Trans. Nonferrous Met. Soc. China 26(2016) 2220-2229
Effects of processing pH stimulation on cooperative bioleaching of chalcopyrite concentrate by free and attached cells
Tang-jian PENG1, Li-juan SHI1, Run-lan YU1,2, Guo-hua GU1,2, Dan ZHOU1, Miao CHEN3, Guan-zhou QIU1,2, Wei-min ZENG1,2,3
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy, Ministry of Education, Central South University, Changsha 410083, China;
3. CSIRO Process Science and Engineering, Box 312, Clayton South, Victoria 3169, Australia
Received 3 May 2015; accepted 4 May 2016
Abstract:
In order to investigate the effects of processing pH stimulation on bioleaching of chalcopyrite by moderate thermophiles, copper leaching rates and the dynamics of microbial community structures of free and attached cells were monitored. The results indicated that when the processing pH values were respectively adjusted to 1.0 and 3.0 on day 14, both free and attached cells experienced an adaptive phase. Meanwhile, the copper leaching rates were 86.9% and 64.0%,respectively, as opposed to a copper leaching rate of 87.5% in the control group without pH stimulation. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis suggested that pH stimulation imposed less impact on the attached organisms than on the free cells, indicating that the attached cells were more resistant to processing pH stimulation than the free cells. Furthermore, adjusting processing pH to 3.0 significantly disrupted both free and attached microbial communities, and the bioleaching system could not recover to the normal status as the control group.
Key words:
chalcopyrite; processing pH stimulation; free cells; attached cells; microbial community; real-time quantitative PCR;
1 Introduction
Chalcopyrite (CuFeS2) is one of the most economically important copper sulfide minerals and is the world’s major source of copper. Due to low reactivity in hydrometallurgical systems, chalcopyrite is considered as one of the most inert copper ores [1,2]. Bioleaching of copper ores offers an attractive alternative to conventional leaching methods and has been successfully applied to leaching of secondary copper sulfides such as bornite, chalcocite and chalcopyrite in many countries. At present, researchers have paid more attention to bioleaching of chalcopyrite with moderately thermophilic microorganisms rather than mesophiles, since moderately thermophilic microorganisms greatly improve the reaction kinetics and thus improve copper extraction during bioleaching [3,4].
Bioleaching is a complex process, involving a number of chemical and physical factors including temperature, pH, mole ratio of Fe3+ to Fe2+, concentrations of dissolved oxygen, carbon dioxide and sulfate, pulp density and particle size of the mineral. Among them, pH is a critical parameter affecting the growth, activity of microorganisms and microbial community, thereby influencing the leaching rate. Especially in bio-heap systems, a sudden rainfall or a sustained drought may lead to significant changes of pH in the system. High pH environments are not only detrimental to the oxidization ability of microorganisms, but also reduce heap permeability on account of ferric ion precipitation in the heap bed [5]. On the other hand, the extreme low pH environments (lower than pH 0.8) would lower the growth rate, oxidative activity and leaching rate [6]. Furthermore, biological oxidation of iron and sulfur involves the movement of hydrogen ions and electrons. For the above reasons, pH plays an important role in iron and sulfur metabolism.
The microbial community changes with temperature, pH, mole ratio of Fe3+ to Fe2+ and concentrations of dissolved oxygen, carbon dioxide, sulfate and metal ions during bioleaching. The current studies about community dynamics during bioleaching mostly focus on free microorganisms in solution [7], while less attention is paid to attached microbes. The extracellular polymeric substances (EPS) secreted by attached microorganisms can concentrate iron (III) ions and accumulate organic compounds, providing a microenvironment, where the concentrations of metal ions and organic substances are higher than those in bioleaching solution [8-10]. Furthermore, the microenvironment has the capacity of maintaining a relatively stable environment against adverse conditions. Thus, the structure and dynamics of attached microbial community may be different from those of free microorganisms when facing with environmental changes.
In this study, processing pH shifts to 1.0 and 3.0 were designed to simulate a sustained drought and a sudden rainfall respectively, and to explore their effects on the cooperative bioleaching behaviors of both free and attached microorganisms. Dynamics of their microbial communities were detected by quantitative real-time polymerase chain reaction (qRT-PCR) [11]. Finally, canonical correspondence analysis (CCA) was applied to examining the relationships between micro- organisms and environmental parameters.
2 Experimental
2.1 Mineral components and preparation
The chalcopyrite concentrate was obtained from Meizhou Yushui Copper Mine in Guangdong, China. The mineral sample was crushed and sieved, with fractional sizes of not larger than 75 μm. XRD analysis showed that it consisted of chalcopyrite (62.6%), steatite (14.2%), sphalerite (15%), galena (5%) and others (3.2%).
2.2 Microorganisms enrichment and acclimation
Acid mine drainage (AMD) samples were collected from several copper mines in China. The samples were inoculated into the culture medium at 45 °C for the enrichment of moderate thermophiles. The culture medium was modified with 9K medium [12] containing the following compounds: 3.0 g/L (NH4)2SO4, 2.1 g/L Na2SO4, 0.5 g/L MgSO4·7H2O, 0.05 g/L K2HPO4, 0.1 g/L KCl and 0.01 g/L Ca(NO3)2. The enrichment culture was acclimated to adapt to increasing concentrations of chalcopyrite concentrates from 1% to 10% pulp densities in a 3 L glass cylindrical reactor equipped with a mechanic stirrer operated at 500 r/min, 45 °C and initial pH 2.0 (see Section 2.3). After acclimation, it was investigated through PCR-denaturing gradient gel electrophoresis (DGGE), and the enrichment culture consisted of four types of moderate thermophiles: Acidithiobacillus caldus, Leptospirillum ferriphilum, Sulfobacillus thermosulfidooxidans and Ferroplasma thermophilum [13]. This mixed culture was subcultured every two months and maintained as an active culture in our laboratory.
