DOI:10.19476/j.ysxb.1004.0609.2018.03.24
磷酸盐沉淀法除铁过程热力学分析
肖 超1,曾 理2,李义兵1,肖连生2
(1. 桂林理工大学 材料科学与工程学院 广西高校有色金属清洁冶炼与综合利用重点实验室,桂林 541004;
2. 中南大学 冶金与环境学院,长沙 410083)
摘 要:
除铁是锌湿法冶金过程的重要步骤。磷酸盐沉淀法相比于传统的铁矾、针铁矿和赤铁矿沉淀法具有明显的优势。针对磷酸盐沉淀法的从硫酸锌溶液中净化除铁过程进行热力学分析,绘制了298 K时Men+-P-H2O(Me:Zn(II),Cu(II),Fe(II),Fe(III),Ni(II))系组浓度对数-pH图,利用热力学平衡图对磷酸盐沉淀法的从硫酸锌溶液中净化除铁和磷酸铁碱分解过程进行热力学分析。结果表明:pH值为0~5.0时磷酸盐形成由易至难依次为 Fe(III)>>Cu(II)>Fe(II) >>Zn(II)>Ni(II);整个pH值范围可以分为难溶磷酸盐稳定区、Me(OH)n稳定区;高pH区磷酸盐中的Me转变为稳定的Me(OH)n,实现磷酸盐碱分解。验证实验表明,加入1.0倍理论量的磷酸钠,控制沉淀pH值为2.0,铁、锌、铜、镍沉淀率分别为98.9%、3.5%、2.8%、0.7%;FePO4与其2.0倍物质量的NaOH反应,产物为Fe(OH)3、Na2HPO4,磷浸出率为96.8%,分解液pH为11.3,实验与理论相符。
关键词:
文章编号:1004-0609(2018)-03-0637-07 中图分类号:TF841.1 文献标志码:A
除铁是锌湿法冶炼过程的关键工序[1]。现有除铁工艺[2]主要包括铁矾法、针铁矿法、赤铁矿法。铁矾法是目前锌冶炼过程的主要除铁工艺。该方法除铁效果好、易于操作,但是铁矾渣容易夹带锌、铅等重金属造成有价金属损失和环境污染[3-5]。针铁矿法要求沉铁过程Fe(III)浓度约为1 g/L,使得操作较为复杂,同样产生大量夹带重金属的针铁矿渣,容易对环境造成危害[6-8]。赤铁矿法产生的铁渣重金属夹带少,稳定性好,铁含量高,可以作为钢铁和铁氧体的原材料[9-10]。但赤铁矿法反应温度高(为160~200 ℃),设备投资大,不利于工业应用[11-12]。窦明民等[13]研究表明磷酸盐沉淀法能够在pH值为1.5,温度30~60 ℃,加入1.0倍理论量的磷酸,反应30~60 min,即可沉淀99 %的铁,锌夹带率低。GAROLE等[14]发现磷酸盐沉淀法相比现有的沉淀法除铁工艺,具有操作温度低,反应速度快等优点,具有优异的工业应用前景。含铁的铜、镍、钴等溶液同样可以采用磷酸盐法除铁。杨秋菊等[15]在室温条件下采用磷酸盐沉淀法除去99%的铁,铜的损失率仅2%。杨振宁等[16]研究表明,磷酸盐沉淀法可以从硫酸镍溶液中选择性除去99 %的Fe(III)、Al、Cr(III),镍损失率小于10%。NORTH等[17]研究发现磷酸盐可以从含钴溶液中选择性除铁,钴几乎不损失。磷酸盐作为沉淀剂成本较高,因此还需考虑从磷酸铁渣中磷的利用,以降低工艺运行成本。杨秋菊等[15]采用0.8倍理论量的NaOH分解铁渣,几乎回收了所以的磷。徐志峰等[18]同样采用氢氧化钠分解法从磷酸盐沉淀物中回收磷。磷酸盐除铁工艺主要包括除铁和磷回收两部分。
