Trans. Nonferrous Met. Soc. China 26(2016) 2257-2262
High-temperature diffusion behavior of ZrC in C matrix and its promotion on graphitization
Zhong-wei ZHANG1,2, Qiang ZHEN3, Feng ZHENG3, Fei LU3, Ce-wen NAN1, Rong LI3, Jun-shan WANG2
1. State Key Laboratory of New Ceramics & Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China;
2. Science and Technology on Advanced Functional Composites Laboratory, Aerospace Research Institute of Materials and Processing Technology, Beijing 100076, China;
3. Research Center of Nano Science and Technology, Advanced Material Composite and Dispersion Technology Engineering Research Center of Ministry of Education of China, Shanghai University, Shanghai 200444, China
Received 27 August 2015; accepted 14 June 2016
Abstract:
C/C composite material is widely used in aerospace field and others, however, it is easy to be oxidized at high temperature. In order to improve the oxidation resistance, ZrC is introduced as an oxidation inhibitor used in matrix modification of C/C composite material. Flat plate samples of ZrC/C composite materials were prepared by hot-pressing sintering. The degree of graphitization increases with rising sintering temperature, and layer structure of carbon matrix is observed clearly in the sample treated at 2273 K. Diffusion behavior of Zr in C matrix at high temperature is studied, which can be generally expressed as D=3.382×10-11 exp[2.029×105/(RT)]. The diffusion of Zr in C matrix leads to the over-saturation of C in the micro area and the oversaturated C precipitates as graphite. This continuous process promotes the transformation of carbon to graphite.
Key words:
ZrC; carbon material; diffusion; graphitization;
1 Introduction
Carbon materials (graphite, carbon/carbon composites) are considered to be promising light-weight, high temperature-resistant structural materials [1,2], which are widely used in the advanced technology fields of aeronautic and aerospace due to the excellent electrical conductivity, thermal conductivity, low thermal expansion coefficient, and high-temperature strength retention [3,4]. However, the carbon materials are easily oxidized in the high-temperature and oxidized environment, which leads to poorer performance and limited application. To improve the anti-oxidation property of carbon materials, the generally accepted method is the use of oxidation resistant coating [5,6] or matrix modification [7-9]. ZrC not only has higher melting point (>3000 °C) and better compatibility with carbon, but also the oxide itself has enough high melting point and relatively low vapor pressure. It has been confirmed that the ablative rate of ZrC-modified C/C composite is decreased obviously as compared with that of original material, which results in the enhanced ablation resistance of the C/C composite [10-12]. Moreover, it is also discovered that ZrC could catalyze and induce graphitization at high temperatures [13,14].
The graphitization degree is one of the most important structural parameters for carbon materials. After high temperature graphitization treatment, the microstructure of carbon materials could be improved to obtain high electrical conductibility, heat conductivity and mechanical property [15-17]. Nowadays, few efforts were taken on the effect of ZrC modified carbon materials on the microstructure, and the mechanism of the ZrC catalytic graphitization was rarely investigated. In this work, flat plate sample of ZrC/C composite material was successfully prepared, and the diffusion kinetic behavior of Zr in C at high temperature was studied. Furthermore, the mechanism of ZrC promotion on graphitization of carbon was also discussed.
2 Experimental
2.1 Synthesis of ZrC modified carbon material
Flat plate sample of ZrC modified carbon material was prepared by the hot-pressing sintering method. In a typical synthesis, ZrC powder was compressed into a wafer (d=10 mm, h=3 mm) at around 300 MPa, and then the ZrC wafer was embedded in the active carbon and sintered at 1600 °C for 4 h at a heating rate of 5 °C/min with the protection of high purity argon. After that, the ZrC wafer was transferred into a graphite mould. To avoid contamination, a layer of carbon paper was adhered to the inner wall of the graphite mould and a small amount of carbon powder was added to it in advance. Then, the graphite mould was placed in a hot-pressing sintering furnace with an axial pressure of 30 MPa. Finally, the graphite mould was first calcined to 473 K at a heating rate of 5 K/min, maintained at that temperature for 180 min, followed by a second calcination at a heating rate of 10 K/min up to 2073, 2173 and 2273 K, respectively (holding time, 180 min), and then it was naturally cooled to room temperature. It was noted that the high purity argon was used as the protective atmosphere in the whole sintering process.