2.3 Bioleaching experiments
Bioleaching experiments were conducted in 3 L glass cylindrical reactors equipped with mechanic stirrers operated at 500 r/min. Chalcopyrite concentrate (10% pulp density) and 2500 mL modified 9K medium were added into each reactor. The mixed culture was centrifuged at 5000 r/min for 15 min to obtain the pellets. Then, the pellets were resuspended with 10 mL modified 9K medium, added to the reactors and adjusted to obtain a cell density of approximately 2×107 cell/mL. In order to keep the constant temperature at (45±0.2) °C, the reactors were placed in thermostatic baths. Air was blown from the bottom of the reactors at a rate of 360 mL/min. Deionized water was added to compensate for the water loss by evaporation. Bioleaching of chalcopyrite concentrate was conducted in three stirred tank reactors: reactors A, B and C. Reactor A was carried out at initial pH 2.0 while reactors B and C were performed at initial pH 2.0 for the first 14 days until the mixed culture almost entered into the late exponential phase, then the processing pH values were respectively adjusted to 1.0 with 10 mol/L H2SO4 and 3.0 with 10 mol/L NaOH to simulate a sustained drought or a sudden rainfall. Each bioleaching experiment was carried out in triplicate.
Samples were withdrawn to analyze the concentrations of copper, ferric and total iron in solution every two days, while pH and redox potential (φEh) were analyzed each day. These parameters were determined as described in Ref. [3]. Bioleaching residues were removed periodically by filtration. The components of the mineral samples and ore residues were analyzed by X-ray diffraction (XRD) (Panalytical, X’Pert Pro, Netherlands) with Cu Kα radiation (40 kV, 35 mA). The ore surface was characterized by scanning electron microscope (SEM), which was performed on an FEI Quanta 400 field emission, environmental scanning electron microscope (ESEM) under high vacuum conditions. Separation of free and attached microorganisms was shown in Section 2.4.1. Free and attached cells were counted by using a blood cell counting chamber under an optical microscope (Olympus, Tokyo, Japan).
2.4 Community structure analysis
2.4.1 DNA extraction from free and sessile cells
For collecting of free cells, the leaching solution was settled for 1.5 h and the supernatant was decanted. Then, 10 mL solution was centrifuged at 2000 r/min for 4 min to remove the residue. After that, the liquid was centrifuged at 12000 r/min for 10 min to collect the free cells.
Harvesting of sessile cells was performed as follows: the leaching solution was settled for 1.5 h and the supernatant was decanted. Then, the thickened sludge was centrifuged at 2000 r/min and 4 °C for 4 min and the supernatant was removed. The obtained pellets were resuspended with 10 mL acidified water (pH=2.0) in a 50 mL centrifuge tube. After that, 1 g of glass beads with a diameter of 0.2 mm was added into the tube. The tube was vortexed on a vortexer for 8 min at the maximum speed. After that, the mixture was centrifuged at 2000 r/min for 2 min to separate the ore residue and the solution. The solution was decanted and the ore residue was resuspended again. These steps, including resuspension, vortexing and centrifugation, were repeated twice. Finally, about 30 mL solution was obtained and centrifuged at 12000 r/min for 10 min to collect the sessile microorganisms.
Extraction, visualisation and purification of genomic DNA were performed as described in Ref. [11]. The purified genomic DNA samples were stored at -20 °C as the template for quantification.
2.4.2 Design and specificity of PCR primers
As stated in Section 2.2, the acclimated enrichment culture consisted of A. caldus, L. ferriphilum, S. thermosulfidooxidans and F. thermophilum. Specific primers for the four species were designed based on their 16S rRNA gene sequences and listed in Table 1. All primers were synthesized by Sangon (Sangon Biological Engineering Technology & Services, Co., Ltd., Shanghai, China). The specific fragments were amplified by conventional PCR and then checked by ethidium bromide-UV detection on 1.5% agarose gel to ensure the size of PCR products and the specificity of primers. DNA products were sequenced by Sangon and checked by BLAST analysis in GenBank. These primers were also tested for the requirements imposed by real-time quantitative PCR.
2.4.3 Conventional PCR and real-time quantitative PCR
The conventional PCR was carried out with a T-Gradient Thermoblock (Biometra,  , Germany). The PCR program, purification of PCR products and the calculation of DNA copy numbers were described by ZHANG et al [15]. The PCR products were diluted serially from 1×109 to 1×103 copy/μL and used as template DNA to construct standard curves. qRT-PCR was performed by using an iCycler iQ real-time PCR detection system (Bio-Rad Laboratories Inc., Hercules, USA). The qRT-PCR program was one cycle of 94 °C for 5 min, and then 40 cycles of 94 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s. The reaction mixture contained 12.5 μL of SYBR green real-time PCR Master Mix (Toyobo Co., Ltd., Japan), 1 μL of 10 pmol of each primer, 5 μL of template DNA, and deionized water was added to a total of 25 μL. All tests were conducted in triplicate.
, Germany). The PCR program, purification of PCR products and the calculation of DNA copy numbers were described by ZHANG et al [15]. The PCR products were diluted serially from 1×109 to 1×103 copy/μL and used as template DNA to construct standard curves. qRT-PCR was performed by using an iCycler iQ real-time PCR detection system (Bio-Rad Laboratories Inc., Hercules, USA). The qRT-PCR program was one cycle of 94 °C for 5 min, and then 40 cycles of 94 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s. The reaction mixture contained 12.5 μL of SYBR green real-time PCR Master Mix (Toyobo Co., Ltd., Japan), 1 μL of 10 pmol of each primer, 5 μL of template DNA, and deionized water was added to a total of 25 μL. All tests were conducted in triplicate.
