佳,萃取率均在30%以下。这主要是由于二价的硫酸根离子在溶液中可以与稀土离子形成溶解度小的稀土硫酸盐,降低了萃取过程中稀土离子与含有萃取剂的有机相的反应几率,在两相界面处产生固体颗粒阻碍萃取传质过程。故在(NH4)2SO4溶液中,硫酸根离子的存在不利于稀土离子向有机相中迁移,从而阻碍萃取过程的进行,导致萃取率偏低。
由图5(a)可知,NH4NO3溶液中,以正辛烷-正辛醇(体积比7:3)和二氯甲烷为稀释剂时,N,N,N′,N′-四丁基-3-氧戊二酰胺对3种稀土离子的萃取率均很高。这可能是正辛烷-正辛醇(体积比7:3)和二氯甲烷的极性比其他6种稀释剂的大,有利于形成稀土配合物及进入极性的有机相。其中,以正辛烷-正辛醇(体积比7:3)为稀释剂时,对La离子和Y离子的萃取率最高,分别为85%和98%;以二氯甲烷为稀释剂时,对Gd离子的萃取率最高(98%)。其余6种稀释剂中,N,N,N′,N′-四丁基-3-氧戊二酰胺对3种稀土离子的萃取效果不佳,萃取率均在30%以下。
由图5(b)可知,在NH4NO3溶液中,以N,N,N′,N′-四己基-3-氧戊二酰胺作为萃取剂,对La离子都有很好的萃取效果,萃取率均在60%以上。乙酸乙酯作为稀释剂对La离子的萃取率最高,为92%。N,N,N′,N′-四己基-3-氧戊二酰胺对Y离子和Gd离子的萃取率均能达到98%以上,说明7种稀释剂在NH4NO3溶液中对N,N,N′,N′-四己基-3-氧戊二酰胺萃取Y离子和Gd离子几乎没有影响,这是因为水相溶液中硝酸根离子的存在,在萃取过程中产生了同离子效应[32],有利于Y离子和Gd离子的萃取。

图3 7种稀释剂在NH4Cl溶液中对3种萃取剂萃取稀土离子的影响
Fig. 3 Effects of seven diluents on extraction of rare earth ions by N,N,N,N′-tetrabutyl-3-oxy-glutaramide(a), N,N,N′,N′- tetrahexyl-3-oxy-glutaramide(b) and N,N,N′,N′-tetraoctane-3- oxy-glutaramide(c) three extractants in NH4Cl solution
由图5(c)可知,在NH4NO3溶液中,以N,N,N′,N′-四辛基-3-氧戊二酰胺作为萃取剂,以乙酸乙酯为稀释剂,对La的萃取率最高,为88%;对Y离子的萃取率最高(98%);对Gd离子的萃取率均能达到88%以上。
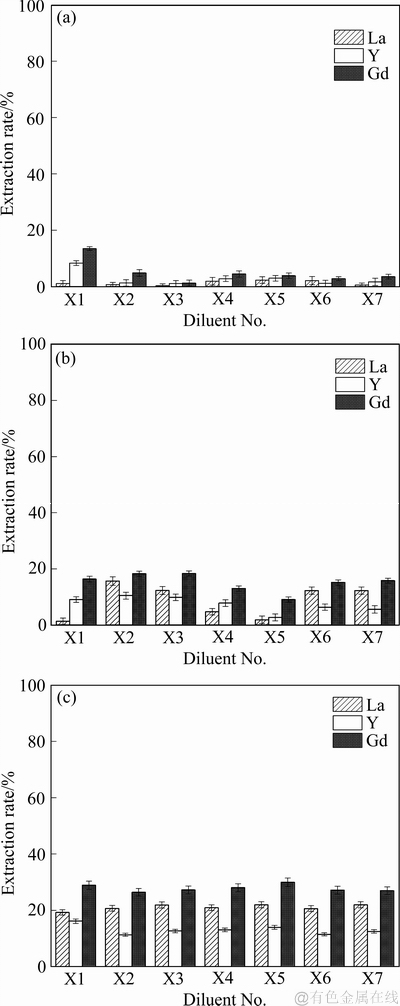
图4 7种稀释剂在(NH4)2SO4溶液中对3种萃取剂萃取稀土离子的影响
Fig. 4 Effects of seven diluents on extraction of rare earth ions by N,N,N,N′- tetrabutyl-3-oxy-glutaramide(a), N,N,N′,N′- tetrahexyl-3-oxy-glutaramide(b) and N,N,N′,N′-tetraoctane-3- oxy-glutaramide(c) three extractants in (NH4)2SO4 solution
