文章编号:1004-0609(2014)04-1008-06
镀锌层表面磷化/硝酸铈封闭后处理复合膜的生长机理
林碧兰1,卢锦堂2,李月婵1
(1. 厦门理工学院 材料科学与工程学院,厦门 361021;
2. 华南理工大学 材料科学与工程学院,广州 510640)
摘 要:
通过磷化/硝酸铈封闭后处理技术在镀锌层表面形成复合膜以提高其耐蚀性能。采用SEM、EDS、XPS和XRD研究复合膜的显微组织、化学成分、元素价态和相组成,并从热力学角度探讨复合膜的生长机理。结果表明:磷化镀锌层经硝酸铈封闭处理后,针片状磷化膜的间隙被铈盐膜覆盖,形成了连续完整的复合膜;在封闭过程中,部分磷化膜发生溶解,针片状磷化膜的边缘变得粗糙模糊,溶解的磷酸根离子又与Ce3+结合,生成不溶的水合磷酸铈,覆盖在复合膜表面,其中一部分水合磷酸铈与复合膜牢固结合,一部分为絮状疏松且可被漂洗下来;延长封闭时间,溶解的磷化膜增多,絮状化合物也增多并几乎覆盖整个复合膜表面;复合膜中含有Zn3(PO4)2·4H2O、CePO4·xH2O、CeO2和Ce(OH)4。
关键词:
中图分类号:TG174.44;TQ153.1 文献标志码:A
Growth mechanism of composite coatings obtained by phosphating and cerium nitrate post-sealing on galvanized steel
LIN Bi-lan1, LU Jin-tang2, LI Yue-chan1
(1. School of Materials Science and Engineering, Xiamen University of Technology, Xiamen 361021, China;
2. School of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, China)
Abstract: To enhance the corrosion resistance of galvanized steel, a composite coating was formed by phosphating and cerium nitrate post-sealing treatment. The microstructure, chemical composition, element valence and phase of the composite coatings were investigated by SEM, EDS, XPS and XRD. And the growth mechanism of the composite coating was discussed based on thermodynamics. The results show that after post-sealing the phosphated galvanized steel with cerium nitrate, the gaps of the needle-like zinc phosphate crystals are covered with cerium salts, and thus, the continuous and intact composite coatings form. During post-sealing process, the edges of phosphate crystals become rough and fuzzy due to the dissolution of phosphate. The dissolved phosphate ions simultaneously combine with cerium ions, and the corresponding insoluble CePO4·xH2O compounds form and cover on the composite coatings. A part of compounds bond strongly, and the other parts are flocculent and will be rinsed easily. Both the dissolved zinc phosphate and the new-formed flocculent compounds increase with the increase of post-sealing time, and finally the flocculent compounds almost completely cover the composite coatings. The composite coatings are composed of Zn3(PO4)2·4H2O, CePO4·xH2O, CeO2 and Ce(OH)4.
