
J. Cent. South Univ. (2019) 26: 813-823
DOI: https://doi.org/10.1007/s11771-019-4051-5
Adsorption properties of a novel 3D graphene/MgO composite for heavy metal ions
ZHOU Ying(周颖)1, LIANG Chun-yan(梁春艳)1, YU Jin-gang(于金刚)1, 2, JIANG Xin-yu(蒋新宇)1, 2
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Hunan Province for Water Environment and Agriculture Product Safety,
Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
A novel three-dimension (3D) graphene/MgO composite was synthesized through self-assembly and embedding MgO nanoparticle in reduced graphene in situ. Fourier transform infrared (FT-IR) spectroscopy, thermal gravimetric analysis (TGA), scanning electron microscopy (SEM), transmission electron microscope (TEM), powder X-raydiffraction (XRD) and X-rayphotoelectron spectroscopy(XPS) were employed to characterize the prepared 3D graphene/MgO composite. The adsorption performance of some metal ions on 3D graphene/MgO was investigated. The results showed that the adsorption capacity was greater than 3D graphene and the maximum adsorption capacity at 25°C was found to be 358.96 mg/g, 388.4 mg/g and 169.8 mg/g for Pb2+, Cd2+ and Cu2+, respectively. The adsorption kinetic conformed to the pseudo-second-order kinetic model and the adsorption isotherm was well described by Langmuir model. The thermodynamic constants revealed that the sorption process was endothermic and spontaneous in nature. Based on the results of the removal of heavy metal ions from metal smelting wastewater, it can be concluded that the prepared 3D graphene/MgO composite is an effective and potential adsorbent.
Key words:
adsorption; heavy metal ion; 3D graphene; MgO;
Cite this article as:
ZHOU Ying, LIANG Chun-yan, YU Jin-gang, JIANG Xin-yu. Adsorption properties of a novel 3D graphene/MgO composite for heavy metal ions [J]. Journal of Central South University, 2019, 26(4): 813–823.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4051-51 Introduction
Emission of heavy metal ions into water bodies becomes more and more serious due to the increasing development of industries. Especially, the emission of untreated wastewater from smelter containing heavy metal ions was one of the most serious problems. Heavy metal ions in water will cause public health problems because of its toxicity and bioaccumulation [1]. Among these heavy metals, lead ion, cadmium ion, cupric ion and zinc ion are common poisonous. It has therefore become an urgent issue to remove heavy metals from water. Various approaches, including oxidation [2], reverse osmosis [3], chemical precipitation [4], membrane filtration [5], ion exchange [6], electrochemical treatment [7] and adsorption [8–10] have been reported to remove heavy metals in water. Some of these conventional methods suffer from flaws such as toxic sludge formation, high sensitivity to pH of solution, corrosion problems and also some of the processes are not economically feasible [11–13]. However, adsorption is considered as an effective and widely application over other methods, owing to its low cost [14], high effectiveness and easy operates [15–17]. Active carbon, magnetite, carbon nanotubes, chitosan and metal oxides have been reported as adsorbents for heavy metals.
To date, graphene has attracted increasing attention in many different fields due to their novel electronic, mechanical [18], thermal properties [19] and it is also considered as a suitable adsorbent owing to its large specific surface area, chemical stability, flexibility and π-electron rich structure [20, 21]. Pristine graphene usually is not directly used to remove heavy metals, because it can only provide van der Waals force to bind heavy metals. However, the adsorption performance can be improved by introducing functionalized groups onto it. For example, graphene oxide (GO), as a derivatives of graphene, exhibits great adsorption capacity due to the oxygen groups introduced and an extremely high surface area. Even though, there still have some problems that prevent its massive use, such as difficult segregation, easy agglomeration and poor block mechanical strength.
Recently, studies have been focused on the fabrication of 3D architectures of graphene, which possess great surface area, abundant interconnected micropores or mesopores and remain less aggregated [22]. These properties are benefit for adsorption and offer new chance to design new graphene-based materials [23, 24]. The most common method for preparing 3D graphene oxide is hydrothermal, in which process graphene oxide will be reduced to reduced graphene oxide [21], which tend to form irreversible agglomerates. By incorporation of nanomaterials into graphene sheets, the problem can be improved, meanwhile the full use of the advantages of both graphene and nanoparticles would be obtained [25]. In theory, the 3D graphene/nanoparticales gel could be prepared if the nanoparticales homogeneously are dispersed in the aqueous suspension during the reduction and self-assembly [26]. Some composite of graphene and nanoparticales, such as 3D TiO2/graphene hydrogel [27], 3D SiO2/graphene [28] and 3D graphene/MnO2 [29], have been reported to be applied in different fields. However, there is no report about the composite of graphene and magnesium oxide (MgO) nanoparticles, which have caused a lot of attention in water treatment due to its alkaline, nontoxic and environmentally friendly [30].
In the present study, a 3D graphene/MgO composite was synthesized through self-assembly and embedding MgO nanoparticle in reduced graphene in situ. The characterization results are used to evaluate the influences of MgO nanoparticle on the morphology, structure and adsorption ability of the material. In addition, the adsorption performance of lead ions onto 3D graphene/MgO was investigated. The adsorption kinetics and thermodynamics were also investigated to understand the adsorption process. Moreover, the as-prepared adsorbent was applied to the treatment of metal ions from metal smelting wastewater.
2 Materials and methods
2.1 Materials and reagents
Nature flaky graphite was purchased from Shenzhen Nanotech Port Co., Ltd. with >97% purity. Magnesium nitrate hexahydrate (Mg(NO3)2·6H2O) and urea were purchased from Tianjin Fengchuan Chemical Reagent Technologies Co., Ltd. Pb(NO3)2, Cd(NO3)2·4H2O and CuSO4·5H2O were used as the sources for heavy metals and were obtained from Tianjin Kermel Chemical Reagent Co., Ltd. All the reagents used were of analytical grade and used without further purification. Deionized water was used throughout.