已有的研究表明,磷酸盐沉淀法能够在常温条件下高效除铁,且铁渣通过碱分解后磷酸盐沉淀剂能够回用,而针对硫酸锌浸出液采用磷酸盐除铁工艺过程的热力学研究报道较少,本文作者拟对磷酸盐除铁工艺处理锌浸出液的除铁和磷回收过程的热力学进行研究。硫酸锌溶液中主要的金属成分为Zn(II)、Fe(II)、Fe(III)、Cu(II)、Co(II)、Ni(II)。通过热力学计算,依次绘制对应条件下的热力学平衡图,并对结果进行分析以期能够深化对过程的认识,并对磷酸盐除铁工艺提供理论指导和实验验证。
1 热力学计算与实验方法
1.1 热力学计算
Men+-PO43--H2O系中,溶液中可能存在的物质包括H+、OH-、Zn2+、ZnOH+、Zn(OH)2(a)、Zn(OH)3-、Zn(OH)42-、Fe2+、Fe(OH)2(a)、Fe(OH)3-、Fe(OH)42-、Cu2+、CuOH+、Cu(OH)2(a)、Cu(OH)3-、Cu(OH)42-、Fe3+、FeOH2+、Fe(OH)2+、Fe(OH)3(a)、FeSO4+、Fe(SO4)2-、Ni2+、NiOH+、Ni(OH)2(a)、Ni(OH)3-、PO43-、HPO42-、H2PO4-、H3PO4、SO42-、HSO4-、H2SO4。体系可能存在的沉淀物包括Zn3(PO4)2、Cu3(PO4)2、Ni3(PO4)2、Fe3(PO4)2、FePO4、Zn(OH)2、Cu(OH)2、Ni(OH)2、Fe(OH)3、Fe(OH)2。溶液中可能存在的平衡对应的平衡常数以及计算关系列于表1。
表1 Men+-P-H2O系化学反应及其平衡常数(298 K)
Table 1 Equilibrium reactions and constants for Men+-P-H2O system at 298 K
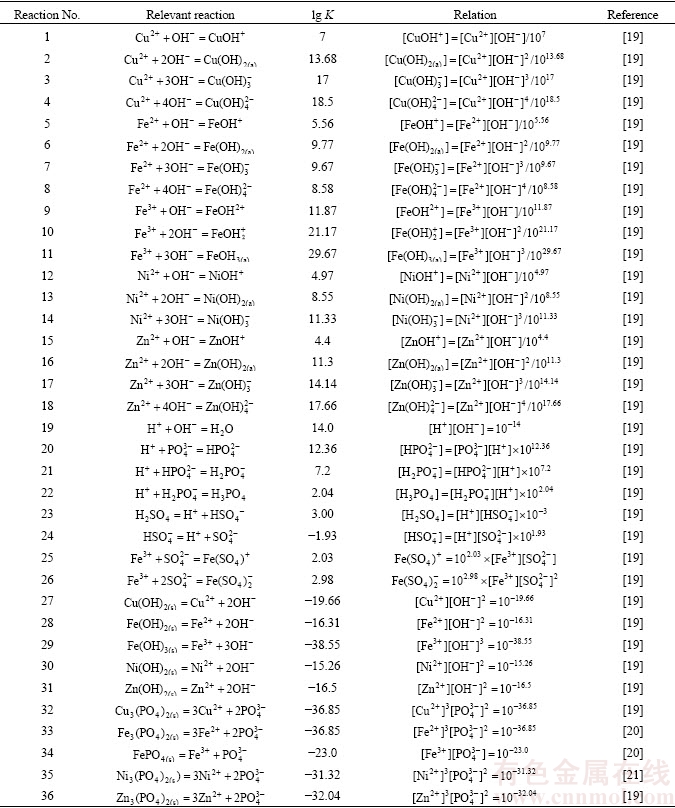
表1中式(1)~(26)表示体系中离子的平衡,式 (27)~(36)表示体系的溶解平衡。设[Me]T、[P]T、[S]T分别为溶液中游离Me、P、S总浓度,“[ ]”为溶液中各游离组分的浓度,由于计算缺乏相关的物质活度系数,故本研究计算过程以浓度代替活度。根据质量守恒定律,溶液中Me、P、S总量如下:
[Cu]T=[Cu2+]+[CuOH+]+[Cu(OH)2(a)]+[Cu(OH)3-]+[Cu(OH)42-] (37)
[Fe(II)]T=[Fe2+]+[FeOH+]+[Fe(OH)2(a)]+[Fe(OH)3-]+[Fe(OH)42-] (38)
[Fe(III)]T=[Fe3+]+[FeOH2+]+[Fe(OH)2+]+[Fe(OH)3(a)]+[FeSO4+]+[Fe(SO4)2-] (39)
[Ni]T=[Ni2+]+[NiOH+]+[Ni(OH)2(a)]+[Ni(OH)3-] (40)
[Zn]T=[Zn2+]+[ZnOH+]+[Zn(OH)2(a)]+[Zn(OH)3-]+[Zn(OH)42-] (41)
[P]T=[PO43-]+[HPO42-]+[H2PO4-]+[H3PO4] (42)
[S]T=[SO42-]+[HSO4-]+[H2SO4]+[FeSO4+]+2[Fe(SO4)2-] (43)