Key words: galvanized steel; composite coatings; phosphate coatings; cerium nitrate; post-sealing; growth mechanism
磷化是提高镀锌层耐蚀性和作为涂装底层的重要技术之一[1-2]。针状、片状、颗粒状等晶形磷化膜上有孔隙,通过磷化工艺的改进以降低磷化膜的孔隙率、或通过封闭后处理以形成复合膜,均可改善膜层的耐蚀性[3-4]。ZENG等[5]研究发现,钙盐添加剂可使AZ31铝合金表面磷酸锌转化膜的晶化更完全,改变了磷化膜的化学组成,并大幅提高磷化膜的耐蚀性。AMINI等[6]研究发现,环境友好型十二烷基硫酸钠可显著改进镁合金表面磷化膜的耐蚀性。LIN等[7-8]研究发现,钼酸盐添加剂可大幅降低磷化膜的孔隙率,钼酸盐封孔后处理可显著提高磷化膜的耐蚀性。
稀土盐对金属具有很好的缓蚀效果,尤其是稀土铈盐[9-10]。锌、镀锌钢表面铈盐处理的研究成果较多,但单一铈盐膜较薄且容易开裂剥落,难以大幅度提高耐蚀性[11-12]。本文作者前期采用硝酸铈封闭磷化的热镀锌钢,初步发现复合膜的耐蚀性大幅提高甚至优于铬酸盐钝化膜的[13]。本文作者采用SEM、EDS、XPS、XRD等研究了复合膜的微观组织和化学成分,并讨论了复合膜的生长机理。
1 实验
实验材料为自制热镀锌钢,镀锌层厚度约为50 μm[13]。热镀锌钢的磷化工艺如下:ZnO 1.2 g/L、NaNO3 15.0 g/L、85%H3PO4 11 mL/L、pH 3.0、温度45 ℃。硝酸铈封闭后处理的工艺为:Ce(NO3)3·6H2O 20 g/L、H2O2 35 mL/L、pH 3.8、温度45 ℃。为了避免污染硝酸铈溶液,热镀锌钢从磷化液中取出后先用蒸馏水漂洗,再浸入硝酸铈溶液中处理,自然干燥。
用XL2302FEG 型扫描电镜观察膜层的显微组织,用DX24 型能谱仪分析膜层微区的化学成分,用Dmax/IIIA型X射线衍射仪(Cu Kα靶)分析膜层的相组成,用AXIS ULTRA型X射线光电子能谱仪(XPS)及XPS高分辨技术(Mg Kα为X射线源)分析膜层的元素组成和价态。XPS高分辨谱的组分有Zn 2p、O 1s、P 2p和Ce 3d,为确定膜层中Ce3+和Ce4+的信号,Ce 3d的结合能范围为875~920 eV。
为方便起见,将磷化试样用P表示,将磷化+硝酸铈封闭后处理的试样用P+Ce表示(代号P和Ce后面的数字表示磷化时间和硝酸铈处理时间(s))。
2 结果与讨论
2.1 复合膜的显微组织
图1所示为P30和P30+Ce30表面的SEM像。表1是相应微区的化学成分。由图1可见,经硝酸铈短时封闭处理后,磷酸锌晶体的边缘由平滑清晰变得粗糙模糊,晶体附近生成粗糙的絮状物。由表1可见,经硝酸铈封闭处理后,整个锌层表面均含有Zn、P、O、Ce,即整个锌层表面均有含铈化合物。其中磷酸锌晶体孔隙处的Ce、P化合物可能来自溶解的磷酸锌,从图2也可以清晰看到磷酸锌晶体的溶解。
图3所示为经硝酸铈封闭较长时间的磷化试样的SEM像。由图3可见,封闭60 s后,复合膜表面的絮状物增多,针状磷酸锌晶体变得模糊;封闭600 s,絮状膜明显增多、变得较密,并且几乎覆盖了整个磷化膜表面。EDS结果(Ce: 56.6%、P: 0.8%、O: 19.6%、Zn: 23.0%,质量分数)表明,絮状物中Ce含量极高。

图1 P30(a)和P30+Ce30(b)表面的SEM像
Fig. 1 SEM images of P30 (a) and P30+Ce30 (b) surfaces
表1 图1中不同微区的化学成分
Table 1 Chemical composition of different micro-sites shown in Fig. 1
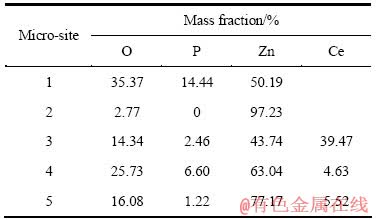

图2 溶解磷酸锌晶体的高倍SEM像
Fig. 2 High-magnification SEM image of dissolved zinc phosphate crystals