2.4.4 Canonical correspondence analysis (CCA)
Canonical correspondence analysis (CCA) is a multivariate method to illustrate the relationships between community compositions and environmental variables. In this study, CCA was utilized to inquire into the potential correlations between microorganisms in the bioleaching system (based on the relative abundance by qRT-PCR) and environmental parameters (pH, φEh, concentrations of Cu2+, Fe3+ and Fe2+), and to explore the influences of leaching parameters on the community compositions in different stages of bioleaching [16].
Table 1 Primers used for conventional PCR and qRT-PCR
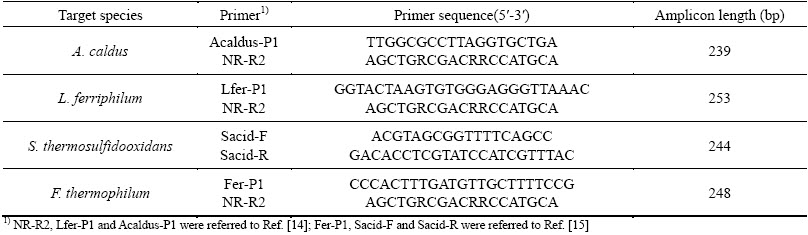
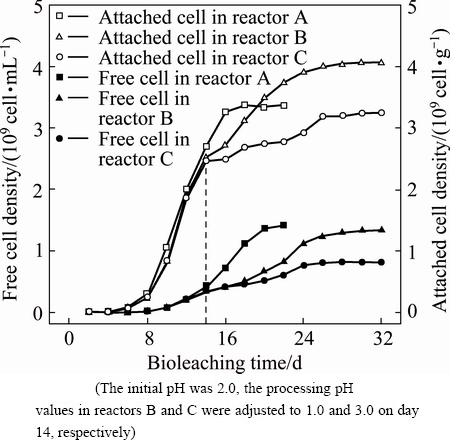
Fig. 1 Variations of free and attached cell densities during bioleaching of chalcopyrite by mixed moderate thermophiles in reactors A, B and C
3 Results and discussion
3.1 Effects of initial and processing pH shifts on bioleaching of chalcopyrite concentrate
Bioleaching of chalcopyrite concentrate by an enrichment culture of mixed moderate thermophiles was carried out at initial pH 2.0 and processing pH shifted from 1.41 to 1.0 and 3.0 for reactors B and C, respectively, as described in Section 2.3. Figure 1 shows the variation of free cell densities in solution and attached cell amounts on the mineral surface in reactors A, B and C. Due to the same condition before processing pH stimulation on day 14, the variations of free and attached cells in the three reactors were very similar. On day 14, the densities of free and attached cells respectively increased to about 4.00×108 cell/mL and 2.50×109 cell/g. It was also noticed that the growth of free cells began to accelerate when attached cells gradually entered the stationary phase. When the processing pH values in reactors B and C were respectively adjusted to 1.0 and 3.0, the cell densities changed very little during the first two days, indicating that the microbes experienced a phase of adjustment. After that, the amounts of both free and attached cells in reactor C still varied rather slowly, which suggested that processing pH of 3.0 was considerably unfavorable to the microbial community. On the contrary, microorganisms in reactor B displayed a distinct trend after 16 d. The free cell density in reactor B increased faster than that in reactor C but did not outnumber that in reactor A. On the other hand, the attached cell density in reactor B finally exceeded that in reactor A. This implied that microbes gradually recovered from the sudden change of the environment, and the attached cells were more resistant to environmental changes.
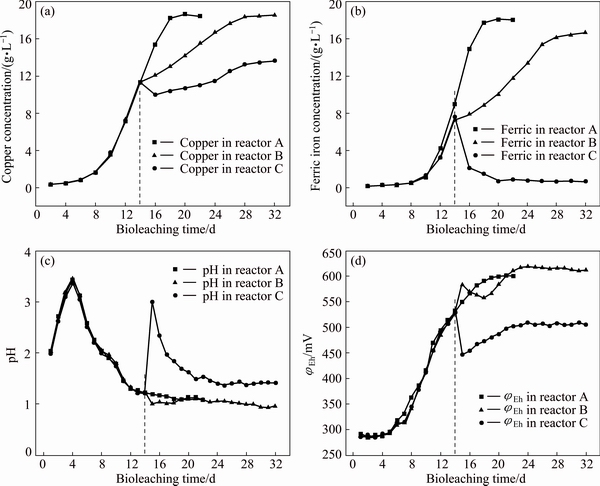
Fig. 2 Variations of copper concentration (a), ferric iron concentration (b), pH (c) and φEh (d) in solution during bioleaching of chalcopyrite by mixed moderate thermophiles in reactors A, B and C
In Fig. 2(a), the copper extraction at initial pH 2.0 and processing pH shifting to 1.0 and 3.0 was presented. In reactor A, the copper leaching rate at 22 d was 87.5% (18.65 g/L). In reactor B, the copper leaching rate was slowed down and the final copper leaching rate was 86.9%. The slowdown of copper leaching rate could be attributed to inhibition of growth and activity of the mixed culture by lowered pH (Fig. 2(c)) [17,18]. In reactor C, the concentration of copper dropped during days 14 and 16, which may result from locking of some extracted copper with the jarosite formed [19]. The final leaching rate of copper in reactor C was 64.0%. The stimulation of processing pH shifting to 1.0 and 3.0 slowed down and extended bioleaching time to 32 d.