根据同时平衡[20],可以将表1中的式(1) ~ (26)分别带入式(37)~(42),则[Me]T只与[Men+]、[OH-]、[SO42-]、[PO43-]的量相关。水相[Men+]与对应氢氧化物、磷酸盐沉淀形成离解平衡,已知[P]T、[S]T和溶液pH求解得到对应的[PO43-]、[SO42-]、[OH-]代入式(27)~(36)即可求解对应[Men+]值,最终求解得出[Me]T。
1.2 验证实验方法
实验所采用的试剂均为分析纯,水为去离子水。采用硫酸盐和稀硫酸配制得到Zn、Fe(III)、Cu、Ni浓度分别为80.55、10.25、0.51和0.13 g/L,pH值为1.5的模拟料液。以8.21 mol/L磷酸钠溶液为沉淀剂,5.0 mol/L的NaOH或H2SO4调节溶液的pH值。磷酸盐沉淀实验是在搅拌条件下缓慢加入1.0倍沉铁理论量的磷酸钠溶液,控制溶液温度为35 ℃,过程保持溶液pH值温度,加料完毕后继续搅拌1.0 h,然后过滤、洗渣,分析滤液中金属含量。金属沉淀量为溶液中原有金属量与残留量差值;金属沉淀率为金属沉淀量与原金属量的百分比。
将磷酸铁(Fe 29.88 %, P 16.56 %)与水按照液固比(L/S)10 mL/1 g浆化,加入设定量的氢氧化钠固体,在90 ℃反应5.0 h,然后过滤、洗渣得到含磷溶液,分析溶液中磷浓度。磷浸出率为溶液中磷与磷酸铁中磷总量的百分比。
溶液成分采用ICP-AES分析。
2 结果与讨论
对Me2+-P-H2O系的热力学进行研究,以论证磷酸盐法对硫酸锌体系除铁的和磷酸铁渣回收磷的原理;研究溶液中Me、磷浓度和pH值变化对磷酸盐沉淀形成的影响,碱用量对磷酸铁分解的影响;通过实验验证理论计算结论。
2.1 Men+-P-H2O系
图1表示溶液[P]T=0.02 mol/L、[S]T=1.0 mol/L对应[Me]T与溶液pH的关系,虚线①~⑤表示对应磷酸盐与氢氧化物的转化pH值。结果表明:pH值增加时磷酸盐转变为氢氧化物,Fe(III)、Cu(II)、Zn(II)、Ni(II)、Fe(II)的磷酸盐与氢氧化物转化的pH值分别为5.0、8.0、9.5、10.0、10.5,显然FePO4最易被碱分解;pH 0.0~5.0磷酸盐沉淀形成优先顺序依次为Fe(III)>>Cu(II)>Fe(II)>>Zn(II)>Ni(II),磷酸盐沉淀法可以从含锌、铜、镍溶液中除铁;Fe(III)优先于Fe(II)形成磷酸盐沉淀,说明Fe(II)氧化为Fe(III)有利于磷酸盐除铁。可以推断得出,溶液锌、铜、镍浓度分别为1.0、0.1、1.0 mol/L,在pH值为2.0~3.0,磷酸盐法可以选择性将Fe(III)降至1×10-2.0~1×10-3.5 mol/L,实现锌、铜、镍与铁分离。
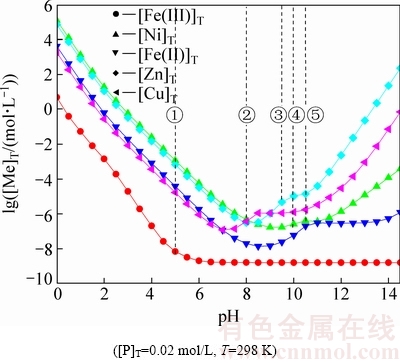
图1 Men+-P-H2O系统lg[Me]T与pH值的关系
Fig. 1 Relationship between lg[Me]T and pH of Men+-P-H2O system
2.2 溶液中[Fe(III)]T对磷平衡浓度的影响
图2所示为溶液中[Fe(III)]T对[P]T平衡浓度的影响。结果表明,溶液中残留Fe(III)越低,对应平衡[P]T浓度越高;同等Fe(III)浓度下,pH为1.5时,对应[P]T浓度更低;当溶液[Fe]T=1×10-2.3 mol/L(0.28 g/L),pH值为1.5时,溶液对应[P]T浓度为3×10-4 mol/L(0.01 g/L),再减少铁量,溶液中残留[P]T浓度快速增加。这说明,在较高平衡pH值和提高溶液[Fe(III)]T时,可以减少P的残留。为了减少磷残留,建议除铁终点浓度[Fe(III)]T≥0.28 g/L。
