
Nucleation and growth of high purity aluminum grains in directional solidification bulk sample without electromagnetic stirring
ZHANG Jiao(张 佼)1, 2, SHU Da(疏 达)1, RAO Qun-li(饶群力)3,
SUN Bao-de(孙宝德)1, CHEN Gang(陈 刚)4
1. State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200030, China;
2. School of Materials Science and Engineering, Hebei University of Technology, Tianjin 300132, China;
3. Instruments Analysis Center, Shanghai Jiao Tong University, Shanghai 200030, China;
4. Analysis and Testing Center For Inorganic Materials, Shanghai Institute of Ceramics Chinese Academy of Sciences,
Shanghai 200037, China
Received 26 April; accepted 25 July 2005
Abstract: A self-made directional solidification device was used to fabricate d 80 mm high purity aluminum ingots. SEM and AFM were used to detect the shape of grain boundaries. The orientation of the grain was studied by X-ray diffractometry. The results show that the nucleation points locate at the intersections of three adjacent grains. The lattice orientation of grains does not alter in the horizontal direction, but gradually approaches the optimum growth direction in the vertical direction during the growth process. All the grains suffer the competition and only the one whose orientation is closest to the preferred direction can occupy the final growth space.
Key words:
high purity aluminum; directional solidification; grain nucleation; lattice orientation; competitive growth;
1 Introduction
The crystal properties were determined by grain morphology and lattice orientation in directional solidification[1]. In fact the grain growth is a complex dynamic 3-D process affected by the temperature field, solute field and flow field. However, most of the solidification processes undergo at high temperature and are unobservable, it is very difficult to investigate the grain growth. Just for this, the model transparent alloy of succinonitrile-acetone is often used for observation of the dynamic process[2-4]. In experiments, the transparent alloy is filled in the narrow gap between two glass slides so that the solidification more or less approximately occurs in two dimensions. The results show that the lattice orientation will gradually deviate from the direction of the temperature gradient as the growth rate increases. Because the 2-D growth is quite different from the real materials processing conditions, No?l et al[5, 6] and Kauerauf et al[7, 8] investigated the growth process of 3-D cylindrical samples, and observed that new grains nucleated at grain boundaries, and the competition growth occurred during the polycry- stalline cell growth. But the sample was still small and was only 10 mm in diameter.
Until now, the researches on high purity aluminum (HPA) solidification always focus on the influence of solidification mode on macro concentration distribution in ingots. Osono et al[9] employed a cold copper crucible induction furnace to prepare HPA under ultra-high vacuum condition, d60 mm single crystal was obtained and the purity of ingot was determined by residual resistance ratio. Although the single crystal grew up through grains competition, the nucleation and growth process were not discussed in his work. Gandin[10] compared the solidification morphology of bulk ingots of 99.99% aluminum with Al-Si alloy fabricated by simple directional solidification device, and put the emphasis only on the columnar-to-equiaxed transition. FAN and LI[11] researched the lattice orientation of continuous casting d8 mm stick. Because of the small size in diameter and high velocity in growth, the results can not completely show the whole process of competition growth. The other researches on crystalline texture and lattice[12, 13] were only concentrated on the deformation process.
In the present work, d 80 mm HPT ingots were fabricated under different growth rates by a self-made directional solidification configuration. Through the detection of AFM and XRD, the characteristics of grain nucleation and growth in HPT bulk ingots were studied.
2 Experimental
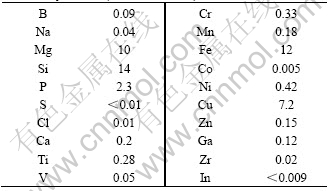
The raw material used in the present work was 99.99% aluminum produced by three-layers refined method. Table 1 lists the constitution analyzed by glow discharge mass spectrometry (GDMS) for 20 elements. The raw materials was cut to suitable size and etched by HF-HNO3 solution, then rinsed in distilled water and ethyl alcohol, respectively.
Table 1 Inpurity components of 99.99% pure aluminum detected by GDMS (mass fraction, %)
The material was melted and solidified by the self-made directional solidification configuration as shown in Fig.1, and the crucible used here was made of 99.999% graphite. Fig.1(a) shows the structure sketch of the furnace, and Fig.1(b) shows the structure of solidification part. According to the analysis of hot zone, adiabatic zone and cold zone, this device can be regarded as Bridgman configuration that the thermal conductivity coefficient of the crucible wall changes along with the height. The small coil at the bottom of the crucible acts as booster heater[14]. With the small coil heating, the solid-liquid interface will present convex platform, otherwise, the interface displays concave meniscus. The S/L interface with or without small coil heating marked by the growth striation is shown in Fig.2.