As reported previously in Ref. [20], both the kinetics and efficiency of the copper extraction were closely related to the concentration of ferric iron in solution. As shown in Fig. 2(b), in reactor A, the concentration of ferric iron amounted for 18 g/L at the end of bioleaching. Nevertheless, in reactors B and C the concentration of ferric iron significantly differed from that in reactor A. Specifically, when adjusting processing pH to 1.0, the release of ferric iron slowed down, which indicated that extremely low pH inhibited the iron-oxidizing activity of microorganisms. When the processing pH was adjusted to 3.0, the content of ferric iron presented a sharp drop, and kept very low till the end. As the concentration of ferric iron dropped, the φEh value decreased too (Fig. 2(d)). The decrease of ferric iron concentration was partly due to the accelerated precipitation of ferric ions since high pH greatly promoted the formation of large amounts of jarosite, hydroxyl and sulfate complexes (Fig. 2(c)) [21]. Moreover, consumption of ferric iron by reaction with metal sulfide and less ferric iron produced further reduced the available ferric ions in solution. All these factors together resulted in a lowered concentration of ferric iron and finally severely inhibited copper extraction.
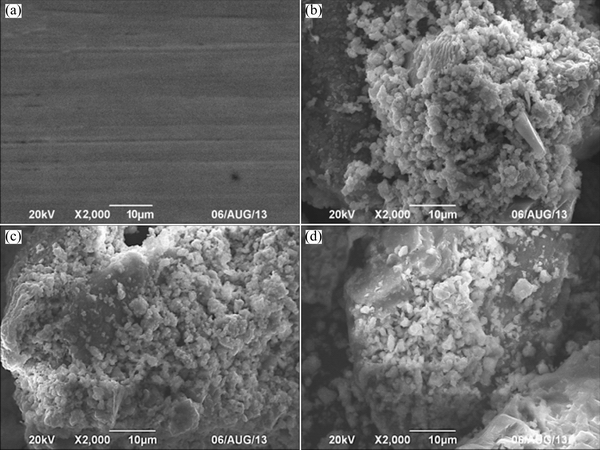
Fig. 3 SEM images of mineral samples of raw mineral sample (a) and ore residues after bioleaching for 22 d in reactors A (b), B (c) and C (d)
Table 2 Residue analysis for different processing pH stimulations in different bioleaching stages

3.2 Effect of processing pH stimulation on ore residues during bioleaching of chalcopyrite concentrate
Raw mineral samples and leached residues were analyzed by SEM (Fig. 3) and XRD (Table 2). Figure 3 shows that after being bioleached for 22 d, the surface of mineral samples was significantly corroded. Table 2 presents the components of raw mineral and leached residues in the three reactors. As it can be seen, the proportions of chalcopyrite in the residues were consistent with copper extraction shown in Fig. 2(a). As bioleaching continued, most components were dissolved but steatite could not be dissolved, therefore the proportion of steatite in leached residues kept increasing.
During bioleaching of chalcopyrite, jarosite precipitation and S0 membrane were the main passivation layers, which greatly slowed down bioleaching rate [22]. It was also reported that the yield of the passivation layer can be significantly influenced by microorganisms, ferric iron, and pH [23]. In this study, the passivation substance was mainly jarosite and formed in the middle and late stages of bioleaching. At initial pH 2.0, the mass fraction of jarosite in leaching residue was 18.8%. However, S0 was not found in all the ore residues. As reported by GAUTIER et al [24], attached microorganisms triggered the dissolution of the passivation layer. The attached cells on the surface of chalcopyrite could directly oxidize the passivation layer and could effectively eliminate S0 membrane.
Table 2 also shows the component of ore residues after processing pH stimulation. The ore residues before adjusting pH were collected, and due to the similarity to that in reactor A, the components are not shown. When adjusting pH to 1.0, analysis of ore residues showed 27.3% chalcopyrite residue on day 22. Compared to 13.9% residual in reactor A, these indicated processing pH 1.0 stimulation obviously decreased the copper leaching rate. However, at the end of bioleaching (day 32), there was only 15.4% residue left, indicating that the final copper leaching rate was close to that in reactor A, which is in consistence with the final copper concentration (Fig. 2(a)). Meanwhile, the content of jarosite decreased. This was because the release of Fe3+ was postponed since low pH stimulation inhibited the growth and activity of microorganisms (Fig. 2(b)). When adjusting processing pH to 3.0, there were still 30.5% and 28.2% of chalcopyrite left on day 22 and day 32, respectively. The stimulation of high pH greatly inhibited the growth of microorganisms and reduced concentration of Fe3+ (Fig. 2(b)), and this led to a low leaching rate of copper and small change in content of jarosite from 15.8% on day 22 to 16.3% on day 32.
3.3 Dynamics of free and attached microbial community structures under processing pH stimulation during bioleaching of chalcopyrite concentrate
During bioleaching, reactor A was designed as a control to explore the effects of pH stimulation on bioleaching behaviors and dynamics of microbial communities. DNA was extracted from free cells in reactor A on the 7th, 12th, 17th and 22nd day during bioleaching. As shown in Fig. 4, at initial pH 2.0, A. caldus, L. ferriphilum, S. thermosulfidooxidans and F. thermophilum could be all detected on day 7, accounting for 53.9%, 18.5%, 4.6% and 23%, respectively. Subsequently, the proportion of A. caldus decreased sharply to 24.1%, while the proportions of L. ferriphilum and F. thermophilum increased to 30.2% and 35.7%, respectively. Thereafter, the proportion of L. ferriphilum decreased, which may be resulted from the inhibition of accumulated organic substances on L. ferriphilum since it is an obligate autotroph. The organic substances mainly originated from microbial metabolism and remains. On the contrary, F. thermophilum became dominant and amounted to 92% on day 22. The dominance of Ferroplasma in the later stage of bioleaching was observed in all our experiments (Figs. 4 and 5). Ferroplasma is extremely acidophilic, and often prospered at extremely low pH [25]. In addition, as bioleaching continued, the organic substances would inhibit the growth and leaching ability of autotrophic bioleaching microorganism, but facilitated the growth of F. thermophilum [26].