2.2 Instruments and Apparatus
FT-IR spectroscopy was recorded using KBr tablets on a Nicolet 6700 FT-IR spectrometer in the range of 400–4000 cm–1. TGA was investigated by using a SDT Q600 V8.0 Build 95 thermal analyzer under air and in the range of 25–800 °C at the heating rate of 10 °C/min. SEM measurements were taken using a MIRA3 TESCAN. TEM was conducted on a Tecnai G2 F20. XRD pattern was obtained with a Rigaku D/max 2550. XPS was conducted on a VG ESCALab220i-XL. The specific surface area and porous microstructure of the materials were analyzed with nirtogen adsorption- desorption measurement and barrett-joyner- halenda (BJH) method using Kubo 1000 apparatus.
2.3 Preparation of graphene oxide (GO)
GO was prepared by oxidation of natural graphite powder according to a modified Hummers’ method [31]. Briefly, a portion of natural graphite powder (300 mg), KMnO4 (1.5 g) were placed into a 100 mL round-bottomed flask, and the mixture of 4 mL of phosphoric acid and 36 mL concentrated sulphuric acid was added into the flask dropwise in ice bath. Then the mixture was vigorously magnetic stirred at 50 °C for 12 h. After cooling to room temperature, the product was cooled by ice and subsequently added 10 mL of 30% H2O2 into the dispersion with the color changed from brown to bright yellow. The dispersion was washed with 1 mol/L HCl aqueous solution three times, and then washed and centrifuged with deionized water repeatedly until the resulting solution reached neutral. Ultimately, the product was dispersed in deionized water to obtain graphene oxide aqueous.
2.4 Synthesis of 3D graphene/MgO composite
Firstly, graphene oxide aqueous (20 mL) was transferred to a 25 mL Teflon-linedstainless-steel autoclave and kept at 180 at hool°C for 2 h. Then the product was washed three times with ultra-pure water. Secondly, amount of 0.2769 g magnesium nitrate hexahydrate and 0.1296 g urea were dissolved in 5 mL deionized water, respectively. The urea solution was then added dropwise to the solution of magnesium nitrate in ultrasonic conditions. After that, the as-prepared 3D graphene hydrogel was soaked in the solution and impregnated for 24 h, followed which the mixture was sealed in a 25 mL Teflon–lined autoclave and maintained at 180 °C for 6 h. After being cooled to room temperature, the obtained sample was washed thoroughly with ultra-pure water until to neutral. Finally, the 3D graphene/MgO composite was obtained through freeze-drying and calcination at 600 °C for 2 h.
2.5 Batch adsorption experiment
Batch adsorption experiment was performed to investigate the adsorption behavior of 3D graphene/ MgO composite for lead ions. In a typically experiment, a series of 50 mL flakes containing 5.0 mg of adsorbent and 20.0 mL solution with different concentration lead ions were placed in the thermostat shaker for a desired time in a suitable temperature. Afterwards, the adsorbent was filtered and 5 mL of residual solution was collected. The initial and final concentration of lead ions was measured by atomic absorption spectrophotometry. In adsorption kinetic experiment, 80 mg/L lead ions solution and temperature of 25 °C were selected. Each point of different time intervals (varying from 2 to 60 min) was obtained from an individual flask. In adsorption isotherm experiment, the flasks were agitated for 60 min in the temperatures 25 °C and the concentration of lead ions was from 20 to 150 mg/L. All the experimental results were an average of three replicate tests.
The amount of lead ions adsorbed (qe) and removal efficiency (R) was calculated by the following equations:
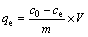 (1)
(1)
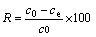 (2)
(2)
where qe (mg/g) is the equilibrium adsorption capacity; m (mg) is the mass of the absorbent used; co and ce (mg/L) are the initial and equilibrium concentrations of the lead ions, respectively; V (L) is the volume of the lead ions solution.
3 Results and discussion
3.1 Characterization of absorbent
Figure 1 shows the synthesis procedure of 3D graphene/MgO composite.
The FT-IR spectra of GO, 3D graphene and 3D graphene/MgO composite were shown in Figure 2. The characteristic peaks of GO appeared at 3402 cm–1 (O—H in carboxyl group), 1734 cm–1 (C=O in carboxyl group), 1620 cm–1 (C=C in the aromatic ring), 1394 cm–1 (C—OH group) and 1141 cm–1 (C—O—C in the epoxide group), respectively. Compared with the spectrum of GO, the characteristic peak of carboxyl group at 1734 cm–1 was almost disappeared in the spectra of 3D graphene and 3D graphene/MgO, and the intensity of other oxygenated functional groups was reduced, which indicated the reduction of GO. The characteristic bonds occurred at 589 cm–1 and 462 cm–1 confirmed the presence of Mg—O groups, which confirmed the successful synthesis of the composite.
The mass proportion of graphene and MgO was determined by TGA shown in Figure 3. All materials were treated under a heating rate of 10 °C/min in air. For the curve of MgO, the mass loss below 350 °C maybe caused by the evaporation of surface adsorbed water, crystalline water and the thermal decomposition of the Mg5(CO3)4(OH)2·4H2O. The mass loss of 3D graphene/MgO after 350 °C may be the combustion of graphene in air. Therefore, the content of graphene and MgO in the 3D graphene/MgO was 71.92% and 16.06%, respectively.