The investigation on the lattice orientation of the grains is performed by X-ray diffraction (Cr Ka) pole figures which were obtained by ![]() -scan method. The sample’s position is confirmed by
-scan method. The sample’s position is confirmed by ![]() ,
, ![]() and
and ![]() . In this experiment,
. In this experiment, ![]() is 54.74°, while
is 54.74°, while ![]() , i.e. half of the 2q, is 32°. When the value of
, i.e. half of the 2q, is 32°. When the value of ![]() changes from 0° to 360°, the data collection was carried at an interval of 5°. After that, the pole figure can be calculated according to 72 groups of data. The real S/L interface was obtained by outpouring the residual melt during crystal growth, and the interface was scanned by atom force microscope (AFM) and SEM. The ingots were heated at 650℃ to melt the grain boundaries and then the columnar grains were peeled off[15].
changes from 0° to 360°, the data collection was carried at an interval of 5°. After that, the pole figure can be calculated according to 72 groups of data. The real S/L interface was obtained by outpouring the residual melt during crystal growth, and the interface was scanned by atom force microscope (AFM) and SEM. The ingots were heated at 650℃ to melt the grain boundaries and then the columnar grains were peeled off[15].
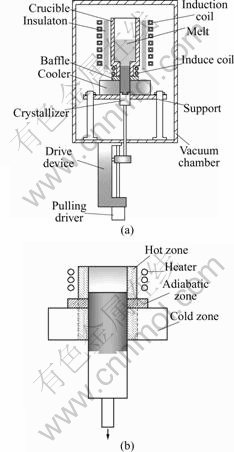
Fig.1 Sketch of self-made experiment setup: (a) Structure sketch of furnace; (b) Structure of solidification part
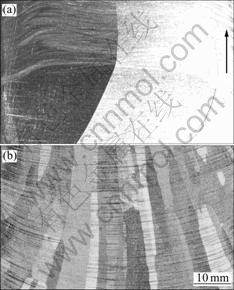
Fig.2 Influence of small coil on S/L interface shape during crystal growth (arrow indicates growth direction): (a) With small coil; (b) Without small coil
3 Results and discussion
3.1 Grain nucleation
The ingots grown at different rates were cut along the cylindrical axis and the surface was etched by HF- HNO3 solution in order to reveal the grain structure as shown in Fig.3. From metallographic analysis, it is found that the grain size in the bottom part of the ingots is only several millimeters and these small grains can not grow up finally. New grains nucleate at the corners of adjacent grains layer-by-layer until the large grain appears. In addition, during the whole growth process, no bifurcation appears on the top of the grain which divides the original into two new grains. When the small grains at the bottom grow to a certain extent, the grain combination phenomenon that the growth space of several small grains is replaced by a new large grain occurs, as shown in Fig.4. On the other hand, continuous and in situ observation of the solid-liquid interface by optical methods was carried out through the transparent alloy in Refs.[5, 6], and it is found that the grooves of grains can propagate to form new grain boundary, at the same time, new grains also nucleate at the grain boundary grooves. Comparing with the solid-liquid interface and metallograph in this case, however, does not occur.