液中对N,N,N′,N′-四辛基-3-氧戊二酰胺萃取Y离子的对Y离子的萃取率均能达到90%以上,说明7种稀释剂在NH4NO3溶影响很小,说明不同稀释剂对N,N,N′,N′-四丁基-3-氧戊二酰胺萃取Gd的影响也不大,其中以正辛烷-正辛醇(体积比7:3)为稀释剂时,对Gd离子的萃取率最高,为98%。
通常稀释剂的极性大有利于萃取过程的进行,但由图5(b)、(c)可知,7种稀释剂在NH4NO3溶液中对N,N,N′,N′-四辛基-3-氧戊二酰胺萃取Y离子和Gd离子的影响很小,萃取率均在90%以上,故可以认为除了稀释剂的极性,空间位阻效应和萃取剂在稀释剂中的聚合等因素都可以影响萃取剂的萃取效果。
2.3 萃取剂浓度对萃取的影响
混合稀释剂正辛烷-正辛醇(体积比7:3)的极性最大,且由于正辛醇的存在增大了萃取剂的溶解度,有利于萃合物更加容易进入有机相中,所以3种萃取剂对稀土离子的萃取效果都很好。图6和7所示分别为NH4Cl和NH4NO3溶液中萃取剂浓度对萃取稀土离子的影响,萃取剂浓度以有机相和水相的体积比表示,稀土离子选用La、Y、Gd离子为代表,稀释剂选用正辛烷-正辛醇(体积比7:3),萃取剂分别为C1、C2和C3。
由图6可知,NH4Cl溶液中,在所研究的萃取浓度范围内,随着萃取剂浓度增大,3种萃取剂对La、Y、Gd离子的萃取率均有不同程度的增大,在同一浓度(相比)下,对La离子的萃取率最低。这是因为La的原子半径在选用的3种稀土离子中较大,正电荷密度相对较小,结合硬碱的能力较弱,而本实验中选用的三种酰胺类萃取剂属于硬碱。其中,由图6(a)可知,N,N,N′,N′-四丁基-3-氧戊二酰胺作为萃取剂,La离子的萃取率均在20%以下,而Y离子和Gd离子在相比为4:6时,萃取率达到最大96%并几乎恒定,故选择最佳相比为4:6。由图6(b)可知,N,N,N′,N′-四己基-3-氧戊二酰胺作为萃取剂,相比为5:5时萃取率达到最大,La离子的萃取率为29%,Y离子为91%,Gd离子为92%。由图6(c)知,N,N,N′,N′-四辛基-3-氧戊二酰胺作为萃取剂,相比为5:5时达到最大,La离子的萃取率为73%,Y离子为95%,Gd离子为92%。故选择5:5的相比作为N,N,N′,N′-四己基-3-氧戊二酰胺和N,N,N′,N′-四辛基-3-氧戊二酰胺的最佳萃取条件。
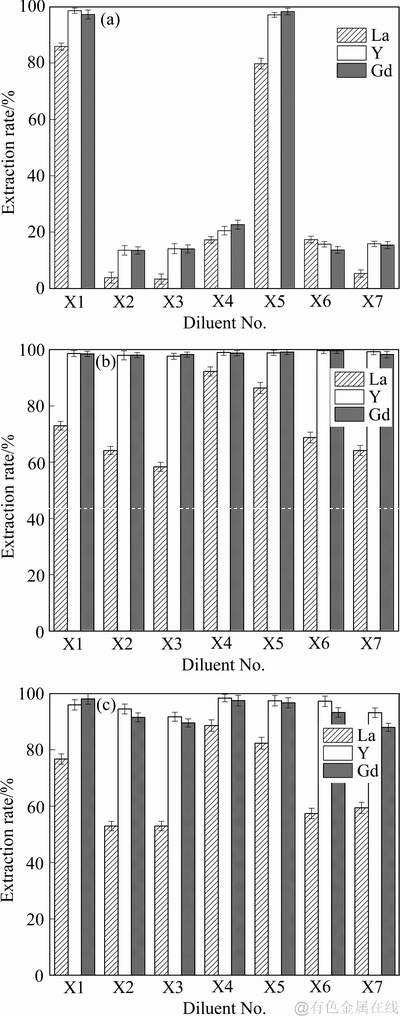
图5 7种稀释剂在NH4NO3溶液中对3种萃取剂萃取稀土离子的影响
Fig. 5 Effects of seven diluents on extraction of rare earth ions by N,N,N,N′- tetrabutyl-3-oxy-glutaramide(a), N,N,N′,N′- tetrahexyl-3-oxy-glutaramide(b) and N,N,N′,N′-tetraoctane-3- oxy-glutaramide(c) three extractants in NH4NO3 solution