2.2 Characterization
The fracture micro-morphology of the flat plate sample of ZrC modified carbon composite was observed on a field emission scanning electron microscope (FESEM, JSM-6700F). Line scanning and spot sampling of fracture section were determined using energy dispersive spectrometry (EDS) equipped on FESEM.
3 Results and discussion
3.1 Microstructure of flat plate sample
Figure 1 shows the SEM images of C matrix layers, ZrC layers and their interface layers of fracture section of flat plate samples treated at different temperatures. From Figs. 1(b, e, h), it can be seen that with increasing of the heat treatment temperature, the layer structures could be observed in the C matrix, especially that treated at 2273 K, indicating that the graphitization degree of the C matrix could increase with the rise of heat treatment temperature. However, no significant changes are discovered in the ZrC layers as temperature is increased in Figs. 1(c, f, g). In addition, the ZrC flat plates are bonded tightly to C matrix in the diffusion interface layers, as shown in Figs. 1(a, d, g).
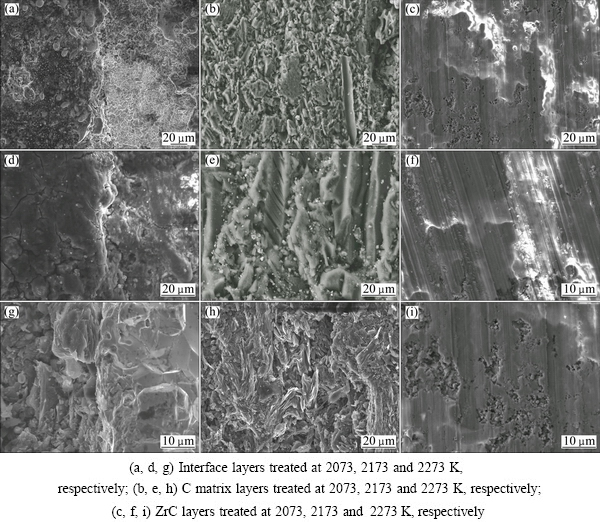
Fig. 1 Microstructures of ZrC/C samples sintered at high temperatures
3.2 Diffusion of Zr in C matrix
Figure 2 shows the schematic plan of flat plate model of ZrC modified C matrix. In the study of Zr diffusion behavior, we assume that the contact diffusion plane of Zr element is infinitely large in two dimensions, and the Zr element diffuses along one dimensional direction vertical to the diffusion plane.
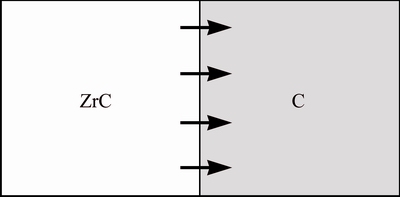
Fig. 2 Schematic plan of ZrC/C flat plate mode
Figure 3 shows the line scanning energy spectrum of ZrC modified C matrix flat plate sample after the high temperature treatment (HTT). Due to the presence of C element in ZrC, only the diffusion behavior of Zr element in C matrix is considered and that of C element is excluded. From Fig. 3, we know that the content of Zr element gradually declines as the diffusion distance increases using the interface as the baseline, and δ is the reaction layer thickness of the Zr element diffusion in the C matrix.