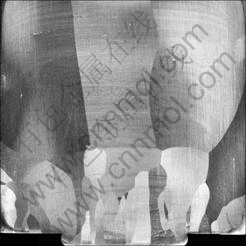
Fig.3 Metallograph of longitudinal section of d 80 mm ingot
Based on the fact that the nucleation point of grain is always located at the corner of grain boundary, in order to investigate the grain nucleation conditions, the solid-liquid interface was obtained by outpouring method and scanned by SEM and AFM respectively. The photos are shown in Figs.5 and 6. It is clear from
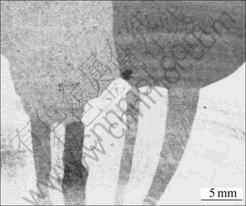
Fig.4 Metallograph showing grain combination during solidification
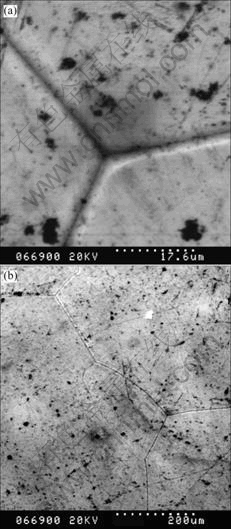
Fig.5 Morphologies of growth interface obtained by outpouring method: (a) Grain boundary on S/L interface; (b) Grain grown at three-grain corner
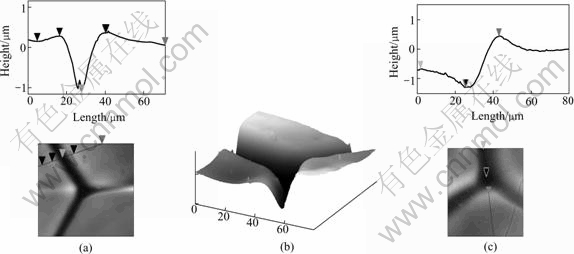
Fig.6 Section analysis of grain boundaries of bi-grain and tri-grain by atomic force microscope: (a) Contour of bi-grain boundary; (b) Morphology of tri-grain boundary intersection; (c) Contour of tri-grain boundary intersection
Fig.5(a) that the interface looks smooth and ridge–free except the oxide contamination and grain boundaries. Because of the low impurities concentration, most of the impurities accumulate at the boundary, and there are not so many impurities to form groove on the top of grain. Through analyzing the distribution of grains, new grain nucleating at the triple-grain corner can be found as shown in Fig.5(b). At the primary stage of growth, the new grain grows along the boundary grooves arrowed in Fig.5. After a period of development, the boundary of new grain begins to bulge to original grain and occupy its growth space.
Under AFM, the grain boundaries intersect the interface and form grooves deeply in the solid while the solid regions sides adopt a sand-dune shape. This situation is similar to that observed in Refs.[5, 16]. But the limitation of their experiments hindered them to study the details of the grooves and the dune. Through the detection of AFM, the cross-section of the grooves is figured out and the height and width of the dune is measured in the present work, as shown in Fig.6. It can be seen from Fig.6(a) that the dune of the boundary side is 0.3 mm in height and 1.1 mm in width. The height from the bottom of groove to the top of the dune is 1.1 mm, and the width between the peaks of the two dunes can reach 27 mm. Fig.6(b) shows the intersection of the three grains. It is obvious that the height and shape of the three contiguous grains are different. However, the rim of the boundary grooves all presents smooth arc. The exact measurement of the three grains intersection is shown in Fig.6(c). The measurement results indicate that the trap at the intersection is 1.7 mm and about 0.5 mm deeper than the two grain boun- daries, the dune is wider and higher, too. As a matter of course, there must be a layer of melt adhere to the groove wall when pouring the melt, so it is reputed that the real width and depth of boundary groove are larger than the values measured in the present experiments.
The trap and the grooves are actually full of melt which is rich of impurity and has the low melting point. The melt in the three grains intersection trap receives the solute segregated from solid around it, and its concentration is larger than that of the two grains boundary groove. This causes the trap of intersection to become deeper than groove between two grains. During directional solidification, there exists positive thermal gradient in the solid and the melt in front of the S/L interface. The tip of the trap which penetrates into the low temperature part of the solid has lower temperature than the interface. Because of the small space, the melt stays in the trap without flow. Under this condition, only two kinds of solidification mode can occur. Firstly, if the thermal field is steady, the cross growth of circumjacent grains forces the atoms of the melt to arrange along their lattice structure. Secondly, if the thermal field is unstable, the constitutional supercooling caused by temperature fluctuation will nucleate new grains, and then the melt freezes along the new grains growth. As shown in Fig.7, the complete grains obtained by peeling off ingots have a conic bottom composed by three or more slant faces. All the slant faces display the limitation of the wall of the neighboring grains to the solidification process of the melt in the trap.
3.2 Competitive growth of grains
When the new grain nucleates at the top of the trap, the grain will grow along the wall of the grooves. If the temperature field keeps steady, the grain develops like that shown in Fig.8(a). If the tempera- ture field is suitable to form convex solid-liquid interface, the newborn grain will grow like that shown in Fig.8(b), and at the same time the morphology of the boundary interfaces changes concomitantly. From Fig.3, it can be deducted that all the grains will suffer the competition growth from nucleation to end. Fig.7 displays the cross-sections of the ingot at different heights, and the development of the three grains marked in the figures demonstrates the competition progress. In the 2-D and cylindrical crucible experiments of transparent alloy[2, 6], the similar phenomenon also appeared. Due to the difference in height of the grains around the new born, the new grain will occupy the growth way of the lower grain if the lattice orientation of the newborn grain is closer to the normal direction of (100) face. If several grains are suppressed, the combination of grains will occur.