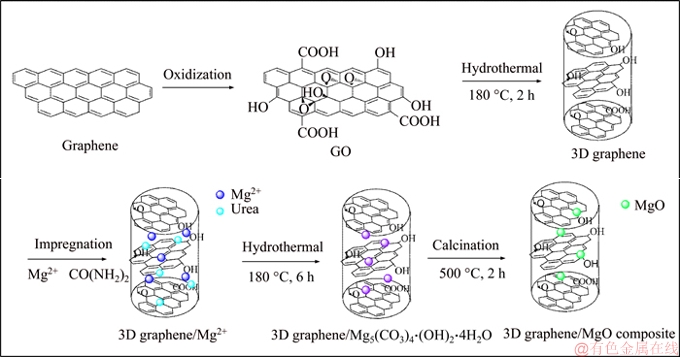
Figure 1 Schematic diagram of preparation of 3D graphene/MgO composite
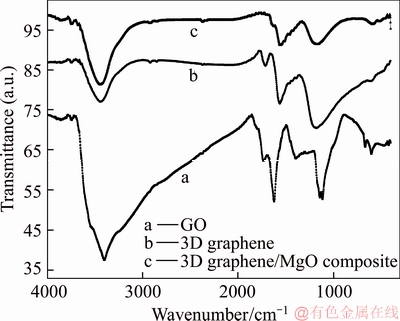
Figure 2 FT-IR curves of GO, 3D graphene and 3D graphene/MgO composite
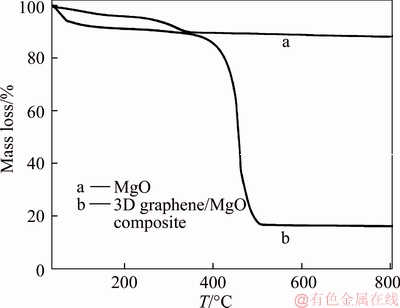
Figure 3 TGA curves of MgO and 3D graphene/MgO composite
SEM and TEM were used to investigate the morphology of the as-prepared 3D graphene/MgO. From the SEM images in Figures 4(b) and (c), it can be seen that MgO as a supported material was densely incorporated into the graphene nanosheets layers, which minimized the aggregation of grapheme sheets. The same results also can be seen in the TEM images of 3D graphene/MgO shown in Figures 4(e)–(f). Meanwhile, compared with the GO shown in Figures 4(a) and (d), the surface of 3D graphene/MgO became rougher and thicker, which may damage lattice and result in more active sites for metal ions.
The crystal structure of 3D graphene/MgO composite was investigated with XRD. As shown in Figure 5, the diffraction peaks at 42.90°, 62.26°and 77.26°in the composite corresponded to the (200), (220) and (222) planes of MgO, respectively. What’s more, the diffraction peak at 25.6° corresponded to the (002) reflection of graphitic carbon of 3D graphene. This also confirmed the existence of MgO. And the pore structure was further studied by nitrogen adsorption/desorption isotherm shown in Figure 6. The curve showed a typical IV isotherm. The calculated specific surface areas and average pore size of 3D graphene/MgO were 274.5 m2/g and 1.93 nm, respectively. The big specific surface areas and massive mesoporous were favorable for removing heavy metal ions.
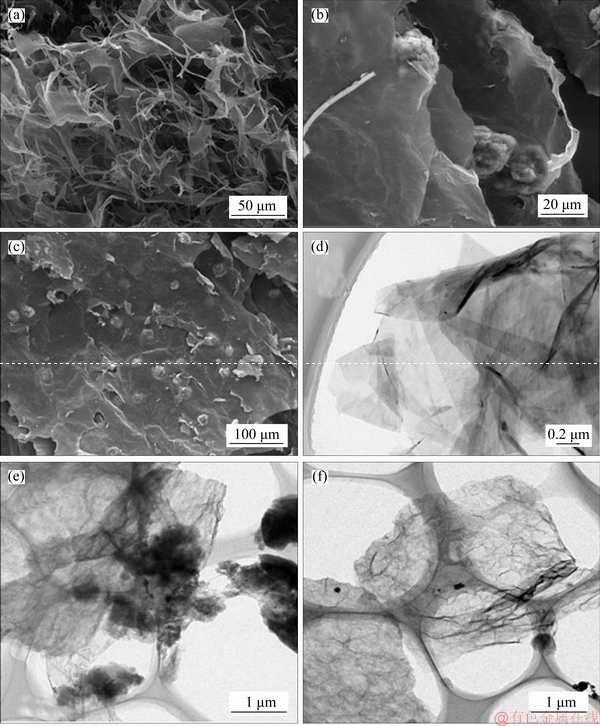
Figure 4 SEM image of GO (a), SEM images of 3D graphene/MgO composite (b, c), TEM image of GO (d), TEM images of 3D graphene/MgO composite (e, f)
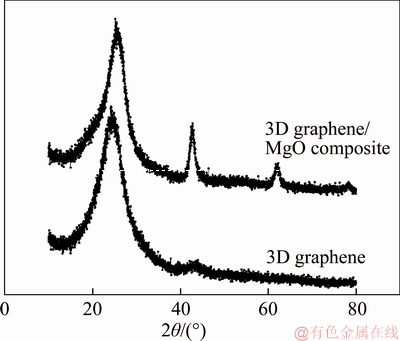
Figure 5 X-ray diffraction patterns of 3D graphene and 3D graphene/MgO composite
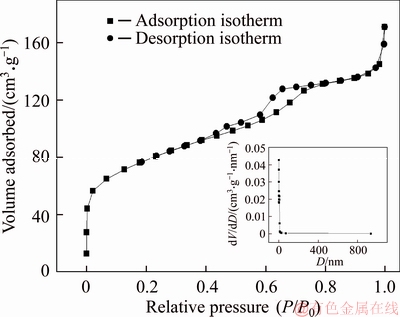
Figure 6 Nitrogen adsorption-desorption isotherm and BJH pore distribution (inset) of 3D graphene/MgO composite
XPS was conducted to investigate the surface composite and chemical state of 3D graphene/MgO. As shown in the XPS spectrum (Figure 7(a)), the peaks at 286.6, 533 and 1304 eV corresponded to C 1s, O 1s and Mg 1s bonds, indicating the incorporation of MgO into graphene. The high resolution spectrum of C 1s peak shown in Figure 7(b) was divided into four peaks at 284.9,286.0, 287.5 and 289.1 eV, represented C—C, C—O, C=O and C(O)O bonding, respectively. It was clear to see that the intensity of C=O and C(O)O peaks were very weak. This meant the reduction of GO in the hydrothermal process, which was consistent with the results of FT-IR.