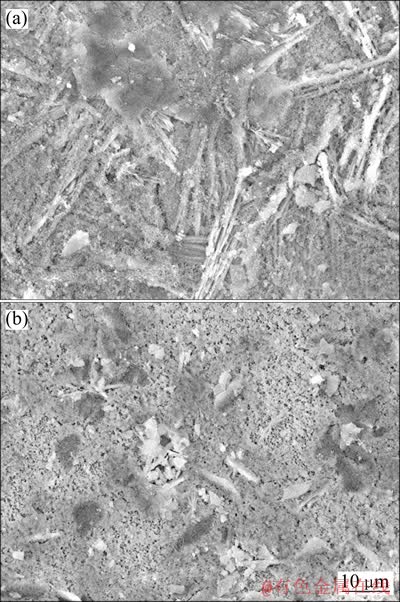
图3 P30+Ce60(a)和P30+Ce600(b)表面的SEM像
Fig. 3 SEM images of P30+Ce60 (a) and P30+Ce600 (b) surfaces
图4所示为漂洗后P300+Ce600的SEM像。表2为相应微区的化学成分。由图4可见,最外层的絮状铈盐化合物被漂洗下来;底层的磷酸锌晶体仍为放射型的针片状,且清晰可见,占据了大部分的锌层表面,表明只有部分磷酸锌发生溶解。由表2可见,经水漂洗后,整个锌层表面仍含有Ce和P,表明部分Ce、P化合物的机械强度较高,与复合膜牢固结合。另外,微区2没有传统单一铈盐膜的开裂脱落现象,从而形成了连续完整的复合膜。
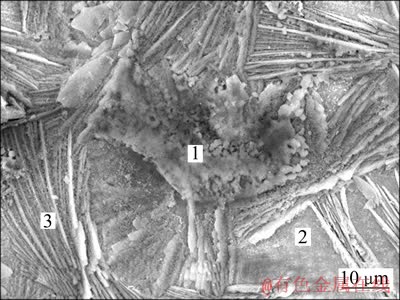
图4 P300+Ce600经水漂洗后的SEM像
Fig. 4 SEM image of rinsed P300+Ce600
表2 图4中不同微区的化学成分
Table 2 Chemical composition of different micro-sites shown in Fig. 4

2.2 复合膜的化学组成
图5所示为复合膜表面的XPS全谱。由图5可见,复合膜表面含有Zn、P、O和Ce。同时,XPS分析得到复合膜表面各元素的相对含量(摩尔分数,%)为Zn 4.2、P 16.4、O 66.5和Ce 12.6,复合膜表面Ce含量极高。

图5 复合膜表面的XPS全谱图
Fig. 5 XPS spectra of composite coatings surfaces
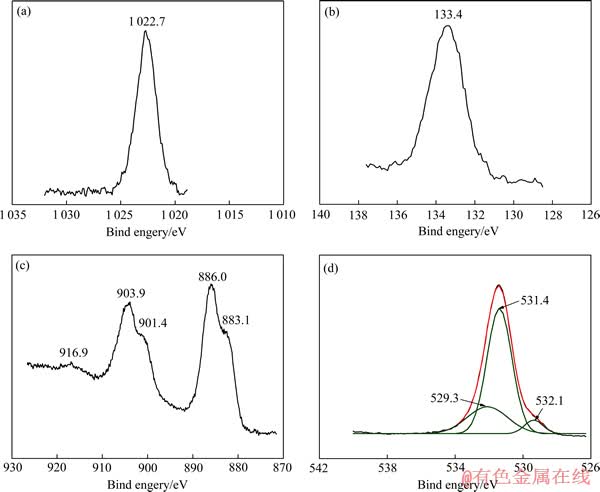
图6 复合膜表面Zn 2p(a), P 2p(b), Ce 3d(c)和O 1s (d)的高分辨XPS谱
Fig. 6 High resolution XPS spectra of Zn 2p (a), P 2p(b), Ce 3d (c)and O 1s (d) of composite coating
图6所示为复合膜表面Zn 2p、Ce 3d、O 1s和P 2p的高分辨XPS谱。由图6可见,Zn 2p谱上结合能为1022.6 eV的Zn2+峰与Zn3(PO4)2中的Zn2+峰(1022.7 eV)一致;P 2p谱上结合能为133.4 eV的P5+峰,不是Na3PO4中的P5+峰(132.3 eV) [14],但与Zn3(PO4)2和CePO4中的P峰(133.4 eV)一致[15]。
一般地,Ce 3d的XPS高分辨谱由3个特征区组成:880~890 eV与Ce 3d5/2相关;890~910 eV为Ce 3d5/2和Ce 3d3/2的混合区域;917 eV为Ce3d3/2的卫星峰,这是Ce4+的标志[16]。由图6(c)可见,Ce 3d谱也含有3个特征区:883.1~886 eV处的Ce 3d5/2,901.4~903.9 eV处Ce 3d5/2和Ce 3d3/2的混合区及916.9 eV处Ce3d3/2的卫星峰,说明复合膜表面含有Ce3+和Ce4+化合物。
由图6(d)可见,经分峰拟合后,O 1s谱由结合能为532.1、531.4和529.3 eV的 3个小峰组成。532.1 eV对应于H2O和Ce—OH中的O 1s峰(均为532.2 eV);531.4 eV对应于Zn3(PO4)2和CePO4中的O 1s峰(分别为531.5和531.0 eV);529.3 eV对应于CeO2中的O 1s峰(为529.2 eV)。
XPS结果表明,磷化/硝酸铈封闭后处理复合膜表面可能有Zn3(PO4)2·4H2O、CePO4·xH2O、CeO2和(或) Ce(OH)4,这与XRD的研究结果相一致(见图7)。
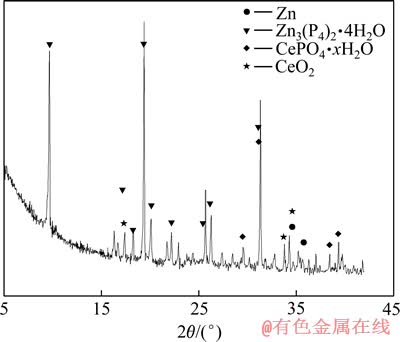
图7 复合膜的XRD谱
Fig. 7 XRD spectra of composite coatings
2.3 复合膜的生长机理
在Ce(NO3)3水溶液中添加氧化性极强的H2O2会使部分Ce3+氧化成Ce4+,溶液变成淡黄色,铈以Ce3+和Ce4+的形式存在。20 g/L、pH=3.8的Ce(NO3)3·6H2O (含H2O2)溶液中,[OH-]约为6.3×10-11 mol/L,Ce3+与Ce4+的总浓度为4.6×10-2 mol/L。