由图7可知,NH4NO3溶液中,在所研究的萃取浓度范围内,随着萃取剂浓度增大,3种萃取剂对La、Y、Gd离子的萃取率均有不同程度的增大。由图7(a)可知,N,N,N′,N′-四丁基-3-氧戊二酰胺作为萃取剂,对La离子和Y离子的萃取率在相比为4:6时达到92%,对Gd离子的萃取率达到97%。且继续增大相比,萃取率几乎不变。由图7(b)可知,N,N,N′,N′-四己基-3-氧戊二酰胺作为萃取剂,对La离子和Y离子的萃取率在相比为5:5时分别达到94%和99%;对Gd离子的萃取率在相比为4:6时达到99%。由图7(c)可知,N,N,N′,N′-四辛基-3-氧戊二酰胺作为萃取剂,对Y离子的萃取率在相比为5:5时达到92%;对Gd离子的萃取率在相比为4:6达到99%。且继续增大相比,萃取率几乎不变。
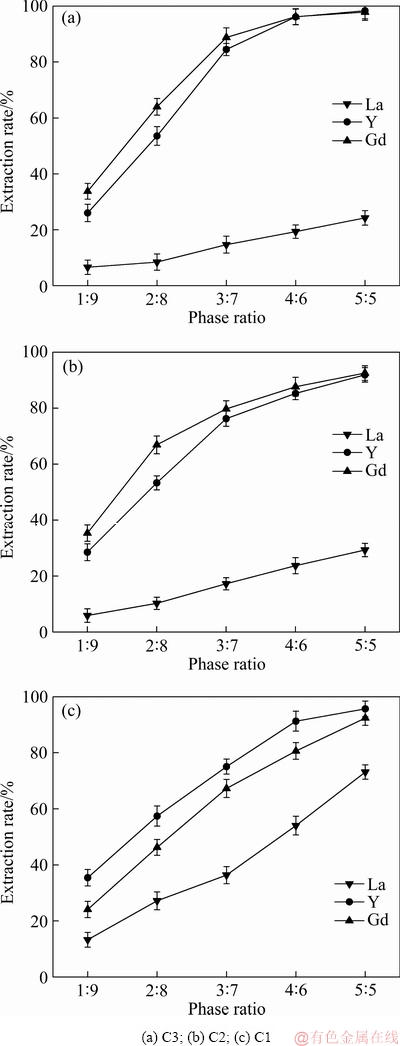
图6 NH4Cl溶液中萃取剂浓度对萃取稀土离子的影响
Fig. 6 Effects of extractant concentration on extraction of rare earth ions in NH4Cl solution

图7 NH4NO3溶液中萃取剂浓度对萃取稀土离子的影响
Fig. 7 Effect of extractant concentration on extraction of rare earth ions in NH4NO3 solution
3 结论
1) 在被考察的7种稀释剂中,强极性的稀释剂正辛烷-正辛醇(体积比7:3)适合3种萃取剂在NH4Cl溶液中进行萃取,其萃取率明显优于其余6种稀释剂的萃取率。正辛烷-正辛醇(体积比7:3)和二氯甲烷作为稀释剂适合N,N,N′,N′-四丁基-3-氧戊二酰胺在NH4NO3溶液中对稀土离子的萃取;所考察的7组稀释剂对N,N,N′,N′-四己基-3-氧戊二酰胺和N,N,N′,N′-四辛基-3-氧戊二酰胺在NH4NO3溶液中的萃取效果影响较小,其萃取率都在90%以上。
2) 在正辛烷-正辛醇(体积比7:3)作为稀释剂时,N,N,N′,N′-四丁基-3-氧戊二酰胺在相比为4:6时最适用于稀土离子的萃取;在NH4Cl溶液中,N,N,N′,N′-四己基-3-氧戊二酰胺和N,N,N′,N′-四辛基-3-氧戊二酰胺在相比为5:5时最适用于稀土离子的萃取。NH4NO3溶液中,N,N,N′,N′-四己基-3-氧戊二酰胺和N,N,N′,N′-四辛基-3-氧戊二酰胺在相比为4:6时最适用于稀土离子的萃取。
REFERENCES
[1] 池汝安, 王淀佐. 稀土矿物加工[M]. 北京: 科学出版社, 2014.
CHI Ru-an, WANG Dian-zuo. Rare earth mineral processing[M]. Beijing: Science Press, 2014.
[2] 黄静丽. 世界稀土资源储量分布及供需现状分析[J]. 中国集体经济, 2015(6): 109-110.
HUANG Jing-li. The distribution of rare earth resources in the world and the present situation of supply and demand[J]. China Collective Economy, 2015(6): 109-110.