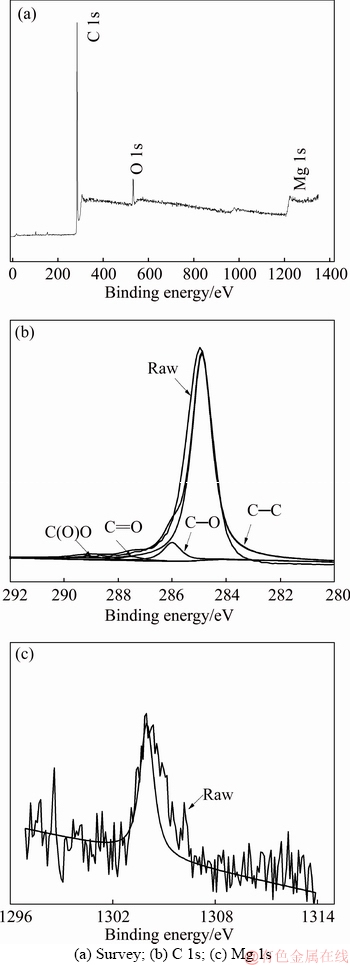
Figure 7 XPS spectra of 3D graphene/MgO composite:
3.2 Contrast experiment
The contrast experiment was conducted to investigate the performance of 3D graphene/MgO composite. The adsorption amount of 3D graphene and 3D graphene/MgO composite for Pb2+ was 93.5 mg/g and 358.96 mg/g, for Cd2+ was 11.2 mg/g and 388.4 mg/g, and for Cu2+ was 35.2 mg/g and 169.8 mg/g, respectively. It was notable that the enhance of adsorption capacity after incorporation of MgO. Therefore, the results proved that the composite was promising in removal of heavy metal ions.
3.3 Adsorption kinetics
Adsorption is a process of dynamic balance, the adsorption rate and the equilibrium time can be obtained from the adsorption kinetics study, and the kinetics parameters reveal the mechanism that controls the adsorption process. In this study, the pseudo-first-order and pseudo-second-order modelswere used to fit the kinetic data. The linear forms of the pseudo-first-order kinetic and the pseudo- second-order kinetic model are expressed as:
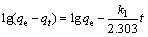 (3)
(3)
 (4)
(4)
where qt (mg/g) and qe (mg/g) stand for the amount of metal ions adsorbed at time t (min) and equilibrium, respectively; k1 (min–1) and k2 (g·mg–1·min–1) are the rate constants of the pseudo- first-order adsorption and pseudo second-order adsorption, respectively.
Figure 8 shows the kinetics of lead ions removal by 3D graphene/MgO composite and the adsorption parameters were given in Table 1. As seen from Figure 8, adsorption reached equilibrium at 10 min, therefore, the contact time of 60 min was chosen to insure the adsorption equilibrium. From Table 1, for the pseudo-first-order kinetic equation, the correlation coefficients (R2) was small (0.9056), and the experiment adsorption capacity (300.3 mg/g) was greater different with the calculated adsorption capacity (144.1 mg/g). However, for the pseudo-second-order kinetic model, the correlation coefficients (R2) was higher than 0.99, and the experiment adsorption capacity matched the calculated adsorption capacity (313.5 mg/g) well, indicating the adsorption process could described well by the pseudo-second-order kinetic model. It meant that chemical action was the rate-controlling step in the adsorption process.
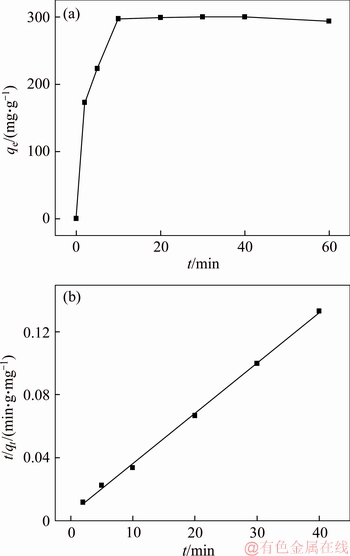
Figure 8 Effect of time on adsorption of Pb2+ onto 3D graphene/MgO composite (a) and pseudo-second-order kinetic model (b)
Table 1 Kinetic parameters for adsorption of Pb2+ on 3D graphene/MgO composite at 25 °C

3.4 Adsorption isotherm
The interaction between adsorbent and adsorbate could be described by adsorption isotherms. In this study, the experiment data were simulated by Langmuir and Freundlich isotherm model. The Langmuir isotherm model assumes amonolayer and homogeneous distribution of adsorbate on the surface of adsorbent. The liner equation of Langmuir model is expressed as
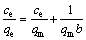 (5)
(5)
where ce (mg/L) is the equilibrium concentration; qe (mg/g) is the mass of metal ions adsorbed at equilibrium; qm(mg/g) is the theoretical maximum adsorption capacity of the adsorbent; b is the Langmuir constant. What’s more, equilibrium parameter or separation factor (RL) can help to judge whether the adsorption process is favorable. RL is defined as
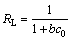 (6)
(6)
where c0 (mg/L) is the highest initial concentration of adsorbate in solution; the value of RL indicates the adsorption isotherm is unfavorable (RL>1), linear (RL=1), favorable (0<>L<1) or irreversible (RL=0).