Ce3+-e → Ce4+ (1)
H2O2+2e → 2OH- (2)
将磷化热镀锌板浸入上述硝酸铈溶液中,磷酸锌晶体的间隙发生锌的阳极溶解
Zn-2e →Zn2+ (3)
微阴极发生H2O2的还原反应(见式(2))或析氢反应(pH=3.8)
2H++2e →H2 (4)
微阳极过程使溶液中的[Zn2+]增多;微阴极过程使锌层表面附近区域溶液中的pH增大,即[OH-]增多,形成了一个碱性环境,这将有利于氢氧化物的沉积。
Zn(OH)2、Ce(OH)3和Ce(OH)4的浓度积Ksp分别为3×10-17、1.5×10-20和2×10-48 [17-18],其饱和溶解度分别为2.0×10-6、4.9×10-6和9.5×10-11 mol/L。Zn2+是通过溶解锌层来提供的,增量有限,而且硝酸铈溶液中的[OH-]明显小于Zn(OH)2的饱和溶解度(6.3×10-11 mol/L << 4.0×10-6 mol/L),故需溶解较多锌层及溶液pH需增大较多,才能引起Zn(OH)2的沉积。同理,溶液pH增大较多才能使Ce(OH)3沉积。但硝酸铈溶液中的[OH-]仅略小于Ce(OH)4的饱和溶解度(6.3×10-11 mol/L≈3.8× 10-10 mol/L),故pH的微量增加即可促进Ce(OH)4的形成
Ce4+ + 4OH- →Ce(OH)4 (5)
即Ce(OH)4较Ce(OH)3和Zn(OH)2优先沉积。同时,Ce(OH)4逐渐转化成稳定的氧化物
Ce(OH)4 →CeO2+2H2O (6)
因此,XRD谱上可检测到较多的CeO2,而无法检测到Ce2O3、ZnO、Ce(OH)3、Zn(OH)2、Ce(OH)4,但实际上可能存在少量未脱水的Ce(OH)4。
Zn3(PO4)2·4H2O和CePO4的Ksp分别为9.1×10-33和1.1×10-24 [18],其饱和溶解度分别为1.5×10-7和1.0×10-12 mol/L。磷化的镀锌层与上述硝酸铈溶液接触时,部分磷酸锌晶体发生溶解
Zn3(PO4)·4H2O →3Zn2++2PO43-+4H2O (7)
溶液中的饱和[PO43-]为3.0×10-7 mol/L、远大于CePO4的饱和溶解度(1.0×10-12 mol/L)。同时,硝酸铈溶液中的[Ce3+]也远大于CePO4的饱和溶解度。因此,磷化热镀锌钢浸入上述硝酸铈溶液中,必将形成水合铈盐化合物CePO4·xH2O
Ce3++PO43-+xH2O →CePO4·xH2O (8)
因此,XRD和XPS分析均可检测到CePO4·xH2O,SEM和EDS分析可观察到磷酸锌晶体的溶解、新生成的含Ce和P的絮状化合物以及磷化膜间隙处有含Ce和P的化合物。
综上分析,CeO2的形成是由于氧化性极强的H2O2将Ce3+氧化成Ce4+、微阴极过程使锌层附近区域溶液中的pH增大、Ce(OH)4的饱和溶解度极小以及Ce(OH)4的稳定化脱水。CePO4·xH2O的形成是由于Zn3(PO4)2·4H2O的溶解以及CePO4的饱和溶解度极小。随着封闭时间的延长,Zn3(PO4)2·4H2O不断溶解、PO43-不断得到供给,CePO4·xH2O不断在复合膜表面沉积,并由疏松絮状转变成较密的颗粒状。其中,先沉积的(内层的)CePO4·xH2O与底层复合膜牢固结合,不易被漂洗下来,也不易识别;而后沉积的(外层)颗粒状的CePO4·xH2O极易被漂洗下来。图8所示为镀锌层表面磷化膜和复合膜的结构示意图,硝酸铈封闭后处理使磷化膜的显微结构和化学组成发生显著变化。
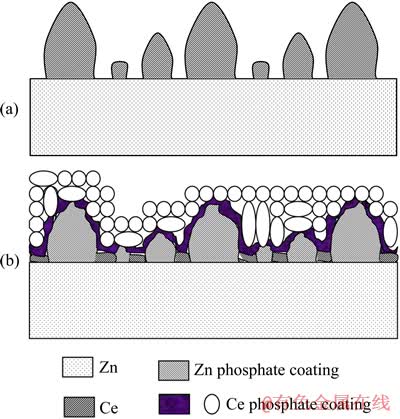
图8 镀锌层表面磷化膜(a)和复合膜(b)的示意图
Fig. 8 Schematic diagrams of phosphate coatings (a) and composite coatings (b) on galvanized steel
3 结论
1) 磷化热镀锌层经硝酸铈封闭处理后,无裂纹的铈盐化合物膜填补了磷化膜的间隙,从而形成连续完整的复合膜;同时,部分磷酸锌晶体发生溶解,使针片状磷化膜的边缘变得模糊并提供PO43-以形成更难溶的水合磷酸铈化合物,其中一部分化合物牢固覆盖在复合膜上,而另一部分为疏松状的、可被漂洗下来的;延长封闭时间复合膜表面絮状颗粒增多并最终覆盖了整个表面。
2) 复合膜中含有Zn3(PO4)2·4H2O、CePO4·xH2O、CeO2和Ce(OH)4。CeO2的形成是由于氧化性极强的H2O2将Ce3+氧化成Ce4+、微阴极过程使锌层附近区域溶液中的pH增大、Ce(OH)4的饱和溶解度极小、及其稳定化脱水;CePO4·xH2O的形成是由于Zn3(PO4)2·4H2O的溶解及CePO4的饱和溶解度极小。