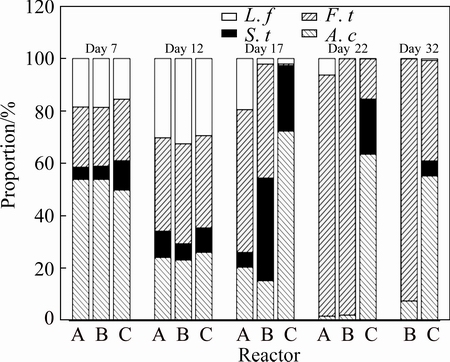
Fig. 4 Dynamics of free microbial communities in solution in reactors A, B and C

Fig. 5 Variations of attached microbial communities in reactors A, B and C
With respect to the experiment under processing pH stimulation, the initial pH was 2.0, and the processing pH values were respectively adjusted to 1.0 and 3.0 on day 14, DNA was extracted from free cells in solution on the 7th, 12th, 17th, 22nd and 32nd day during bioleaching. Due to the same condition (initial pH 2.0) in the first 13 days of bioleaching, the dynamics of free microbial communities in the three reactors were very similar. As shown in Fig. 4, when the processing pH was adjusted from 1.41 to 1.0 to simulate a sustained drought, the structure of free microbial community in reactor B changed largely. The proportion of free L. ferriphilum decreased to only 2.1%, as opposed to 19.5% in reactor A. In contrast, the proportion of free S. thermosulfidooxidans in reactor B increased to 39.2%, while it was 5.6% in reactor A. The proportions of A. caldus and F. thermophilum also declined compared with that in reactor A. These indicated that S. thermosulfidooxidans showed great advantage over the other three species to low pH stimulation. This may be probably because S. thermosulfidooxidans is a gram-positive species, being able to maintain a firm cell structure against pH shock [27]. When pH was adjusted from 1.36 to 3.0 to simulate a sudden rainfall, the species diversity decreased significantly. The free microbial community mainly consisted of A. caldus and S. thermosulfidooxidans, accounting for 72.3% and 25%, respectively. Only a very small quantity of L. ferriphilum and F. thermophilum were detected. As bioleaching continued, the proportion of A. caldus decreased slightly, but it was still the predominant one (over 55%), which was mainly attributed to pH around 1.5 in the solution.
The dynamics of attached microbial community in reactor A was investigated and shown in Fig. 5. In the early stage of bioleaching, A. caldus was the major species in the three reactors. On day 12, the proportion of A. caldus decreased slightly, but was still the predominant one. The dominance of sessile A. caldus in the early stage of bioleaching may be due to the formation of elemental sulfur on the surface of chalcopyrite and that A. caldus could effectively use elemental sulfur as an energy source. Meanwhile, the oxidation of elemental sulfur could offer more growth energy to the sulfur-oxidizing microorganisms than the oxidation of ferrous iron to iron-oxidizing microorganisms, which could meet the demand of attached A. caldus cells [28]. The proportion of A. caldus decreased quickly at the end of bioleaching (day 17 and day 22), and this could be attributed to the toxicity of ferric ions and organic substances to A. caldus since it is an obligate sulfur-oxidizing species.
The attached microbial community in reactors B and C also varied after respectively adjusting processing pH values to 1.0 and 3.0 on day 14. However, compared with the free microbial communities (Fig. 4), the shifts of attached cells were slighter. To be specific, in reactor B, the proportion of attached S. thermosulfidooxidans was 40.2% on day 17, while in reactor A the species accounted for 23.7% of the community. A difference of 16.5% between proportions of S. thermosulfidooxidans in reactors A and B was observed. However, the proportion of free S. thermosulfidooxidans in reactor B was 39.2% on day 17, as opposed to 5.6% in reactor A. There was a difference of 33.6%, much larger than 16.5%. Similar trends could be also observed as processing pH was adjusted to 3.0, especially when comparing variations of F. thermophilum and A. caldus in reactors C and A. These further suggested that the attached cells were more resistant to variations of pH than the free microbes. Still and all, adjusting pH to 3.0 also greatly influenced the microbial structure of attached cells. As can be seen from Fig. 5, the proportions of attached L. ferriphilum and F. thermophilum were reduced greatly to considerably low values after the pH was adjusted to 3.0.
The parameters in bioleaching system are shifty, and the microbial community varies with variations of pH, φEh and metal ions during bioleaching. The microorganisms in the solution are susceptible to environmental changes. Our results showed that the microbial compositions of free cells varied a lot after the processing pH values were adjusted to 1.0 or 3.0 (Figs. 4 and 5). Especially at processing pH 3.0, the microbial structures were almost disrupted. The disruption of microbial structure resulted in a lowered leaching rate, which was in accordance with the sudden decrease of copper and ferric iron concentrations (Figs. 2(a) and (b)). It was also shown that the shifts of attached microorganisms were slighter than those of free cells, no matter the processing pH values were adjusted to 1.0 or 3.0. This was not unexpected since EPS had the capacity of maintaining a relatively stable microenvironment to buffer processing pH stimulation [29]. In fact, the EPS secreted by sessile cells not only acted as a buffer zone against environmental variations, but also provided a microenvironment for biochemical reactions. The attached cells play very critical roles in bioleaching [24,30]. Therefore, maintaining a steady micro-environment for attached cells is of great significance during bioleaching.