The Freundlich model assumes a multilayer adsorption, which is expressed as
 (7)
(7)
where ce (mg/L) and qe (mg/g) are the concentration of metal ions and mass of metal ions adsorbed at equilibrium, respectively, kf (mg/g·(mg/L)n) is the constant that represents the adsorption capacity, n is the constant reflecting the degree and the favorability of adsorption. The adsorption is poor when the n values are less than 1, while the adsorption is favorable when n is between 1 and 10.
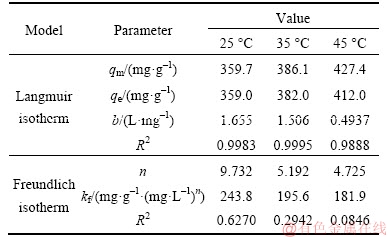
The fitting equilibrium curves from the Langmuir and Freundlich isotherm model were given in Figure 9 and relative parameters were listed in Table 2. According to Table 2, the adsorption process fitted Langmuir isotherm model better than Freundlich isotherm model, and the theoretical capacities (qm) at different temperatures in Langmuir equation were also matched well with the experiment adsorption capacity than Freundlich isotherm model, which meant a monolayer adsorption for lead ions adsorbed. Moreover, the maximum adsorption capacity calculated from the Langmuir isotherm model was 427.4 mg/g at 318 K. The values of RL (0.0037– 0.0331) showed that the adsorption was favorable.
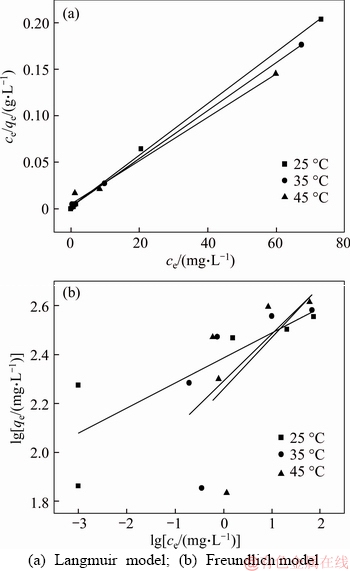
Figure 9 Adsorption isotherm for Pb2+on 3D graphene/ MgO composite:
Table 2 Langmuir and Freundlich isotherms parameters for adsorption of Pb2+on 3D graphene/MgO composite
3.5 Adsorption thermodynamics
Thermodynamic parameters, standard Gibbs free energy changes (△GΘ), standard enthalpy changes (△HΘ) and standard entropy changes (△SΘ) were used to further understand the adsorption process and calculated using the following equations:
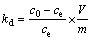 (8)
(8)
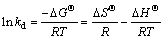 (9)
(9)
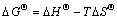 (10)
(10)
where c0 (mg/L) and ce (mg/L) are the initial and equilibrium concentrations of metal ions; V (L) is the volume of the solution; m (g) is the dosage of the adsorbent; kd is the thermodynamic equilibrium constant; T(K) is the absolute temperature and R(8.314 J/mol K) is the ideal gas constant. The values of △SΘ and △HΘ can be calculated from the intercept and slope of the plots of lnkd versus 1/T (shown in Figure 10), respectively, and are given in Table 3.
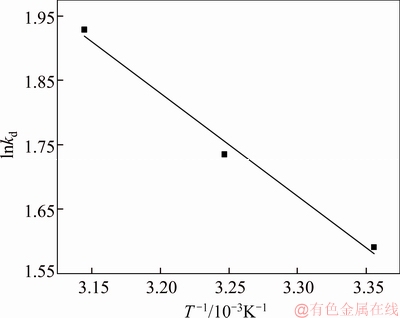
Figure 10 Plots of lnkd versus 1/T for adsorption of Pb(II)
Table 3 Thermodynamic parameters for adsorption of Pb2+ on 3D graphene/MgO composite
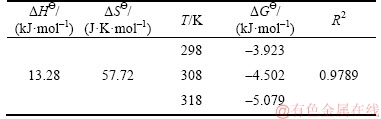
From Table 3, it was clear to see that the values of △GΘ were negative and decreased with the increase of temperature, suggesting that the adsorption was feasible and spontaneous and more favorable at higher temperature. The positive value of △HΘ indicated that the adsorption was an endothermic process and the positive value of △SΘ revealed an increasing disorder and randomness at the solid–solution interface during the adsorption process.
3.6 Application in real sample
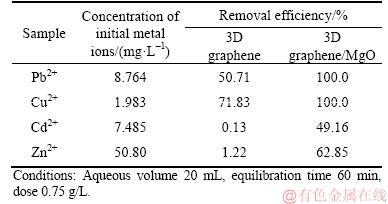
In order to investigate whether the 3D graphene/MgO composite was efficient for treating real wastewater, the real wastewater containing different concentrations of heavy metal ions was obtained from a local smelter. In brief, 15 mg of 3D graphene/MgO composite was added to 20 mL real wastewater samples at 25 °C. After adsorption equilibrium, the removal efficiency was calculated. And for comparison, the adsorption experiment of 3D graphene was performed in the same condition. The removing efficiencies of 3D graphene and 3D graphene/MgO for real heavy metal ions were calculated and listed in Table 4. From Table 4, the removal efficiencies of 3D graphene/MgO composite were 100.0%, 100.0%, 49.16% and 62.85% for Pb2+, Cu2+, Cd2+ and Zn2+, respectively. And for 3D graphene, the removing efficiencies were 50.71%, 71.83%, 0.13% and 1.22% for Pb2+, Cu2+, Cd2+ and Zn2+, respectively. It was obvious that the removal efficiencies of 3D graphene/MgO composite were great higher than those of 3D graphene and the removal efficiencies for different heavy metal ions were different. On the one hand, these different removal efficiencies may due to the selective adsorption of the 3D graphene/ MgOcomposite. On the other hand, the reason why the removal efficiencies for Cd2+ and Zn2+ were lower was because of their higher initial concentrations and saturated adsorption. From the above, 3D graphene/MgO composite was efficient for the removal of heavy metal ions in the real wastewater.