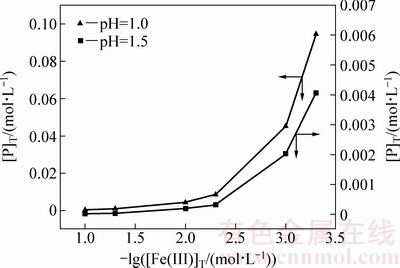
图2 Fe(III)浓度对溶液中[P]T浓度的影响
Fig. 2 Effect of Fe(III) concentration on [P]T
2.3 溶液中[P]T对Fe(III)平衡浓度的影响
图3所示为溶液中[P]T浓度对Fe(III)行为的影响,虚线⑥~⑨表示不同[P]T浓度对应FePO4与Fe(OH)3转化pH值。结果表明,[P]T浓度越高,FePO4稳定区越大,分解为Fe(OH)3的pH越高;随着[P]T和pH提高,对应[Fe]T减少。可以推断,当[P]T为0.0025 mol/L,pH值为2.0~2.5,溶液中[Fe(III)]T降至1×10-2 mol/L(0.56 g/L),若要深度除铁([Fe]T=1×10-4.5 mol/L) 则需要提高溶液中[P]T浓度。综合考虑控制溶液中[P]T 浓度为0.0025 mol/L,除铁终点[Fe(III)]T约为0.56 g/L较好。
图3 溶液磷浓度对体系中[Fe(III)]T的影响
Fig. 3 Effect of P concentration on [Fe(III)]T in system
2.4 碱度对FePO4稳定性的影响
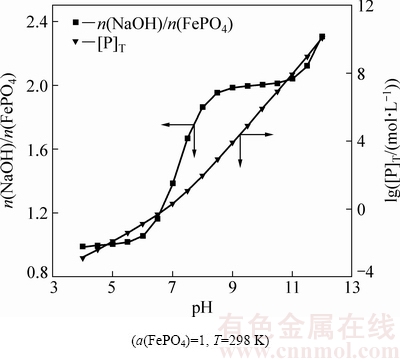
图4 NaOH用量对FePO4分解的影响
Fig. 4 Effect of NaOH dosage on decomposition of FePO4

图5 pH值对体系磷离子形态的影响
Fig. 5 Effect of pH on phosphorus speciation in system
图4 所示为NaOH加入量对FePO4分解的影响。用n(NaOH)/n(FePO4)表示NaOH加入量与FePO4物质量的倍数,[P]T表示溶液最大平衡磷浓度,设a(FePO4)=1 (FePO4过量)。结果表明,随着n(NaOH)/n(FePO4)的增加溶液平衡pH值增大,溶液磷浓度增加,说明提高NaOH用量利于FePO4分解和得到高浓度的含磷溶液。结合图4、5可知,n(NaOH)/n(FePO4)=1.0时溶液pH值为4~6,磷的主要存在形态为NaH2PO4;n(NaOH)/n(FePO4)为1.0~2.0时,随着NaOH加入量的增加,溶液pH和磷浓度快速增加,对应磷的主要存在形态为NaH2PO4、Na2HPO4;n(NaOH)/n(FePO4)≈2.0,增加NaOH用量pH值从8.5快速增加至11.0,溶液磷几乎完全以Na2HPO4存在;再增加NaOH用量时,磷则以Na3PO4存在。说明随着NaOH用量的增加FePO4分解的趋势增加,可能按照以下3种反应方式进行:
FePO4+NaOH+2H2O=Fe(OH)3+NaH2PO4 (44)
FePO4+2NaOH+H2O=Fe(OH)3+Na2HPO4 (45)
FePO4+3NaOH=Fe(OH)3+Na3PO4 (46)
2.5 验证实验
图6表明,随着溶液pH值的增加,金属的沉淀率增加,铁的沉淀率实验值与计算值接近。pH值为2.0时,铁、锌、铜、镍沉淀率分别为98.9%、3.5%、2.8%、0.7%,铁与锌、铜、镍的沉淀分离效果好。