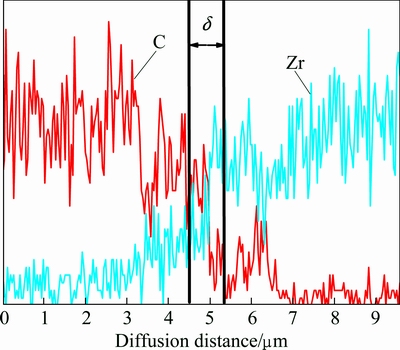
Fig. 3 Line scanning EDS pattern at ZrC-C intersurface
Assuming that the content of Zr element is 100% on the ZrC diffusion interface side, then the content of Zr element is 0 on the C matrix side. Figure 4 shows the relation curve between the diffusion distance and content of Zr element in the C matrix. From Fig. 4, the diffusion depth of Zr element increases with the increasing of the heat treatment temperature, and at the same time, the content of Zr element increases at various diffusion distances. These results suggest that the temperature plays an important role in promoting Zr element diffusion. The reason could be explained as follows: with the rise of the temperature, the thermal motion of Zr element will be strengthened and the movement velocity will be accelerated, which results in the longer diffusion distance.
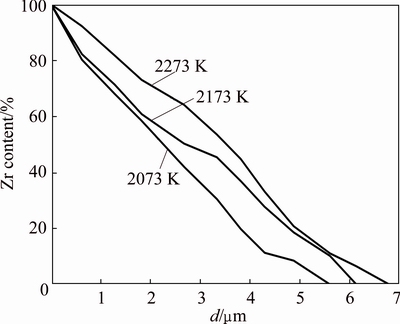
Fig. 4 Zr content as a function of diffusion distance
The diffusion process between ZrC and C matrix belongs to solid mass transfer. The expression of diffusion coefficient could be written as Eq. (1) because vacancy diffusion and interstitial diffusion are the main diffusion phenomenon [18]:
D=D0exp[-Q/(RT)] (1)
where Q is the diffusion activation energy; D0 is the frequency factor; R is the gas constant with a value of 8.3143 J/(mol·K); T is the absolute temperature.
Based on the condition of diffusion region, the expression of diffusion coefficient is changed by BHANUMURTHY et al [19]:
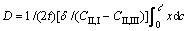 (2)
(2)
where t is the diffusion time, δ is the reaction layer thickness, (CII,I-CII,III) is the content difference across the reaction layer, where CII,I is the content of phase II in the boundary phase I-II and CII,III is the content of phase II in the boundary phase II-III, and c' is the average content of reaction layer interface.
The reaction layer width, δ, and the diffusion coefficient, D, treated at different temperatures are given in Table 1.
Equation (1) of diffusion coefficient could be transform to
ln D=ln D0-[Q/(RT)] (3)
Figure 5 shows the relationship between ln D and the temperature. The diffusion activation energy, Q=2.029×105 J/mol, and the frequency factor, D0= 3.382×10-11 m2/s, of the Zr element in the C matrix could be calculated from Fig. 5, therefore, the diffusion coefficient could be expressed as
D=3.382×10-11exp[2.029×105/(RT)] (4)
Table 1 Reaction layer δ and diffusion coefficient D of Zr in C matrix at different temperatures
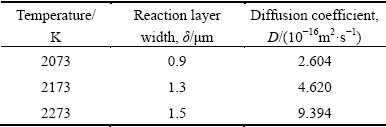
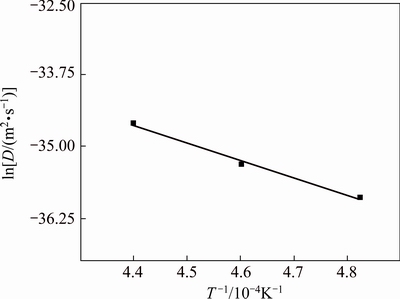
Fig. 5 Relation between ln D and 1/T
3.3 Mechanism of ZrC promotion on C graphitization
The micro-morphology of C matrix of ZrC doping carbon materials is shown in Fig. 6. It can be seen that the carbon crystallites around ZrC have high graphitization degree and good orientation arranged in layer structure, indicating that ZrC has catalytic effect of graphitization for carbon.