Table 4 Application and characteristics of residual heavy metal ions from metal smelting wastewater
3.7 Adsorption mechanism
The experiment data showed that the adsorption capacity was increased at 25–45 °C, indicating the co-existence of physisorption and chemisorption in the adsorption process [32]{Vukovi , 2011 #2355}. The high specific surface area and abundant pore structure of 3D graphene/MgO provided active sites for heavy metal ions, and the remnant oxygen-containing functional groups can interact with heavy metal ions, including complexation and ion exchange [33, 34]. Mg ions in the MgO crystal lattice also can exchange with Pb2+ [35] as minor magnesium ions could be detected in the solution after adsorption. However, this exchange just played a small part of the role since the concentration of Mg ions kept the same with the increasing of adsorption capacity. Therefore, GO with higher specific surface area and abundant active sites played the key role in the adsorption process.
, 2011 #2355}. The high specific surface area and abundant pore structure of 3D graphene/MgO provided active sites for heavy metal ions, and the remnant oxygen-containing functional groups can interact with heavy metal ions, including complexation and ion exchange [33, 34]. Mg ions in the MgO crystal lattice also can exchange with Pb2+ [35] as minor magnesium ions could be detected in the solution after adsorption. However, this exchange just played a small part of the role since the concentration of Mg ions kept the same with the increasing of adsorption capacity. Therefore, GO with higher specific surface area and abundant active sites played the key role in the adsorption process.
4 Conclusions
1) In the present study, for the first time, MgO was successfully incorporated into graphene sheets through a green and effective hydrothermal process, which was confirmed by lots of technologies, such as Fourier transform infrared (FT-IR) spectroscopy, thermal gravimetric analysis (TGA), scanning electron microscopy (SEM), transmission electron microscope (TEM), powder X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS).
2) The adsorption experiment showed a quick equilibrium and the data fitted the pseudo-second- order kinetic model and Langmuir isotherm well. The maximum adsorption capacities were 358.96, 388.4 and 169.8 mg/g for Pb2+, Cd2+ and Cu2+, respectively. In addition, the thermodynamic parameters indicated an endothermic and spontaneous adsorption process.
3) The adsorption experiment in real wastewater demonstrated the efficient application of the composite for removing heavy metal ions, which showed higher adsorption capacity for heavy metals than that of 3D graphene. Therefore, 3D graphene/MgO composite could be used as a promising adsorbent for removing heavy metal ions.
References
[1] WANG Jun-ping, MA Xiao-xing, FANG Guo-zhen, PAN Ming-fei, YE Xiao-ke, WANG Shuo. Preparation of iminodiacetic acid functionalized multi-walled carbon nanotubes and its application as sorbent for separation and preconcentration of heavy metal ions [J]. Journal of Hazardous Materials, 2011, 186(2): 1985–1992. DOI: 10.1016/j.jhazmat.2010.12.087.
[2] BARAKAT M. New trends in removing heavy metals from industrial wastewater [J]. Arabian Journal of Chemistry, 2011, 4(4): 361–377. DOI: 10.1016/j.arabjc.2010.07.019.
[3] CHAN B, DUDENEY A. Reverse osmosis removal of arsenic residues from bioleaching of refractory gold concentrates [J]. Minerals Engineering, 2008, 21(4): 272–278. DOI: 10.1016/j.mineng.2007.10.003.
[4] YUAN Yang, ZHANG Guang-hui, LI Yang, ZHANG Guo-liang, ZHANG Feng-bao, FAN Xiao-bin. Poly (amidoamine) modified graphene oxide as an efficient adsorbent for heavy metal ions [J]. Polymer Chemistry, 2013, 4(6): 2164–2167. DOI: 10.1039/C3PY21128B.
[5] ATALAYA J, ISACSSON A, KINARET J M. Continuum elastic modeling of graphene resonators [J]. Nano Letters, 2016, 8(12): 4196–4200. DOI: 10.1021/nl801733d.
[6] KUMAR A S K, JIANGS J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution [J]. Journal of Environmental Chemical Engineering, 2016, 4(2): 1698–1713. DOI: 10.1016/j.jece.2016.02.035.
[7] KU Y, JUNG I L. Photocatalytic reduction of Cr (VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide [J]. Water Research, 2001, 35(1): 135–142. DOI: 10.1016/S0043-1354(00)00098-1.
[8] GAO Ting-ting, YU Jin-gang, ZHOU Ying, JIANG Xin-yu. The synthesis of graphene oxide functionalized with dithiocarbamate group and its prominent performance on adsorption of lead ions [J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 71: 426–432. DOI: 10.1016/ j.jtice.2016.11.033.
[9] WANG Zhian, ZHANG Xiumei, WU Xiongwei, YU Jin-gang, JIANG Xin-yu, WU Zhi-liang, HAO Xin. Soluble starch functionalized graphene oxide as an efficient adsorbent for aqueous removal of Cd(II): The adsorption thermodynamic, kinetics and isotherms [J]. Journal of Sol-Gel Science and Technology, 2017, 82: 440–449. DOI: 10.1007/s10971-017-4313-3.