图7表明,随着NaOH用量增大,浸出液pH值和磷浸出率增加。当NaOH 用量为2.0倍FePO4摩尔量时,浸出液pH值为11.5,磷浸出率达到96.8%,结合图5和文献[15]可知,最终反应产物为Na2HPO4、Fe(OH)3。说明按照反应FePO4+2NaOH+H2O=Fe(OH)3 + Na2HPO4,FePO4可以彻底分解。
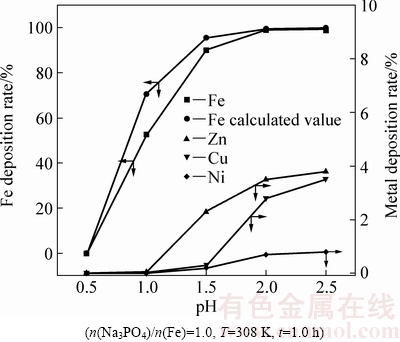
图6 pH值对金属沉淀率的影响
Fig. 6 Effect of pH on metal deposition rate
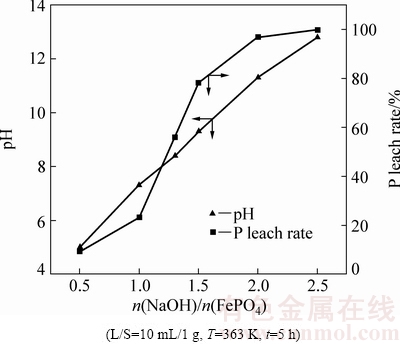
图7 NaOH用量对FePO4分解的影响
Fig. 7 Effect of dosage of NaOH on decomposition of FePO4
3 结论
1) Me-P-H2O系在较低和较高pH值区分别为MePO4和Me(OH)n区,pH值为0~5.0时磷酸盐沉淀形成优先顺序依次为Fe(III) >>Cu(II)>Fe(II)>>Zn(II)>Ni(II),MePO4分解为Me(OH)n的优先顺序依次为Fe(III)>Cu(II)>Zn(II)>Ni(II)>Fe(II)。
2) 维持较高的溶液中Fe(III)浓度和pH值利于减少P的残留,控制溶液残留Fe(III)为0.56 g/L、pH为2.0,P残留量为0.0025 mol/L。
3) n(NaOH)/n(FePO4)=2.0时,FePO4中磷浸出率达到96.8%,反应式为FePO4+2NaOH+H2O=Fe(OH)3+ Na2HPO4。
4) 沉淀平衡pH为2.0,加入1.0倍理论量的磷酸钠,铁与锌、铜、镍的沉淀分离效果好。
REFERENCES
[1] ISMAEL M R C, CARVALHO J M R. Iron recovery from sulphate leach liquors in zinc hydrometallurgy[J]. Minerals Engineering, 2003, 16(1): 31-35.
[2] CLAASSEN J O, MEYER E H O, RENNIE J, SANDENBERGH R F. Iron precipitation from zinc-rich solutions: Defining the Zincor Process[J]. Hydrometallurgy, 2002, 67(1): 87-108.
[3] WANG K, LI J, MCDONALD R G, BROWNER R E. The effect of iron precipitation upon nickel losses from synthetic atmospheric nickel laterite leach solutions: Statistical analysis and modelling[J]. Hydrometallurgy, 2011, 109(1/2): 140-152.
[4] 邵鸿媚, 申晓毅, 张彬彬, 翟玉春. 氧化锌矿溶出液的净化[J]. 东北大学学报(自然科学版), 2015, 36(6): 811-813.