[3] 中国稀土学会. 稀土资源可持续开发利用战略研究[M]. 北京: 冶金工业出版社, 2015.
Chinese Society of Rare Earths. Study on the strategy of sustainable development and utilization of rare earth resources[M]. Beijing: Metallurgical Industry Press, 2015.
[4] CHI Ru-an, TIAN Jun, LI Zhong-jun, PENG Cui, WU Yuan-xin, LI Shi-rong, WANG Cun-wen, ZHOU Zhi-ang. Existing state and partitioning of rare earth on weathered ores[J]. Journal of Rare Earths, 2005, 23(6): 756-775.
[5] TIAN Jun, YIN Jing-qun, CHEN Kai-hong, RAO Guo-hua, JIANG Min-tao, CHI Ru-an. Extraction of rare earths from the leach liquor of the weathered crust elution-deposited rare earth ore with non-precipitation[J]. International Journal of Mineral Processing, 2011, 98: 125-131.
[6] 张臻悦, 何正艳, 徐志高, 余军霞, 张越非, 池汝安. 中国稀土矿稀土配分特征[J]. 稀土, 2016, 37(1): 121-127.
ZHANG Zhen-yue, HE Zheng-yan, XU Zhi-gao, YU Jun-xia, ZHANG Yue-fei, CHI Ru-an. Rare earth partitioning characteristics of China rare earth ore [J]. Chinese Rare Earths, 2016, 37(1): 121-127.
[7] 倪嘉缵, 洪光言. 中国科学院稀土研究五十年[M]. 北京: 科学出版社, 2005.
NI Jia-zuan, HONG Guang-yan. Fifty years of rare-earth research by the Chinese Academy of Sciences[M]. Beijing: Science Press, 2005.
[8] KANAZAWA Y, KAMITANI M. Rare earth minerals and resources in the world[J]. Journal of Alloys and Compounds, 2006, 408: 1339-1343.
[9] 田 君, 尹敬群, 欧阳克氙, 池汝安. 风化壳淋积型稀土矿提取工艺绿色化学内涵与发展[J]. 稀土, 2006, 27(1): 70-72.