[10] ZHOU Ying, YU Jin-gang, JIANG Xin-yu. Removing lead ions from aqueous solutions by the thiosemicarbazide grafted multi-walled carbon nanotubes [J]. Water Science and Technology, 2017, 76(2): 302–310. DOI: 10.2166/wst.2017. 198.
[11] FU Feng-lian, WANG Qi. Removal of heavy metal ions from wastewaters: A review [J]. Journal of Environmental Management, 2011, 92(3): 407–418. DOI: 10.1016/ j.jenvman.2010.11.011.
[12] CHEN Na, TENG Jie, JIAO Fei-peng, JIANG Xin-yu, HAO Xin, YU Jin-gang. Preparation of triethanolamine functionalized carbon nanotube for aqueous removal of Pb(II) [J]. Desalination and Water Treatment, 2017, 71: 191–200. DOI: 10.5004/dwt.2017.20551.
[13] RAY P Z, SHIPLEY H J. Inorganic nano-adsorbents for the removal of heavy metals and arsenic: A review [J]. RSC Advances, 2015, 5(38): 29885–29907. DOI: 10.1039/ C5RA02714D.
[14] SHEELA T, NAYAKA Y A, VISWANATHA R, BASAVANNA S, VENKATESHA T. Kinetics and thermodynamics studies on the adsorption of Zn (II), Cd (II) and Hg (II) from aqueous solution using zinc oxide nanoparticles [J]. Powder Technology, 2012, 217: 163–170. DOI: 10.1016/j.powtec.2011.10.023.
[15] FUTALAN C M, KAN C C, DALIDA M L, HSIEN K J, PASCUA C, WAN Meng-Wei. Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite [J]. Carbohydrate Polymers, 2011, 83(2): 528–536. DOI: 10.1016/j.carbpol.2010.08.013.
[16] YUE Bao-yu, YU Lin-yan, JIAO Fei-peng, JIANG Xin-yu, YU Jin-gang. The fabrication of pentaerythritol pillared graphene oxide composite and its adsorption performance towards metal ions from aqueous solutions [J]. Desalination and Water Treatment, 2018, 102: 124–133. DOI: 10.5004/dwt.2018.21805.
[17] ZHOU Fang, YU Jin-gang, JIANG Xin-yu. Green synthesis of 3D porous graphene/lignin composites with improved adsorption capacity for heavy metal ions in aqueous solution [J]. Desalination and Water Treatment, 2018, 103: 175–181. DOI: 10.5004/dwt.2018.21899.
[18] ATALAYA J, ISACSSON A, KINARET J M. Continuum elastic modeling of graphene resonators [J]. Nano Letters, 2008, 8(12): 4196–4200. DOI: 10.1021/nl801733d.
[19] VARSHNEY V, PATNAIK S S, ROY A K, FROUDAKIS G, FARMER B L. Modeling of thermal transport in pillared- graphene architectures [J]. ACS Nano, 2010, 4(2): 1153–1161. DOI: 10.1021/nn901341r.
[20] HUANG Ke-Jing, YU Sheng, LI Jing, WU Zhi-Wei, WEI Cai-Yun. Extraction of neurotransmitters from rat brain using graphene as a solid-phase sorbent, and their fluorescent detection by HPLC [J]. Microchimica Acta, 2012, 176(3): 327–335. DOI: 10.1007/s00604-011-0719-8.
[21] HAN Qiang, WANG Zong-hua, XIA Jian-fei, ZHANG Xiao-qiong, WANG Hong-wu, DING Ming-yu. Application of graphene for the SPE clean-up of organophosphorus pesticides residues from apple juices [J]. Journal of Separation Science, 2014, 37(1, 2): 99–105. DOI: 10.1002/jssc.201301005.
[22] ZHOU Fang, YU Jin-gang, JIANG Xin-yu. 3D porous graphene synthesised using different hydrothermal treatment times for the removal of lead ions from an aqueous solution [J]. Micro & Nano Letters, 2017, 12(5): 308–311. DOI: 10.1049/mnl.2016.0618.
[23] KABIRI S, TRAN D N, ALTALHI T, LOSIC D. Outstanding adsorption performance of graphene–carbon nanotube aerogels for continuous oil removal [J]. Carbon, 2014, 80: 523–533. DOI: 10.1016/j.carbon.2014.08.092.
[24] ZHOU Fang, FENG Xue-zhen, YU Jin-gang, JIANG Xin-yu. High performance of 3D porous graphene/lignin/sodium alginate composite for adsorption of Cd(II) and Pb(II) [J]. Environmental Science and Pollution Research, 2018, 25: 15651–15661. DOI: 10.1007/s11356-018-1733-8.
[25] GAO Chao, YU Xin-yao, XU Ren-xia, LIU Jin-huai, HUANG Xing-jiu. AlOOH-reduced graphene oxide nanocomposites: One-pot hydrothermal synthesis and their enhanced electrochemical activity for heavy metal ions [J]. ACS Applied Materials & Interfaces, 2012, 4(9): 4672–4682. DOI: 10.1021/am3010434.
[26] CHEN Wu-feng, LI Si-rong, CHEN Chun-hua, YAN Li-feng. Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3d graphene/ nanoparticle aerogel [J]. Advanced Materials, 2011, 23(47): 5679–5683. DOI: 10.1002/adma.201102838.
[27] ZHANG Zhe-ye, XIAO Fei, GUO Yun-long, WANG Shuai, LIU Yun-qi. One-pot self-assembled three-dimensional TiO2-graphene hydrogel with improved adsorption capacities and photocatalytic and electrochemical activities [J]. ACS Applied Materials & Interfaces, 2013, 5(6): 2227–2233. DOI: 10.1021/am303299r.