SHAO Hong-mei, SHEN Xiao-yi, ZHANG Bin-bin, ZHAI Yu-chun. Purification of the digestion solution of zinc oxide ores[J]. Journal of Northeastern University (Natural Science), 2015, 36(6): 811-813.
[5] 何 静, 罗 超, 唐谟堂, 鲁君乐, 王小能, 王 涛. 采用铅黄铁矾去除硫酸体系中的铁[J]. 中国有色金属学报, 2012, 22(10): 2890-2895.
HE Jing, LUO Chao, TANG Mo-tang, LU Jun-yue, WANG Xiao-neng, WANG Tao. Technique of ferrum-removal by lead jarosite from sulfuric acid solution[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(10): 2890-2895.
[6] HAN H, SUN W, HU Y, TANG H. The application of zinc calcine as a neutralizing agent for the goethite process in zinc hydrometallurgy[J]. Hydrometallurgy, 2014, 147(5): 120-126.
[7] 李海军, 杨洪英, 陈国宝, 周立杰, 刘媛媛. 中心复合设计针铁矿法从含钴生物浸出液中除铁[J]. 中国有色金属学报, 2013, 23(7): 2040-2046.
LI Hai-Jun, YANG Hong-ying, CHEN Guo-bao, ZHOU Li-jie, LIU Yuan-yuan. Goethite deironization process for cobaltiferous bioleaching solution by central composite design[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(7): 2040-2046.
[8] 邓永贵, 陈启元, 尹周澜, 张平民. 湿法炼锌浸出液针铁矿法除铁晶种的制备[J]. 中国有色金属学报, 2008, 18(s1): s27-s31.
DENG Yong-gui, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Ping-min. Preparation of goethite seeds for removal of ferrous/ferric ions from leaching solution of zinc in hydrometallurgy process[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(s1): s27-s31.
[9]  D. Zinc recovery from steel-making wastes by acid pressure leaching and hematite precipitation[J]. Hydrometallurgy, 2010, 101(3/4): 171-173.
D. Zinc recovery from steel-making wastes by acid pressure leaching and hematite precipitation[J]. Hydrometallurgy, 2010, 101(3/4): 171-173.
[10] LU J, DREISINGER D. Pressure oxidation of ferrous ions by oxygen and hematite precipitation from concentrated solution of calcium, copper and iron chlorides[J]. Hydrometallurgy, 2013, 140(11): 59-65.
[11] 杨 凡, 邓志敢, 魏 昶, 李存兄, 李兴彬. 采用赤铁矿去除高铁闪锌矿浸出液中的铁[J]. 中国有色金属学报, 2014, 24(9): 2387-2392.
YANG Fan, DENG Zhi-gan, WEI Chang, LI Cun-xiong, LI Xing-bin. Iron-removal by hematite from leaching liquor of high iron sphalerite[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2387-2392.
[12] 杨 凡, 邓志敢, 魏 昶, 李兴彬, 李存兄, 邱伟佳, 朱如龙. 高铁闪锌矿赤铁矿法除铁中锌离子和镁离子的影响及存在机理[J]. 中国有色金属学报, 2014, 24(11): 2906-2913.
YANG Fan, DENG Zhi-gan, WEI Chang, LI Xing-bin, LI Cun-xiong, QIU Wei-jia, ZHU Ru-long. Effect and existence mechanism of zinc ion and magnesium ion in iron removal from high iron sphalerite by hematite process[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(11): 2906-2913.
[13] 窦明民, 周德林. 湿法炼锌除铁新工艺研究[J]. 中国有色冶金, 2000, 29(3): 27-30.
DOU Ming-Min, ZHOU De-lin. A study on new technology for removing iron by phosphate[J]. Non Ferrous Smelting, 2000, 29(3): 27-30.
[14] GAROLE D J, GAROLE V J, DALAL D S. Recovery of metal value from electroplating sludge[J]. Research Journal of Chemical Sciences, 2012, 3(2): 61-63
[15] 杨秋菊, 刘久清, 蒋 彬, 刘海翔. 高铁低铜溶液磷酸盐除铁工艺[J]. 中南大学学报(自然科学版), 2012, 43(10): 3763-3768.