TIAN Jun, YIN Jing-qun, OUYANG Ke-xian, CHI Ru-an. Development progress and research connotation of green chemistry of extraction process of rare earth from weathering crust elution-deposited rare earth ores in China[J]. Chinese Rare Earths, 2006, 27(1): 70-72.
[10] 周晓文, 温德新, 罗仙平. 南方离子型稀土矿提取技术研究现状及展望[J]. 有色金属科学与工程, 2013, 3(6): 81-85.
ZHOU Xiao-wen, WEN De-xin, LUO Xian-ping. The status quo and development trend of the extraction technology of ion-absorbed rare earth deposits in southern China[J]. Nonferrous Metals Science and Engineering, 2013, 3(6): 81-85.
[11] 赵 靖, 汤洵忠. 我国离子吸附型稀土矿开采提取技术综述[J]. 云南冶金, 2001, 30(1): 11-14.
ZHAO Jing, TANG Xun-zhong. Status guo of mining and recovering technologies for ion- absorbed rare earth deposits in China[J]. Yunnan Metallurgy, 2001, 30(1): 11-14.
[12] CHI Ru-an, TIAN Jun. Weathered crust elution-deposited rare earth ores[M]. New York: Nova Science Publishers, 2008.
[13] 罗仙平, 邱廷省, 严 群. 风化壳淋积型稀土矿的化学提取技术研究进展及发展方向[J]. 南方冶金学院学报, 2002, 23(5): 1-6.
LUO Xian-ping, QIU Ting-xing, YAN Qun. Research progress and developing orientation of chemical extraction technology of weathered crust elution-deposited rare earth ores[J]. Journal of Southern Metallurgical College, 2002, 23(5): 1-6.
[14] 周晓文, 温德新, 罗仙平. 南方稀土矿提取技术研究现状及展望[J]. 有色金属科学与工程, 2013, 3(6): 81-85.
ZHOU Xiao-wen, WEN De-xin, LUO Xian-ping. Present situation and prospect of research on extraction technology of rare earth minerals in southern China[J]. Nonferrous Metals Science and Engineering, 2013, 3(6): 81-85.
[15] 黄小卫, 冯宗玉, 赵龙胜, 孙 旭, 彭新林, 徐 旸, 王 猛. 离子吸附型稀土矿高效绿色提取分离新技术[C]//中国稀土学会2017学术年会摘要集. 中国稀土学会, 2017: 1.
HUANG Xiao-wei, FENG Zong-yu, ZHAO Long-sheng, SUN Xu, PENG Xin-lin, XU Yang, WANG Meng. A new technique of high efficient green extraction and separation of ion-absorbed rare earth deposits[C]//Summary of The 2017 Annual Conference of the Chinese Society of Rare Earths. The Chinese Society of Rare Earths, 2017: 1.
[16] 李永绣, 周新木, 刘艳珠, 李东平, 李 静, 唐 群. 离子吸附型稀土高效提取和分离技术进展[J]. 中国稀土学报, 2012, 30(3): 257-264.
LI Yong-xiu, ZHOU Xin-mu, LIU Yan-zhu, LI Dong-ping, LI Jing, TANG Qun. Development of high efficiency extraction and separation technologies of rare earths from ion- absorbed rare earth[J]. Journal of the Chinese Society of Rare Earths, 2012, 30(3): 257-264.
[17] WANG Jun-lian, CHEN Guang, XU Sheng-ming, LI Lin-yan. Synthesis of novel nonsymmetric dialkylphosphinic acid extractants and studies on their extraction-separation performance for heavy rare earths[J]. Hydrometallurgy, 2015, 154: 129-136.
[18] TURANOV A N, KARANDASHEV V K, BAULIN V E. Extraction of lanthanides(Ⅲ) with N,N′-bis (Diphenylphosphinyl-methylcarbonyl)diaza-18-crown-6 in the presence of ionic liquids[J]. Solvent Extraction and Ion Exchange, 2012, 30(3): 18.
[19] LIU Ying-hui, CHEN Ji, LI De-qian. Application and perspective of ionic liquids on rare earths green separation[J]. Separation Science and Technology, 2012, 47(2): 223-232.