The catalytic graphitization is a complex process with both physical and chemical changes. Based on the above analysis, the Zr element has an obvious diffusion behavior in the C matrix. Besides, the diffusion behavior shows significant change at the higher temperature. The phase diagram of Zr-C binary system is shown in Fig. 7. A relatively wide ZrCx solid solution region could be observed. The Zr element diffuses and then the content of Zr reaches a low level, which results in the precipitation of elemental carbon with the formation of graphite. In additional, it can also be seen that the ZrCx solid solution region decreases with the increasing of the temperature, which indicates that the carbon is easy to supersaturate at high temperature and then it precipitates from the ZrCx solid solution region.
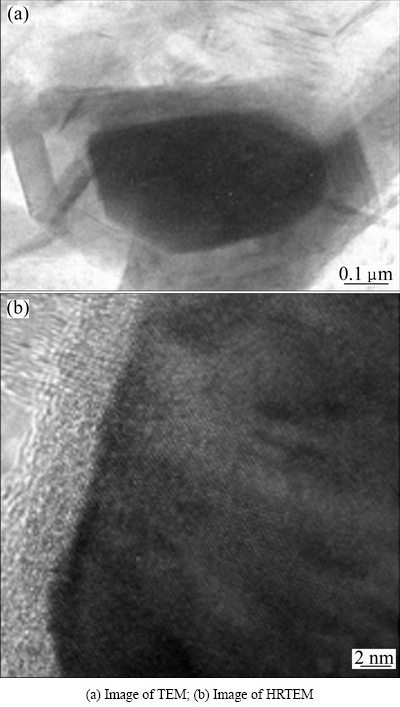
Fig. 6 Microstructures of carbon matrix of ZrC doping carbon materials
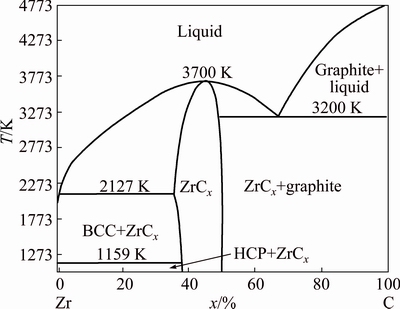
Fig. 7 Binary phase diagram of Zr-C
The chemical reactions of amorphous and graphite carbon obtained by decomposing ZrC are given in Eqs. (5) and (6), respectively. The standard Gibbs free energy curves with temperature changes of Eqs. (5) and (6) are compared in Fig. 8, where the thermodynamic data are taken from available literature [20]. From Fig. 8, the standard Gibbs free energy of Eq. (6) is actually lower at high temperatures, which means that the high temperatures make it more available for the chemical reaction of Eq. (6), so the graphite carbon with lower energy level should be easy to precipitate from ZrC.
ZrC=Zr+C(amorphous) (5)
ZrC=Zr+C(graphite) (6)
When processing high temperature graphitization, Zr diffuses to C matrix and reacts with amorphous carbon to form carbide. With the continuous diffusion of Zr, the graphite carbon and elemental Zr are generated. Then, the Zr diffuses along the surface of carbide particles to the side of amorphous carbon and reacts with it to form more carbide. The entire process is repeated with the Zr migration, which results in catalytic graphitization. It is worth noting that the graphitization degree of C matrix could be improved as the diffusion velocity of Zr element is accelerated at high temperatures.
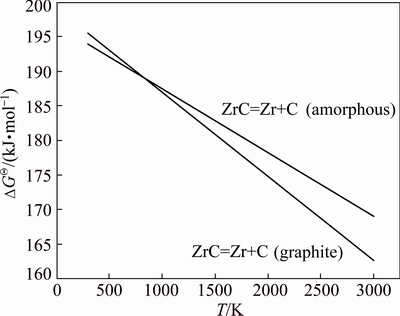
Fig. 8 Standard Gibbs energies of decomposition reaction of ZrC as a function of temperature
4 Conclusions
1) Flat plate sample of ZrC modified carbon material was obtained by using a hot-pressing sintering method. With the increasing of the temperature, the layer structures become more apparent in the C matrix, indicating the increase of graphitization degree of the C matrix.