YANG Qiu-ju, LIU Jiu-qing, JIANG Bin, LIU Hai-xiang. Technique of removing iron by phosphate in high iron-low copper solution[J]. Journal of Central South University (Natural Science), 2012, 43(10): 3763-3768.
[16] 杨振宁, 陈志传, 高大明, 童张法. 电镀污泥中铜镍回收方法及工艺的研究[J]. 环境污染与防治, 2008, 30(7): 58-61.
YANG Zhen-ning, CHEN Zhi-chuan, GAO Da-ming, TONG Zhang-fa. Recovery of copper and nickel from electroplating sludge[J]. Environmental Pollution & Control, 2008, 30(7): 58-61.
[17] NORTH V, WELLS R C. Use of phosphate for separation of cobalt from iron[J]. Industrial & Engineering Chemistry Analytical Edition, 2002, 14(11): 859-860.
[18] 徐志峰, 赵洪兴, 王成彦, 袁文辉, 杨 卜. 由混合电镀污泥硫酸浸出液选择性沉淀分离铬的研究[J]. 有色金属科学与工程, 2015, 6(5): 7-11.
XU Zhi-feng, ZHAO Hong-xing, WANG Cheng-yan, YUAN Wen-hui, YANG Bo. Recovery of chromium from sulphuric acid leaching solutions of mixed electroplating sludge by selective precipitation[J]. Nonferrous Metals Science & Engineering, 2015, 6(5): 7-11.
[19] DEAN J A. LANGE's handbook of chemistry[M]. Fifteenth Edition. New York: McGRAW-HILL, INC, 1999: 1185-1261.
[20] 张 刚, 赵中伟, 曹才放, 黄少波. 磷酸盐分解钼酸钙的热力学[J]. 北京科技大学学报, 2009, 31(11): 1394-1399.
ZHANG Gang, ZHAO Zhong-wei, CAO Cai-fang, HUANG Shao-bo. Thermodynamic analysis on phosphate decomposition of calcium molybdate[J]. Journal of University of Science & Technology Beijing, 2009, 31(11): 1394-1399.
[21] ZHANG M, ZHENG K, JIN J, YU X, QIU L, DING S, LU H F, CAI J, ZHANG P. Effects of Fe(II)/P ratio and pH on phosphorus removal by ferrous salt and approach to mechanisms[J]. Separation and Purification Technology, 2013, 118(6): 801-805.
Thermodynamic analysis on removing iron by phosphate precipitation
XIAO Chao1, ZENG Li2, LI Yi-bing1, XIAO Lian-sheng2
(1. Guangxi Key Laboratory in Universities of Clean Metallurgy and Comprehensive Utilization for Non-ferrous Metals Resources, School of Materials Science and Engineering, Guilin University of Technology, Guilin 541004, China;
2. School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: Removing iron is the key process of extraction zinc by hydrometallurgy. The removing iron from leach solution by means of phosphate precipitation may be a feasible alternative to conventional precipitation processes as jarosite, goethite or hematite. Aiming at removing iron by phosphate precipitation, the logarithm concentration-pH diagram of Men+-P-H2O(Me:Zn(II), Cu(II), Fe(II), Fe(III), Ni(II)) system was established on the basis of thermodynamic data at 298 K, according to the laws of conservation of mass and simultaneous equilibria. Themodynamic analysis was carried out to discuss the process of removing iron from lixivium of zinc calcine by phosphate precipitation and decomposition of iron phosphate by sodium hydroxide. The results show that the ions can be removed respectively by phosphate precipitation in the following order: Fe(III)>>Cu(II)>Fe(II) >>Zn(II)>Ni(II). The whole pH value of the system is divided into two areas. The one is the stable of sparingly soluble phosphate, the other is Me(OH)n. Me(OH)n is gradually superaturated in the solution with the increase of pH value, so the Me(OH)n is deposited as sediment, and phosphate goes into solution. The verification experiment results showed that the precipitation rate of Fe, Zn, Cu and Ni were 98.9%, 3.5%, 2.8% and 0.7%, respectively, by 100% theoretical amounts of sodium phosphate at pH 2.0. When the dosage of NaOH is 2.0 times of the mole ratio of FePO4, FePO4 decomposed into Fe(OH)3 precipitate and Na2HPO4, phosphorus leaching rate is 96.8%, the pH value of leaching liquid is 11.3, which is consistent with the thermodynamic analysis.