[20] SUN Guo-xin, LIU Min, CUI Yu. Synthesis of N,N′-dimethyl-N,N′-dioctyl-3-oxadiglycolamide and its extraction properties for lanthanides[J]. Solvent Extraction and Ion Exchange, 2010, 28(4): 482-494.
[21] SASAKI Yuji, MORITA Keisuke, SAEKI Morihisa. Precious metal extraction by N,N,N′,N′-tetraoctyl-thiodiglycolamide and its comparison with N,N,N′,N′-tetraoctyl-diglycolamide and methylimino-N,N′-dioctylacetamide[J]. Hydrometallurgy, 2017, 169.
[22] WANG Jun-lian, XU Sheng-ming, LI Lin-yan, LI Jing. Synthesis of organic phosphinic acids and studies on the relationship between their structure and extraction-separation performance of heavy rare earths from HNO3 solutions[J]. Hydrometallurgy, 2013, 137(Complete): 108-114.
[23] WANG Jun-lian, CHEN Guang, XU Sheng-ming, YIN Zhi-li, ZHANG Qin. Solvent extraction of rare earth ions from nitrate media with new extractant di-(2,3-dimethylbutyl)- phosphinic acid[J]. Journal of Rare Earths, 2016, 34(7): 724-730.
[24] 李永绣. 离子吸附型稀土资源与绿色提取[M]. 北京: 化学工业出版社, 2014.
LI Yong-xiu. Ion-absorbrd rare earth deposits resources and green extraction[M]. Beijing: Chemistry Industry Press, 2014.
[25] CARLOS P I, DANIELE M C, ELST L V, MULLER R N, IMBERT D, BüNZLI J C G, TOTH E, MASCHMEYER T, PETERS J A. Stability, structure and dynamics of cationic lanthanide(Ⅲ) complexes of N,N′-bis(propylamide) ethylenediamine-N,N′-diacetic acid[J]. Dalton Transactions, 2003(4): 727-737.
[26] 叶国安, 罗永祥, 姜永青, 丁颂东, 陈文俊. 酰胺荚醚对Tc(Ⅶ)的萃取行为研究[J]. 原子能科学技术, 2000, 34(6): 528-533.
YE Guo-an, LUO Yong-xiang, JIANG Yong-qing, DING Song-dong, CHEN Wen-jun. Study on the extraction of technetium with amido podand[J]. Atomic Energy Science and Technology, 2000, 34(6): 528-533.
[27] 张辽宁. 一种新型双环类萃取剂的合成及萃取性能研 究[D]. 济南: 济南大学, 2014.
ZHANG Liao-ning. The synthesis of a new type of bicyclic lactam extractantand its extraction performance study[D]. Jinan: University of Jinan, 2014.
[28] 韦 兵. N,N,N′,N′-四辛基-3-氧戊二酰胺/HNO3萃取稀土元素的动力学研究[D]. 济南: 济南大学, 2016.
WEI Bing. Extraction kinetics study of rare earths by N,N, N′,N′-tetraoctyl-diglycolamide/HNO3[D]. Jinan: University of Jinan, 2016.
[29] MA Mowafy, HF Aly. Synthesis of some N,N,N′,N′- tetraalkyl-3-oxa-pentane-1,5-diamide and their applications in solvent extraction[J]. Solvent Extraction and Ion Exchange, 2007, 25(2): 205-224.
[30] 王君平, 冯 达, 赵洁莹, 康鑫淳, 孙云雨, 朱涛峰, 梁洪泽. 稀释剂对有机膦功能化离子液体萃取钕(Ⅲ)的影响[J]. 稀土, 2016, 37(5): 97-103.
WANG Jun-ping, FENG Da, ZHAO Jie-ying, KANG Xin-chun, SUN Yun-yu, ZHU Tao-feng. Influence of diluent on extracting neodymium(Ⅲ) by phosphoryl- functionailzedionic liquid system[J]. Chinese Rare Earths, 2016, 37(5): 97-103.