2) The diffusion activation energy, Q=2.029×105 J/mol, and the frequency factor, D0=3.382×10-11 m2/s, of the Zr element in the C matrix could be calculated from the energy spectrum, and the diffusion coefficient expression could be written as
D=3.382×10-11exp[2.029×105/(RT)].
The diffusion of Zr in C matrix leads to the over-saturation of C around the ZrC particles and the over-saturated C precipitates as graphite. Then, the Zr diffuses along the surface of carbide particles and reacts with amorphous carbon to form more carbide. The procedure described above is an iterative process and the amorphous carbon gradually changes to graphite carbon, which results in the promotion on graphitization of the C matrix.
References
[1] ZENG Y, XIONG X, WANG D, WU L. Residual thermal stresses in carbon/carbon–Zr-Ti-C composites and their effects on the fracture behavior of composites with different performs [J]. Carbon, 2015, 81: 597-606.
[2] OUYANG H, LI C, HUANG J F, FEI J. Synthesis of carbon/carbon composites by hydrothermal carbonization using starch as carbon source [J]. RSC Advances, 2014, 4: 12586-12589.
[3] LIEW K M, LEI Z X, ZHANG L W. Mechanical analysis of functionally graded carbon nanotube reinforced composites: A review [J]. Composite Structures, 2015, 120: 90-97.
[4] KOVACIK J, EMMER S, BIELEK J. Thermal conductivity of Cu-graphite composites [J]. International Journal of Thermal Sciences, 2015, 90: 298-302.
[5] ZHANG Y L, HU Z, YANG B, REN J, LI H. Effect of pre-oxidation on the ablation resistance of ZrB2-SiC coating for SiC-coated carbon/carbon composites [J]. Ceramics International, 2015, 41: 2582-2589.
[6] WANG X, WENG L, ZHANG W. A SiC/Mo(Six,Al1-x)2 oxidation- resistant coating for carbon/carbon composites [J]. New Carbon Materals, 2014, 29: 126-131.
[7] YUAN H, WANG C, ZHANG S, LIN X. Effect of surface modification on carbon fiber and its reinforced phenolic matrix composite [J]. Applied Surface Science, 2012, 259: 288-293.
[8] GIBSON R F. A review of recent research on mechanics of multifunctional composite materials and structures [J]. Composite Structures, 2010, 92: 2793-2810.
[9] CHEN Y, WANG S, LIU B, ZHANG J. Effects of geometrical and mechanical properties of fiber and matrix on composite fracture toughness [J]. Composite Structures, 2015, 122: 496-506.
[10] SHI X H, HUO J H, ZHU J L, LIU L, LI H J, HU X J, LI M Y L. GUO L J, FU Q G. Ablation resistance of SiC-ZrC coating prepared by a simple two-step method on carbon fiber reinforced composites [J]. Corrosion Science, 2014, 88: 49-55.
[11] WANG S L, LI K, LI H J, ZHANG Y L. Microstructure and ablation resistance of ZrC nanostructured coating for carbon/carbon composites [J]. Materials Letters, 2013, 107: 99-102.
[12] HUANG D, ZHANG M, HUANG Q, WANG L, TANG X, YANG X, TONG K. Fabrication and ablation property of carbon/carbon composites with novel SiC-ZrB2 coating [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 3708-3715.
[13] LIU C, CHEN J, SU Z, YANG X, CAO L, HUANG Q. Pyrolysis mechanism of ZrC precursor and fabrication of C/C-ZrC composites by precursor infiltration and pyrolysis [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 1779-1784.
[14] LIU C, LI K, LI H, ZHANG S, ZHANG Y, WANG B. Synthesis, characterization and ceramization of a carbon-rich zirconium- containing precursor for ZrC ceramic [J]. Ceramics International, 2014, 40: 7285-7292.