[28] MENG Jing-ke, CAO Yuan, SUO Yang, LIU Yu-shan, ZHANG Jian-min, ZHENG Xiu-cheng. Facile fabrication of 3D SiO2@graphene aerogel composites as anode material for lithium ion batteries [J]. Electrochimica Acta, 2015, 176: 1001–1009. DOI: 10.1016/j.electacta.2015.07.141.
[29] LIU Jun-tao, GE Xiao, YE Xin-xin, WANG Guo-zhong, ZHANG Hai-min, ZHOU Hong-jian, ZHANG Yun-xia, ZHAO Hui-ju. 3D graphene/△-MnO2 aerogels for highly efficient and reversible removal of heavy metal ions [J]. Journal of Materials Chemistry A, 2016, 4(5): 1970–1979. DOI: 10.1039/C5TA08106H.
[30] GAO Cui-ling, ZHANG Wen-li, LI Hong-bian, LANG Lei-ming, XU Zheng. Controllable fabrication of mesoporous MgO with various morphologies and their absorption performance for toxic pollutants in water [J]. Crystal Growth and Design, 2008, 8(10): 3785–3790. DOI: 10.1021/cg8004147.
[31] LERF A, HE He-yong, FORSTER M, KLINOWSKI J. Structure of graphite oxide revisited [J]. The Journal of Physical Chemistry B, 1998, 102(23): 4477–4482. DOI: 10.1021/jp9731821.
[32] VUKOVI G D, MARINKOVI
G D, MARINKOVI A D,
A D,  KAPIN S D, RISTI
KAPIN S D, RISTI M
M  , ALEKSI
, ALEKSI R, PERI
R, PERI -GRUJI
-GRUJI A A, USKOKOVI
A A, USKOKOVI P S. Removal of lead from water by amino modified multi-walled carbon nanotubes [J]. Chemical Engineering Journal, 2011, 173(3): 855–865. DOI: 10.1016/j.cej.2011.08.036.
P S. Removal of lead from water by amino modified multi-walled carbon nanotubes [J]. Chemical Engineering Journal, 2011, 173(3): 855–865. DOI: 10.1016/j.cej.2011.08.036.
[33] ZHAO Gui-xia, REN Xue-mei, GAO Xing, TAN Xiao-li, LI Jia-xing, CHEN Chang-lun, HUANG Yu-ying, WANG Xiang-ke. Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets [J]. Dalton Transactions, 2011, 40(41): 10945–10952. DOI: 10.1039/C1DT11005E.
[34] PENG Wei-jun, LI Hong-qiang, LIU Yan-yan, SONG Shao-xian. Comparison of Pb(II) adsorption onto graphene oxide prepared from natural graphites: Diagramming the Pb(II) adsorption sites [J]. Applied Surface Science, 2016, 364: 620–627. DOI: 10.1016/j.apsusc.2015.12.208.
[35] CAO Chang-yan, QU Jin, WEI Fang, LIU Hua, SONG Wei-guo. Superb adsorption capacity and mechanism of flowerlike magnesium oxide nanostructures for lead and cadmium ions [J]. ACS Applied Materials & Interfaces, 2012, 4(8): 4283–4287. DOI: 10.1021/am300972z.
(Edited by HE Yun-bin)
中文导读
一种新的三维石墨烯/MgO复合物对重金属离子的吸附性能研究
摘要:通过水热法在石墨烯自组装和原位还原的过程中嵌入MgO制备了一种新的三维石墨烯/MgO复合物。采用FT-IR、TGA、SEM、TEM、XRD和XPS等测试手段对制备的材料进行了表征。研究了三维石墨烯/MgO复合物对一些金属离子的吸附性能。研究结果表明复合物材料的吸附性能优于三 维石墨烯,25°C时对Pb2+、Cd2+和Cu2+的最大吸附量分别为358.96 mg/g、388.4 mg/g和169.8 mg/g,吸附过程符合准二级动力学和Langmuir等温吸附模型。热力学参数表明吸附过程是一个吸热自发的过程。将三维石墨烯/MgO复合物用于冶炼厂废水样中重金属离子的去除,结果显示材料具有很好的去除效果。
关键词:吸附;重金属离子;三维石墨烯;MgO
Foundation item: Projects(21571191, 51674292) supported by the National Natural Science Foundation of China; Project(2016JJ1023) supported by the Natural Science Foundation of Hunan Province, China; Project(2018TP1003) supported by the Key Laboratory of Hunan Province for Water Environment and Agriculture Product Safety, China
Received date: 2017-11-22; Accepted date: 2018-09-07
Corresponding author: JIANG Xin-yu, PhD, Professor; Tel: +86-731-88879616; E-mail: jiangxinyu@csu.edu.cn; ORCID: 0000-0002- 3680-476X
Abstract: A novel three-dimension (3D) graphene/MgO composite was synthesized through self-assembly and embedding MgO nanoparticle in reduced graphene in situ. Fourier transform infrared (FT-IR) spectroscopy, thermal gravimetric analysis (TGA), scanning electron microscopy (SEM), transmission electron microscope (TEM), powder X-raydiffraction (XRD) and X-rayphotoelectron spectroscopy(XPS) were employed to characterize the prepared 3D graphene/MgO composite. The adsorption performance of some metal ions on 3D graphene/MgO was investigated. The results showed that the adsorption capacity was greater than 3D graphene and the maximum adsorption capacity at 25°C was found to be 358.96 mg/g, 388.4 mg/g and 169.8 mg/g for Pb2+, Cd2+ and Cu2+, respectively. The adsorption kinetic conformed to the pseudo-second-order kinetic model and the adsorption isotherm was well described by Langmuir model. The thermodynamic constants revealed that the sorption process was endothermic and spontaneous in nature. Based on the results of the removal of heavy metal ions from metal smelting wastewater, it can be concluded that the prepared 3D graphene/MgO composite is an effective and potential adsorbent.