REFERENCES
[1] CAO J, SHAO Y W, ZHANG T, MENG G Z. Roles of zinc phosphate on the corrosion of the scratched epoxy coating[J]. Journal of Chinese Society for Corrosion and Protection, 2009, 29(6): 437-441.
[2] Palraj S, Selvaraj M, Jayakrishnan P. Effect of phosphate coatings on the performance of epoxy polyamide red oxide primer on galvanized steel[J]. Progress in Organic Coatings, 2005, 54(1): 5-9.
[3] Teixeira C H S B, Alvarenga E A, Vasconcelos W L, Lins V F C. Effect of porosity of phosphate coating on corrosion resistance of galvanized and phosphated steels. Part Ⅱ: Evaluation of corrosion resistance[J]. Materials and Corrosion, 2011, 62(9): 853-860.
[4] 林碧兰, 孔 纲, 卢锦堂. 热镀锌层上磷酸锌转化膜的生长与耐蚀性[J]. 中国有色金属学报, 2007, 17(5): 800-806.
LIN Bi-lan, KONG Gang, LU Jin-tang. Growth and corrosion resistance of zinc phosphate conversion coatings on hot dip galvanized steel[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(5): 800-806.
[5] Zeng R C, Lan Z D, Kong L H, Huang Y D, Cui H Z. Characterization of calcium-modified zinc phosphate conversion coatings and their influences on corrosion resistance of AZ31 alloy[J]. Surface and Coatings Technology, 2011, 205(11): 3347-3355.
[6] AMINI R, SARABI A A. The corrosion properties of phosphate coating on AZ31 magnesium alloy: The effect of sodium dodecyl sulfate (SDS) as an eco-friendly accelerating agent[J]. Applied Surface Science, 2011, 257(16): 7134-7139.
[7] LIN Bi-lan, LU Jin-tang, KONG Gang. Growth and corrosion resistance of molybdate modified zinc phosphate conversion coatings on hot-dip galvanized steel[J]. Transactions of Nonferrous Metals Society of China, 2007, 17(4): 755-761
[8] Lin Bi-lan, Lu Jin-tang, Kong Gang. Effect of molybdate post-sealing on the corrosion resistance of zinc phosphate coatings on hot-dip galvanized steel[J]. Corrosion Science, 2008, 50(4): 962-967.