[15] WANG C, GAO H, CHEN X, YUAN W Z, ZHANG Y. Enabling carbon nanofibers with significantly improved graphitization and homogeneous catalyst deposition for high performance electrocatalysts [J]. Electrochimica Acta, 2015, 152: 383-390.
[16] PARDO A G, RICO J R, RODRIGUEZ R C, FERNANDEZ J M. Effect of catalytic graphitization on the electrochemical behavior of wood derived carbons for use in supercapacitors [J]. Journal of Power Sources, 2015, 278: 18-26.
[17] XU Z, CAI D, HU Z, WANG F, GAN L. The structural optimization and high electrochemical behavior of porous carbons by graphitization in molten sodium metals [J]. Electrochimic Acta, 2014, 117: 486-491.
[18] LIERMANN H P, GANGULY J. Diffusion kinetics of Fe2+ and Mg in aluminous spinel: Experimental determination and applications [J]. Geochimic et Cosmochimic Acta, 2002, 66: 2903-2913.
[19] BHANUMURTHY K, KALE G B, KHERA S K. Reaction diffusion in the zirconium-iron system [J]. Journal of Nuclear Materials, 1991, 185: 208-213.
[20] ZHOU P, PENG Y, DU Y, WANG S, WEN G, XIE W, CHANG K. A thermodynamic description of the C-Ta-Zr system [J]. Int Journal of Refractory Metals and Hard Materials, 2013, 41: 408-415.
ZrC在C基体的扩散行为及石墨化促进作用
张中伟1,2,甄 强3,郑 锋3,鲁 飞3,南策文1,李 榕3,王俊山2
1. 清华大学 材料科学与工程学院 新型陶瓷及精细工艺国家重点实验室,北京 100084;
2. 航天材料及工艺研究所 先进功能复合材料技术重点实验室,北京 100076;
3. 上海大学 纳米科学与技术研究中心,材料复合与先进分散技术教育部工程中心,上海 200444
摘 要:C/C复合材料广泛应用于航空航天等领域,但在高温环境中极易氧化,可以通过基体改性改善其抗氧化性能,而ZrC作为氧化抑制剂应用已取得良好效果。通过高温热压烧结的方法制备了ZrC/C复合材料的平板模型样品;碳基体的石墨化程度随着烧结温度的升高而增加,2273 K烧结的样品中观察到了明显的层状结构;研究了高温下Zr元素在碳基体中的扩散行为,得到Zr元素在碳基体中扩散的一般表达式为D=3.382×10-11×exp[2.029×105(RT)]。Zr元素在碳基体中的扩散会导致微观区域内碳的过饱和并析出石墨结构的碳,而Zr元素在扩散中和周围无定形碳生成碳化物再析出碳的过程促进了碳基体的石墨化。
关键词:ZrC;C/C复合材料;扩散;石墨化
(Edited by Sai-qian YUAN)
Foundation item: Projects (51272154, 51472156) supported by the National Natural Science Foundation of China; Project (9140C5601010801) supported by the Pre-Research Foundation of General Armaments Department, China
Corresponding author: Qiang ZHEN; Tel: +86-21-66137276; E-mail: qzhen@staff.shu.edu.cn
DOI: 10.1016/S1003-6326(16)64345-5
Abstract: C/C composite material is widely used in aerospace field and others, however, it is easy to be oxidized at high temperature. In order to improve the oxidation resistance, ZrC is introduced as an oxidation inhibitor used in matrix modification of C/C composite material. Flat plate samples of ZrC/C composite materials were prepared by hot-pressing sintering. The degree of graphitization increases with rising sintering temperature, and layer structure of carbon matrix is observed clearly in the sample treated at 2273 K. Diffusion behavior of Zr in C matrix at high temperature is studied, which can be generally expressed as D=3.382×10-11 exp[2.029×105/(RT)]. The diffusion of Zr in C matrix leads to the over-saturation of C in the micro area and the oversaturated C precipitates as graphite. This continuous process promotes the transformation of carbon to graphite.