Key words: phosphate; iron; zinc; precipitation; thermodynamic
Foundation item: Project (51404297) supported by the National Natural Science Foundation of China; Project (2016ZZKT-08) supported by Guangxi Key Laboratory of Universities for Clean Metallurgy Comprehensive Utilization of Nonferrous Metal Resource, China
Received date: 2016-11-29; Accepted date: 2017-05-17
Corresponding author: XIAO Chao; Tel: +86-18008412484; E-mail: shawchao@qq.com
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51404297);广西高校有色金属清洁冶炼与综合利用重点实验室开放基金资助项目(2016ZZKT-08)
收稿日期:2016-11-29;修订日期:2017-05-17
通信作者:肖超,讲师,博士;电话:18008412484;E-mail: shawchao@qq.com
摘 要:除铁是锌湿法冶金过程的重要步骤。磷酸盐沉淀法相比于传统的铁矾、针铁矿和赤铁矿沉淀法具有明显的优势。针对磷酸盐沉淀法的从硫酸锌溶液中净化除铁过程进行热力学分析,绘制了298 K时Men+-P-H2O(Me:Zn(II),Cu(II),Fe(II),Fe(III),Ni(II))系组浓度对数-pH图,利用热力学平衡图对磷酸盐沉淀法的从硫酸锌溶液中净化除铁和磷酸铁碱分解过程进行热力学分析。结果表明:pH值为0~5.0时磷酸盐形成由易至难依次为 Fe(III)>>Cu(II)>Fe(II) >>Zn(II)>Ni(II);整个pH值范围可以分为难溶磷酸盐稳定区、Me(OH)n稳定区;高pH区磷酸盐中的Me转变为稳定的Me(OH)n,实现磷酸盐碱分解。验证实验表明,加入1.0倍理论量的磷酸钠,控制沉淀pH值为2.0,铁、锌、铜、镍沉淀率分别为98.9%、3.5%、2.8%、0.7%;FePO4与其2.0倍物质量的NaOH反应,产物为Fe(OH)3、Na2HPO4,磷浸出率为96.8%,分解液pH为11.3,实验与理论相符。
[4] 邵鸿媚, 申晓毅, 张彬彬, 翟玉春. 氧化锌矿溶出液的净化[J]. 东北大学学报(自然科学版), 2015, 36(6): 811-813.
[5] 何 静, 罗 超, 唐谟堂, 鲁君乐, 王小能, 王 涛. 采用铅黄铁矾去除硫酸体系中的铁[J]. 中国有色金属学报, 2012, 22(10): 2890-2895.
[7] 李海军, 杨洪英, 陈国宝, 周立杰, 刘媛媛. 中心复合设计针铁矿法从含钴生物浸出液中除铁[J]. 中国有色金属学报, 2013, 23(7): 2040-2046.
[8] 邓永贵, 陈启元, 尹周澜, 张平民. 湿法炼锌浸出液针铁矿法除铁晶种的制备[J]. 中国有色金属学报, 2008, 18(s1): s27-s31.
[11] 杨 凡, 邓志敢, 魏 昶, 李存兄, 李兴彬. 采用赤铁矿去除高铁闪锌矿浸出液中的铁[J]. 中国有色金属学报, 2014, 24(9): 2387-2392.
[13] 窦明民, 周德林. 湿法炼锌除铁新工艺研究[J]. 中国有色冶金, 2000, 29(3): 27-30.
[15] 杨秋菊, 刘久清, 蒋 彬, 刘海翔. 高铁低铜溶液磷酸盐除铁工艺[J]. 中南大学学报(自然科学版), 2012, 43(10): 3763-3768.
[16] 杨振宁, 陈志传, 高大明, 童张法. 电镀污泥中铜镍回收方法及工艺的研究[J]. 环境污染与防治, 2008, 30(7): 58-61.
[18] 徐志峰, 赵洪兴, 王成彦, 袁文辉, 杨 卜. 由混合电镀污泥硫酸浸出液选择性沉淀分离铬的研究[J]. 有色金属科学与工程, 2015, 6(5): 7-11.
[20] 张 刚, 赵中伟, 曹才放, 黄少波. 磷酸盐分解钼酸钙的热力学[J]. 北京科技大学学报, 2009, 31(11): 1394-1399.