[31] 崔 玉, 孙国新, 邵 华, 韩景田, 包伯荣. 释剂对双取代单酰胺萃取铀(Ⅵ)的影响[J]. 核技术, 2003(9): 705-707.
CUI Yu, SUN Guo-xin, SHAO Hua, HAN Jing-tian, BAO Bo-rong. Effect of diluents on the extraction of U(Ⅵ) with disubstituted amide[J]. Nuclear Techniques, 2003(9): 705-707.
[32] 孙国新, 韩景田, 李玉兰, 包伯荣, 鲍 猛, 崔 玉, 朱卫平. 稀释剂对N,N-二丁基十二酰胺萃取硝酸平衡的影 响[J]. 化学研究与应用, 1998(3): 40-43.
SUN Guo-xin, HAN Jing-tian, LI Yu-lan, BAO Bo-rong, BAO Meng, CUI Yu, ZHU Wei-ping. Effect of diluents on the extraction equilibrium of nitric acid with N,N-dibutyldodevanamide[J]. Chemical Research and Application, 1998(3): 40-43.
Effects of diluents on extraction of rare earth by 3-oxoglutaramide extractants
MA Chen1, XU Yuan-lai1, MA Chi-yuan1, ZHOU Fang1, WANG Fen1, 2, CHI Ru-an1
(1. Key Laboratory for Green Chemical Process of Ministry of Education, School of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, Wuhan 430074, China;
2. School of Chemistry and Chemical Engineering, Sichuan University of Arts and Science, Dazhou 635000, China)
Abstract: Three kinds of 3-oxa-glutaramide extractants, N,N,N′,N′-tetrabutyl-3-oxy-glutaramide, N,N,N′,N′-tetrahexyl- 3-oxy-glutaramide, N,N,N′,N′-tetraoctane-3-oxy-glutaramide, were synthesized and characterized. The influences of extractant concentration and different diluents on the extraction behavior of rare earth ions with three kinds of 3-oxa-glutaramide extractants were investigated in three ammonium salt solution of NH4Cl, (NH4)2SO4 and NH4NO3. The results screen out suitable diluents and provide support for optimal extraction process. The extraction efficiency of N,N,N′,N′-tetrabutyl-3-oxy-glutaramide is the best when octane-octanol (volume ratio of 7:3) is used as diluent in NH4Cl system. And the single-stage extractants for Y and Gd are 89% and 91%. And extraction efficiency increases with increasing the extractant concentration. The extraction rates of Y and Gd reach the maximum and almost no change under the phase ratio of 4:6. Different diluents have weak effect on the extraction efficiency in the system of (NH4)2SO4 and NH4NO3.
Key words: 3-oxa-glutaramide; extraction; ammonium salt system; rare earth; diluent
Foundation item: Project(51734001) supported by the State Key Program of National Natural Science of China
Received date: 2018-12-13; Accepted date: 2019-11-27
Corresponding author: XU Yuan-lai; Tel: +86-27-87194980; E-mail: xuyuanlai@163.com
(编辑 龙怀中)
基金项目:国家自然科学基金重点基金资助项目(51734001)
收稿日期:2018-12-13;修订日期:2019-11-27
通信作者:徐源来,教授,博士;电话:027-87194980;E-mail:xuyuanlai@163.com
摘 要:为了从风化壳淋积型稀土矿铵盐浸出液中萃取分离出稀土离子,合成3种3-氧戊二酰胺类萃取剂N,N,N′,N′-四丁基-3-氧戊二酰胺、N,N,N′,N′-四己基-3-氧戊二酰胺和N,N,N′,N′-四辛基-3-氧戊二酰胺。考察在NH4Cl、(NH4)2SO4、NH4NO3这3种铵盐溶液中,萃取剂浓度和不同稀释剂类型对3种3-氧戊二酰胺类萃取剂萃取稀土离子的影响,并筛选出合适的稀释剂。结果表明:在NH4Cl溶液中,以正辛烷-正辛醇(体积比7:3)为稀释剂时,N,N,N′,N′-四丁基-3-氧戊二酰胺的萃取效果最好,对Y离子和Gd离子的单级萃取剂分别为为89%和91%,且萃取率随着萃取剂浓度的增大而增大,在相比为4:6时萃取率达到最大并几乎恒定。在(NH4)2SO4、NH4NO3溶液中,不同稀释剂对萃取效果的影响差别则较小。
[1] 池汝安, 王淀佐. 稀土矿物加工[M]. 北京: 科学出版社, 2014.