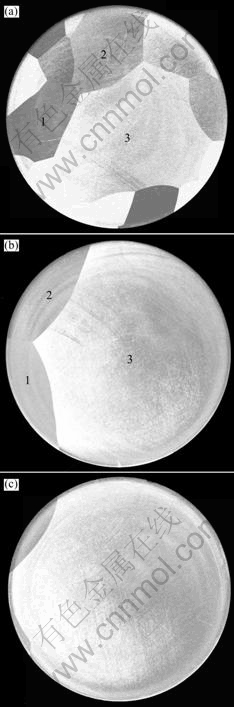
Fig.7 Cross-sections at different heights of d80 mm ingot grown at velocity of 10 mm/h: (a) 30 mm; (b) 60 mm; (c) 80 mm
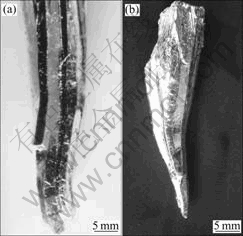
Fig. 8 Complete grains peeled off from ingots(A few grains stick on the large grain in Fig.8(a))
In this paper, different growth velocities are adopted to investigate the competition development. The results indicate that the progress is different from each other when the growth velocity alters, as shown in Fig.9. The faster the growth rate is, the longer the distance is needed for achieving a single crystal. Although the development of grains depends on the growth rate, the trend of competition growth will not change.
3.3 Change of lattice orientation during growth
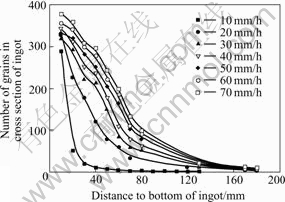
Fig.9 Relationship between number of grains of cross-section at different heights and growth velocities
Al is face centered cubic, and the preferred growth direction for aluminum crystal is [100][17], as in other fcc metals. When the melt freezes on the crystallizer, a layer of fine equiaxed grains firstly forms at the bottom part of the ingot. While the solidification continues, the new grains are born and develop into columnar grains. The growth direction of the columnar grains is near [100], and the only difference between grains is that the angle between growth direction and the [100]. The grain with the smallest angle has the strongest competition ability. As shown in Fig.7, there are fourteen grains at 30 mm height, but only grains 1, 2 and 3 can survive from the competition after 30 mm growth. Among the three grains, there is no doubt that the grain 3 occupies the most of space, and only the grain 3 develops into a single crystal finally. Fig.10 shows (100) pole figures of the three grains. The growth direction of grain 3 is the closest to the preferred direction, and the included angle reaches 79°. The included angle of grains 1 and 2 are far smaller and are only 45° and 61° respec- tively. On the other hand, the included angles of the certain grain is not changeless, it moves towards the preferred direction little by little during the growth process. For example, the angle of grain 3, it increases from 79° at 30 mm height to 83° at 130 mm.
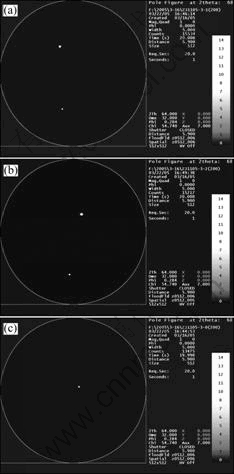
Fig.10 (100) Pole figure of three adjacent grains marked in Fig.7(a): (a) Grain 1; (b) Grain 2; (c) Grain 3
Another point must be addressed that the lattice orientation in horizon does not alter any more during the competition growth process, although the grains look twisted and rotated.
Similar to the results in Ref.[2], the growth velocity can also affect the lattice orientation of the grain grow under 3-D condition. By comparing the lattice orientation of preferred grains grown at different velocity, it can be found that the included angle between growth direction of grain and [100] will expand as the growth velocity increases, as shown in Fig.11.
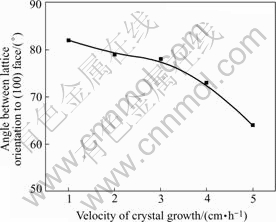
Fig.11 Lattice orientation dependence on growth velocity
4 Conclusions
1) When the HPA grains develop into columnar grain under the 3-D condition, the nucleation point is located at the intersection of three grains.