In our studies, the bioleaching period in reactor B was prolonged but the final copper extraction rate was comparable to that in reactor A. Analysis of microbial community showed that processing pH shift to 1.0 did not thoroughly change the microbial structure, especially that of attached cells. After the cells went through a period of adjustment, the microbial community went back to normal, which was similar to that in reactor A (Figs. 4 and 5). In our previous study, it was concluded that if pH stimulation did not fatally affect microorganisms, the community would recover [13]. This was because highly acidic environment beyond the optimum pH would impose an additional stress on microorganisms since the microbial cytoplasm must be maintained close to neutrality [5]. The processing pH 1.0 stimulation was designed to simulate a sustained drought, and the results indicated that it would influence bioleaching, but this influence could be reversible. However, this was not the case in reactor C. When the processing pH was adjusted to 3.0, both free and sessile microbial compositions were drastically destroyed (Figs. 4 and 5), and the communities could not recovered to the normal statues as in reactor A, even though the shifts of sessile microbial compositions were not so significant as those of free organisms. As a result, the copper leaching rate in reactor C was far less than those in reactors A and B. As we know, the main iron oxidizers in the system, i.e., L. ferriphilum and F. thermophilum both had optimal growth pH values <2.0, hence, the high pH (3.0) strongly inhibited their activity [26]. Furthermore, high pH greatly facilitated the formation of Fe(III) precipitates like jarosite [31]. These precipitates would reduce the available oxidizing agents, cover on the surface of ores and hinder the contact of cells and minerals. As processing pH to 3.0 was designed to simulate a sudden rainfall, it would cause highly adverse impacts on bioleaching and these impacts would last for a long term.
As shown in Fig. 6, CCA presents the relationships between free and attached microbial communities and operational parameters such as pH, φEh, concentions of Fe3+ and Fe2+ during bioleaching. In general, the relationships between environment factors and the four species as well as microbial communities of 14 samples in Figs. 6(a) and (b) were similar. Both free and attached F. thermophilum cells were resistant to high φEh and Fe3+/Fe2+ ratios, low pH and elevated concentrations of Fe3+ and Cu2+, which was the case in the later stage of bioleaching. In contrast, A. caldus and L. ferriphilum could grow well when pH is high and Fe2+ is rich, respectively. Comparatively, S. thermosulfidooxidans was distantly related to all parameters, suggesting that it was relatively insensitive to environmental changes. These results further confirmed the ecological analysis mentioned above. Additionally, the microbial communities of samples in circle A1 from the early stage of bioleaching were mainly affected by high pH and low content of metallic ions. And the microbial communities of samples in circle B1 were up to the concentration of Fe2+. Due to the moderate pH and less harmful substances, samples in circles C1 and C2 which were mainly from the middle stage of bioleaching were affected by all operational parameters. And the samples in circles D1 and D2 were influenced by higher concentrations of metallic ions and low pH.
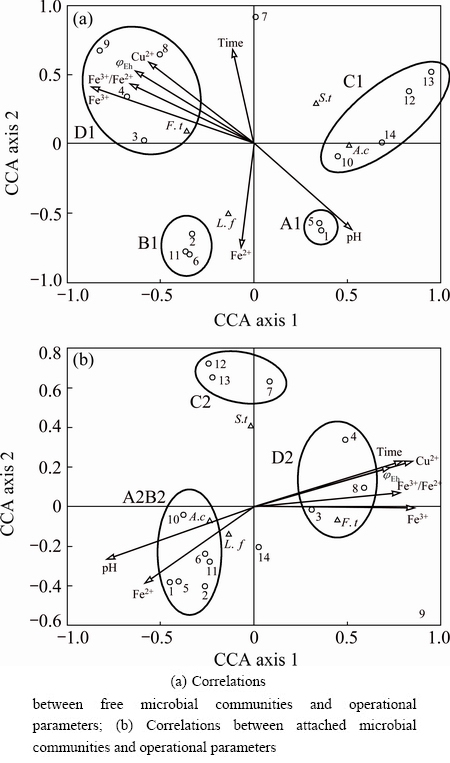
Fig. 6 CCA tri-plots showing correlations between microbial communities and operational parameters (Arrows on graph represent operational parameters. Triangles represent species. Circles represent samples from different stages of bioleaching: 1-4 respectively represent samples on day 7, 12, 17, 22 in reactor A, 5-9 and 10-14 respectively represent samples on day 7, 12, 17, 22 and 32 in reactors B and C)
4 Conclusions
1) An enrichment culture containing moderate thermophiles was utilized to leach chalcopyrite concentrate, and pH stimulation slowed down copper leaching rate and significantly extended the bioleaching period.
2) Both processing pH shifts to 1.0 and 3.0 changed the microbial structures, but processing pH shift to 3.0 imposed much more drastic impacts on both free and attached cells.
3) The attached microbial community was more resistant to pH stimulation.
References
[1] XIA Le-xian, TANG Lu, XIA Jin-lan, YIN Chu, CAI Li-yuan, ZHAO Xiao-juan, NIE Zhen-yuan, LIU Jian-she, QIU Guan-zhou. Relationships among bioleaching performance, additional elemental sulfur, microbial population dynamics and its energy metabolism in bioleaching of chalcopyrite [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 192-198.