[9] Deflorian F, Fedel M, Rossi S, Kamarchik P. Evaluation of mechanically treated cerium (Ⅳ) oxides as corrosion inhibitors for galvanized steel[J]. Electrochimica Acta, 2011, 56(23): 7833-7844.
[10] Hinton B R W, Ryan N E, Arnott D R. The inhibition of aluminum alloy corrosion by rare earth metal cations[J]. Corrosion Australians, 1985, 10(3): 12-17.
[11] Montemor M F,  A M, Ferreira M G S. Composition and behavior of cerium films on galvanized steel[J]. Progress in Organic Coatings, 2001, 43(4): 274-281.
A M, Ferreira M G S. Composition and behavior of cerium films on galvanized steel[J]. Progress in Organic Coatings, 2001, 43(4): 274-281.
[12] Huang H Y, Mansfeld M F. Evaluation of the effects of different process parameters on the corrosion protection provided by cerium-based coatings on galvanized steels[J]. Corrosion, 2009, 65(8): 507-510.
[13] 林碧兰, 卢锦堂. 硝酸铈处理对磷化膜组织和耐蚀性的影响[J]. 表面技术, 2010, 39(4): 47-49.
LIN Bi-lan, LU Jin-tang. Effect of cerium nitrate treatment on morphology and corrosion resistance of phosphate conversion coatings[J]. Surface Technology, 2010, 39(4): 47-49.
[14] Gresch R, Müller-Warmuth W, Dutz H. X-ray photoelectron spectroscopy of sodium phosphate glasses[J]. Journal of Non-Crystalline Solids, 1979, 34(1): 127-136.
[15] Aramaki K. Self-healing mechanism of a protective film prepared on a Ce(NO3)3 pretreated zinc electrode by modification with Zn(NO3)2 and Na3PO4[J]. Corrosion Science, 2003, 45(5): 1085-1101.
[16] Dabala M, Armelao L, Buchberger A. Cerium-based conversion layers on aluminum alloys[J]. Applied Surface Science, 2001, 172(3/4): 312-322.
[17] Ride D. CRC handbook of chemistry and physics[M]. Boca Raton: CRC Press, 1999: 8-111.
[18] Dean J A. Lang’s handbook of chemistry[M]. New York: McGraw-Hill, 1979: 5-7.
(编辑 李艳红)
基金项目:福建省自然科学基金资助项目(2012J05099, 2012J05101);厦门理工学院高层次人才启动项目(YKJ10021R)
收稿日期:2013-07-08;修订日期:2013-09-24
通信作者:林碧兰,副研究员,博士;电话:0592-6291326;E-mail: linbilan@xmut.edu.cn
摘 要:通过磷化/硝酸铈封闭后处理技术在镀锌层表面形成复合膜以提高其耐蚀性能。采用SEM、EDS、XPS和XRD研究复合膜的显微组织、化学成分、元素价态和相组成,并从热力学角度探讨复合膜的生长机理。结果表明:磷化镀锌层经硝酸铈封闭处理后,针片状磷化膜的间隙被铈盐膜覆盖,形成了连续完整的复合膜;在封闭过程中,部分磷化膜发生溶解,针片状磷化膜的边缘变得粗糙模糊,溶解的磷酸根离子又与Ce3+结合,生成不溶的水合磷酸铈,覆盖在复合膜表面,其中一部分水合磷酸铈与复合膜牢固结合,一部分为絮状疏松且可被漂洗下来;延长封闭时间,溶解的磷化膜增多,絮状化合物也增多并几乎覆盖整个复合膜表面;复合膜中含有Zn3(PO4)2·4H2O、CePO4·xH2O、CeO2和Ce(OH)4。
[4] 林碧兰, 孔 纲, 卢锦堂. 热镀锌层上磷酸锌转化膜的生长与耐蚀性[J]. 中国有色金属学报, 2007, 17(5): 800-806.
[13] 林碧兰, 卢锦堂. 硝酸铈处理对磷化膜组织和耐蚀性的影响[J]. 表面技术, 2010, 39(4): 47-49.
[17] Ride D. CRC handbook of chemistry and physics[M]. Boca Raton: CRC Press, 1999: 8-111.
[18] Dean J A. Lang’s handbook of chemistry[M]. New York: McGraw-Hill, 1979: 5-7.