2) All the primary grains of the large diameter ingot fabricated by directional solidification suffer the competition growth, and there always exists a grain that can develop into the preferred grain under the suitable conditions. The preferred grain will become a single crystal finally with the diameter equivalent to the ingot.
3) The growth directions of the primary columnar crystals point to [100] and shift different degrees, and the grain which growth direction shifts the fewest degrees from the preferred direction will develop into the preferred one.
4) As the growth velocity increases, the growth direction of the preferred grain will deflect from [100]. For a grain grown at the fixed velocity, the growth direction will approach to [100] little by little during the growth process.
References
[1] ZHOU Yao-he, HU Zhaung-qi, JIE wan-qi. Solidification Technology[M]. Beijing: China Machine Industry Press, 1998. (in Chinese)
[2] Pocheau A, Georgelin M. Cell tip undercooling in directional solidification[J]. Journal of Crystal Growth, 1999, 206(2): 215-229.
[3] GUO Tai-ming, LI Chen-xi. Characteristics of irregular interface pattern during unidirectional solidification[J]. Journal of Synthetic Crystals, 2003, 32(4): 495-499.
[4] Nagashima K, Furukawa Y. Nonequilibrium effect of anisotropic interface kinetics on directional growth of ice crystals[J]. Journal of Crystal Growth, 1997, 171(4): 577-585.
[5] No?l N, Jamgotchian H, Billia B. Influence of grain boundaries and natural convection on microstructure formation in cellular directional solidification of dilute succinonitrile alloys in a cylinder[J]. Journal of Crystal Growth, 1998, 187(4): 516-526.
[6] No?l N, Jamgotchian H, Billia B. In situ and real-time observation of the formation and dynamics of a cellular interface in a succinonitrile-0.5wt% acetone alloy directionally solidified in a cylinder[J]. Journal of Crystal Growth, 1997, 181(1): 117-132.
[7] Rex S, Kauerauf B, Zimmermann G. Directional solidification of cellular arrays in a transparent organic alloy[J]. Adv Space Res, 2002, 29(4): 511-520.
[8] Kauerauf B, Zimmermann G, Rex S. Directional cellular growth of succinonitrile-0.0075wt% acetone bulk samples. Part 1: Results of space experiments [J]. Journal of Crystal Growth, 2001, 223(2): 265-276.
[9] Osono H, Maeta H, Matsusaka K. Preparation of high perfect aluminum crystal by cold-crucible induction melting in ultra-high vacuum[J]. Materials Transactions, 2002, 43(2): 121-124.
[10] Gandin C A. From constrained to unconstrained growth during directional solidification [J]. Acta Mater, 2000, 48(2): 2483-2501.
[11] FAN Xin-hui, LI Jian-guo. Structure evolution in solidification of CCSC[J]. Chinese Journal of Materials Research, 1999, 4(2): 347-353. (in Chinese)
[12] XIAO Ya-ping, ZHANG Xin-ming, et al. Textures in high purity aluminum foils and AA3004 sheets[J]. Trans Nonferrous Met Soc China, 2003, 13(3): 491-498.
[13] Engler O, Huh M Y. Evolution of the cube texture in high purity aluminum capacitor foils by continuous recrystallization and subsequent grain growth[J]. Mater Sci Eng A, 1999, 271(2): 371-381.
[14] Rosch W R, Fripp A L, et al. Performance testing of a vertical Bridgman furnace using experiments and numerical modeling[J]. Journal of Crystal Growth, 1997, 174(1): 139-152.
[15] Meilan Z, Zhixi C. Study on quantitative relation between grain size and topologic parameter of super-pure aluminum[J]. Journal of Zhejiang University of Technology, 2001, 29(1): 17-23.
[16] Ayers J J, Schaefer R J. Solidification and Casting of Metals[M]. London: The Metals Society, 1979.
[17] Mondolfo L F. Aluminum Alloys: Structure and Properties[M]. New York: Butterworth Press, 1976.
Foundation item: Project(2002AA6070) supported by the Hi-tech Research and Development Program of China
Corresponding autor: SUN Bao-de; Tel: +86-21-62932870; E-mail: bdsun@sjtu.edu.cn
(Edited by LONG Huai-zhong)