[2] WANG Jun, ZHAO Hong-bo, ZHUANG Tian, QIN Wen-qing, ZHU Shan, QIU Guan-zhou. Bioleaching of Pb–Zn–Sn chalcopyrite concentrate in tank bioreactor and microbial community succession analysis [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(12): 3758-3762.
[3] ZENG Wei-min, QIU Guan-zhou, ZHOU Hong-bo, CHEN Miao. Electrochemical behaviour of massive chalcopyrite electrodes bioleached by moderately thermophilic microorganisms at 48 oC [J]. Hydrometallurgy, 2011, 105(3): 259-263.
[4] WANG Yu-guang, SU Li-jun, ZENG Wei-min, QIU Guan-zhou, WAN Li-li, CHEN Xin-hua, ZHOU Hong-bo. Optimization of copper extraction for bioleaching of complex Cu-polymetallic concentrate by moderate thermophiles [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1161-1170.
[5] OJUMU T V, PETERSEN J. The kinetics of ferrous ion oxidation by Leptospirillum ferriphilum in continuous culture: The effect of pH [J]. Hydrometallurgy, 2011, 106(1-2): 5-11.
[6] WATLING H R, COLLINSON D M, SHIERS D W, BRYAN C G, WATKIN E L J. Effects of pH, temperature and solids loading on microbial community structure during batch culture on a polymetallic ore [J]. Minerals Engineering, 2013, 48: 68-76.
[7] LIU Hui, GU Guo-hua, XU Yang-bao. Surface properties of pyrite in the course of bioleaching by pure culture of Acidithiobacillus ferrooxidans and a mixed culture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans [J]. Hydrometallurgy, 2011, 108(1-2): 143-148.
[8] SAND W, GEHRKE T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria [J]. Research in Microbiology, 2006, 157(1): 49-56.
[9] HE Zhi-guo, YANG Yan-ping, ZHOU Shan, HU Yue-hua, ZHONG Hui. Effect of pyrite, elemental sulfur and ferrous ions on EPS production by metal sulfide bioleaching microbes [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1171-1178.
[10] YU Run-lan, OU Yang, TAN Jian-xi, WU Fa-deng, SUN Jing, MIAO Lei, ZHONG Dai-li. Effect of EPS on adhesion of Acidithiobacillus ferrooxidans on chalcopyrite and pyrite mineral surfaces [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(2): 407-412.
[11] WANG Yu-guang, SU Li-jun, ZHANG Li-juan, ZENG Wei-min, WU Jun-zi, WAN Li-li, QIU Guan-zhou, CHEN Xin-hua, ZHOU Hong-bo. Bioleaching of chalcopyrite by defined mixed moderately thermophilic consortium including a marine acidophilic halotolerant bacterium [J]. Bioresource Technology, 2012, 121: 348-354.
[12] ZHOU Hong-bo, ZENG Wei-min, YANG Zhi-feng, XIE Ying-jian, QIU Guan-zhou. Bioleaching of chalcopyrite concentrate by a moderately thermophilic culture in a stirred tank reactor [J]. Bioresource Technology, 2009, 100(2): 515-520.
[13] YU Run-lan, SHI Li-juan, GU Guo-hua, ZHOU Dan, YOU Long, CHEN Miao, QIU Guan-zhou, ZENG Wei-min. The shift of microbial community under the adjustment of initial and processing pH during bioleaching of chalcopyrite concentrate by moderate thermophiles [J]. Bioresource Technology, 2014, 162: 300-307.
[14] LIU C Q, PLUMB J, HENDRY P. Rapid specific detection and quantification of bacteria and archaea involved in mineral sulfide bioleaching using real-time PCR [J]. Biotechnology and Bioengineering, 2006, 94(2): 330-336.
[15] ZHANG Ru-bing, WEI Man-man, JI Hou-guo, CHEN Xin-hua, QIU Guan-zhou, ZHOU Hong-bo. Application of real-time PCR to monitor population dynamics of defined mixed cultures of moderate thermophiles involved in bioleaching of chalcopyrite [J]. Applied Microbiology and Biotechnology, 2009, 81(6): 1161-1168.
[16] JENKINS S N, WAITE I S, BLACKBURN A, HUSBAND R, RUSHTON S P, MANNING D C, O’DONNELL A G. Actinobacterial community dynamics in long term managed grasslands [J]. Antonie van Leeuwenhoek, 2009, 95(4): 319-334.
[17] DOPSON M, LINDSTROM E. Analysis of community composition during moderately thermophilic bioleaching of pyrite, arsenical pyrite, and chalcopyrite [J]. Microbial Ecology, 2004, 48(1): 19-28.
[18] VILCAEZ J, YAMADA R, INOUE C. Effect of pH reduction and ferric ion addition on the leaching of chalcopyrite at thermophilic temperatures [J]. Hydrometallurgy, 2009, 96(1-2): 62-71.
[19] ACEVEDO F. The use of reactors in biomining processes [J]. Electronic Journal of Biotechnology, 2000, 3(3): 10-11.
[20] RODRIGUEZ Y, BALLESTER A, BLAZQUEZ M, GONZALEZ F, MUNOZ J. New information on the sphalerite bioleaching mechanism at low and high temperature [J]. Hydrometallurgy, 2003, 71(1): 57-66.
[21] XIA Le-xian, LIU Jian-she, XIAO Li, ZENG Jia, LI Ban-mei, GENG Mei-mei, QIU Guan-zhou. Single and cooperative bioleaching of sphalerite by two kinds of bacteria—Acidithiobacillus ferriooxidans and Acidithiobacillus thiooxidans [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(1): 190-195.