CHI Ru-an, WANG Dian-zuo. Rare earth mineral processing[M]. Beijing: Science Press, 2014.
[2] 黄静丽. 世界稀土资源储量分布及供需现状分析[J]. 中国集体经济, 2015(6): 109-110.
[3] 中国稀土学会. 稀土资源可持续开发利用战略研究[M]. 北京: 冶金工业出版社, 2015.
[6] 张臻悦, 何正艳, 徐志高, 余军霞, 张越非, 池汝安. 中国稀土矿稀土配分特征[J]. 稀土, 2016, 37(1): 121-127.
[7] 倪嘉缵, 洪光言. 中国科学院稀土研究五十年[M]. 北京: 科学出版社, 2005.
[9] 田 君, 尹敬群, 欧阳克氙, 池汝安. 风化壳淋积型稀土矿提取工艺绿色化学内涵与发展[J]. 稀土, 2006, 27(1): 70-72.
[10] 周晓文, 温德新, 罗仙平. 南方离子型稀土矿提取技术研究现状及展望[J]. 有色金属科学与工程, 2013, 3(6): 81-85.
[11] 赵 靖, 汤洵忠. 我国离子吸附型稀土矿开采提取技术综述[J]. 云南冶金, 2001, 30(1): 11-14.
[13] 罗仙平, 邱廷省, 严 群. 风化壳淋积型稀土矿的化学提取技术研究进展及发展方向[J]. 南方冶金学院学报, 2002, 23(5): 1-6.
[14] 周晓文, 温德新, 罗仙平. 南方稀土矿提取技术研究现状及展望[J]. 有色金属科学与工程, 2013, 3(6): 81-85.
[15] 黄小卫, 冯宗玉, 赵龙胜, 孙 旭, 彭新林, 徐 旸, 王 猛. 离子吸附型稀土矿高效绿色提取分离新技术[C]//中国稀土学会2017学术年会摘要集. 中国稀土学会, 2017: 1.
[16] 李永绣, 周新木, 刘艳珠, 李东平, 李 静, 唐 群. 离子吸附型稀土高效提取和分离技术进展[J]. 中国稀土学报, 2012, 30(3): 257-264.
[24] 李永绣. 离子吸附型稀土资源与绿色提取[M]. 北京: 化学工业出版社, 2014.
[26] 叶国安, 罗永祥, 姜永青, 丁颂东, 陈文俊. 酰胺荚醚对Tc(Ⅶ)的萃取行为研究[J]. 原子能科学技术, 2000, 34(6): 528-533.
[27] 张辽宁. 一种新型双环类萃取剂的合成及萃取性能研 究[D]. 济南: 济南大学, 2014.
[28] 韦 兵. N,N,N′,N′-四辛基-3-氧戊二酰胺/HNO3萃取稀土元素的动力学研究[D]. 济南: 济南大学, 2016.
[30] 王君平, 冯 达, 赵洁莹, 康鑫淳, 孙云雨, 朱涛峰, 梁洪泽. 稀释剂对有机膦功能化离子液体萃取钕(Ⅲ)的影响[J]. 稀土, 2016, 37(5): 97-103.
[31] 崔 玉, 孙国新, 邵 华, 韩景田, 包伯荣. 释剂对双取代单酰胺萃取铀(Ⅵ)的影响[J]. 核技术, 2003(9): 705-707.
[32] 孙国新, 韩景田, 李玉兰, 包伯荣, 鲍 猛, 崔 玉, 朱卫平. 稀释剂对N,N-二丁基十二酰胺萃取硝酸平衡的影 响[J]. 化学研究与应用, 1998(3): 40-43.