[22] FENG Shou-shuai, YANG Hai-lin, XIN Yu, GAO Kai, YANG Ji-wei, LIU Ting, ZHANG Ling, WANG Wu. A novel and highly efficient system for chalcopyrite bioleaching by mixed strains of Acidithiobacillus [J]. Bioresource Technology, 2013, 129: 456-462.
[23] LEAHY M J, SCHWAZ M P. Modelling jarosite precipitation in isothermal chalcopyrite bioleaching columns [J]. Hydrometallurgy, 2009, 98(1-2): 181-191.
[24] GAUTIER V, ESCOBAR B, VARGAS T. Cooperative action of attached and planktonic cells during bioleaching of chalcopyrite with Sulfolobus metallicus at 70 oC [J]. Hydrometallurgy, 2008, 94(1): 121-126.
[25] OKIBE N, JOHNSON D B. Biooxidation of pyrite by defined mixed cultures of moderately thermophilic acidophiles in pH-controlled bioreactors: Significance of microbial interactions [J]. Biotechnology and Bioengineering, 2004, 87(5): 574-583.
[26] ZHOU Hong-bo, ZHANG Ru-bing, HU Pei-lei, ZENG Wei-min, XIE Ying-jian, WU Chang-bin, QIU Guan-zhou. Isolation and characterization of Ferroplasma thermophilum sp. nov., a novel extremely acidophilic, moderately thermophilic archaeon and its role in bioleaching of chalcopyrite [J]. Journal of Applied Microbiology, 2008, 105(2): 591-601.
[27] LI Bo, CHEN Ya-ping, LIU Qian, HU Song-nian, CHEN Xin-hua. Complete genome analysis of sulfobacillus acidophilus strain TPY, isolated from a hydrothermal vent in the pacific ocean [J]. Journal of Bacteriology, 2011, 193(19): 5555-5556.
[28] ZHU Jian-yu, JIAO Wei-feng, LI Qian, LIU Xue-duan, QIN Wen-qing, QIU Guan-zhou, HU Yue-hua, CHAI Li-yuan. Investigation of energy gene expressions and community structures of free and attached acidophilic bacteria in chalcopyrite bioleaching [J]. Journal of Industrial Microbiology & Biotechnology, 2012, 39(12): 1833-1840.
[29] GRANDE B M J, LUCAS L R, LOPEZ A M D C, PEREZ P R, GALVEZ A. Inhibition of planktonic and sessile Salmonella entericacells by combinations of enterocin AS-48, polymyxin B and biocides [J]. Food Control, 2013, 30(1): 214-221.
[30] YANG Hai-lin, FENG Shou-shuai, XIN Yu, WANG Wu. Community dynamics of attached and free cells and the effects of attached cells on chalcopyrite bioleaching by Acidithiobacillus sp [J]. Bioresource Technology, 2014, 154: 185-191.
[31] PLUMB J J, MUDDLE R, FRANZMANN P D. Effect of pH on rates of iron and sulfur oxidation by bioleaching organisms [J]. Minerals Engineering, 2008, 21(1): 76-82.
过程pH刺激对游离和吸附菌协同浸出黄铜矿的影响
彭堂见1,石丽娟1,余润兰1,2,顾帼华1,2,周 丹1,陈 淼3,邱冠周1,2,曾伟民1,2,3
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 生物冶金教育部重点实验室,长沙 410083;
3. CSIRO Process Science and Engineering, Box 312, Clayton South, Victoria 3169, Australia
摘 要:为了研究过程pH刺激对中度嗜热菌浸出黄铜矿的影响,测定浸出过程中铜的浸出率以及游离和吸附菌的微生物群落结构动态变化。结果表明,将浸出第14天浸出过程的pH分别调节至1.0及3.0时,游离菌和吸附菌的生长均出现一个适应期。同时,未调节pH的对照组铜的浸出率为87.5%;而调节过程pH至1.0和3.0时,铜的浸出率分别下降至86.9%和64.0%。实时定量PCR分析表明,pH刺激对吸附菌的影响比对游离菌的影响小,说明吸附菌比游离菌对过程pH刺激具有更强的抗性。此外,调节过程pH至3.0显著破坏了游离菌和吸附菌的群落结构,浸出体系无法恢复至正常状态。
关键词:黄铜矿;过程pH刺激;游离菌;吸附菌;微生物群落结构;实时定量PCR
(Edited by Wei-ping CHEN)
Foundation item: Project (31200382) supported by the National Natural Science Foundation of China; Project (2013FJ4068) supported by the Planned Science and Technology Project of Hunan Province, China; Project supported by Australia CSIRO OCE Science Leader Grant
Corresponding author: Wei-min ZENG; Tel: +86-731-88879212; E-mail: zengweimin1024@sina.com
DOI: 10.1016/S1003-6326(16)64338-8
Abstract: In order to investigate the effects of processing pH stimulation on bioleaching of chalcopyrite by moderate thermophiles, copper leaching rates and the dynamics of microbial community structures of free and attached cells were monitored. The results indicated that when the processing pH values were respectively adjusted to 1.0 and 3.0 on day 14, both free and attached cells experienced an adaptive phase. Meanwhile, the copper leaching rates were 86.9% and 64.0%,respectively, as opposed to a copper leaching rate of 87.5% in the control group without pH stimulation. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis suggested that pH stimulation imposed less impact on the attached organisms than on the free cells, indicating that the attached cells were more resistant to processing pH stimulation than the free cells. Furthermore, adjusting processing pH to 3.0 significantly disrupted both free and attached microbial communities, and the bioleaching system could not recover to the normal status as the control group.


