J. Cent. South Univ. (2016) 23: 1541-1547
DOI: 10.1007/s11771-016-3206-x
Melting, sintering and wetting properties of ZnO–Bi2O3–B2O3 sealing glass
HE Feng(何峰)1, HE Zi-jun(何子君)2, XIE Jun-lin(谢峻林)1, 3, MEI Shu-xia(梅书霞)3, JIN Ming-fang(金明芳)3
1. State Key Laboratory of Silicate Materials for Architectures (Wuhan University of
Technology), Wuhan 430070, China;
2. Department of Materials Engineering, Monash University, VIC 3800, Australia;
3. Material Research and Test Center, Wuhan University of Technology, Wuhan 430070, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
Glasses based on ZnO-Bi2O3-B2O3 system are expected to be a new kind of sealing glasses because of their low melting temperature and other properties. In order to reveal the effect of B2O3 on the rheological behavior of ZnO-Bi2O3-B2O3 system glass melt, the properties of viscosity, thermal expansion, fluxion property and wetting process between cylinder samples and stainless steel were investigated with the rotating crucible viscometer, dilatometer and high-temperature microscope. The structure of sintered glass samples was investigated with scanning electron microscope. The results show that the B2O3 content increasing in B1-B3 at the given temperature between 400 °C and 500 °C leads to the increasing of the sample viscosity. When the amount of B2O3 increases from 5.24% to 9.24% (mass fraction), the coefficients of thermal expansion of glass samples decrease smoothly from 10.94×10-6 to 10.71×10-6 and 10.38×10-6 °C-1 respectively. In the case of sealing temperature, its value increases from 453 °C to 494 °C. ZnO-Bi2O3-B2O3 system low-melting glass powder sintering was with viscous liquid to participate, which could make the densification of glass sample more effective and more efficient. With the content of B2O3 increasing, the wetting angle between the glasses samples and stainless steel could also increase, and the resulting appropriate sealing temperature range is 460-490 °C.
Key words:
ZnO-Bi2O3-B2O3 system glasses; viscosity; sintering; wetting;
1 Introduction
Lead oxide glasses system has become a kind of popular low temperature sealing glasses for commercial use due to their high structural stability, low glass transitional temperature, good thermal and electrical characteristics [1-2]. However, it has been found that lead oxide glass systems should be replaced by lead-free system glasses on account of their toxicity to the environment and human body. In terms of the lead (Pb) toxicity to the environment, many newly lead-free glasses with low melting temperature are being evaluated and estabilished in the research [3-6].
Glasses based on ZnO-Bi2O3-B2O3 system are expected to be a new kind of sealing glasses owing to their low melting temperature. Recently, CHENG et al [7] have studied the structure and crystallization kinetics of Bi2O3-B2O3 glasses and FAN et al [8] have employed infrared and Raman technologies to investigate the structural units in bismuth based glasses. Moreover, BAI et al [9] have investigated the Bi2O3-B2O3 system glasses, particularly their structure. Although Bi2O3 is not a classical glass former, in the presence of conventional glass formers (such as B2O3, SiO2), it may build a glass network of [BiOn] (n=3 and 6) pyramids [10]. In the previous studies [11-12], the structure and the characters of Bi2O3-B2O3 glass system were investigated, but the melting, sintering properties and wetting property of the glass on metals were seldom concerned. In this study, the viscosity, sintering and wetting properties of ZnO-Bi2O3-B2O3 glasses were studied as a function of B2O3 content.
2 Experimental
2.1 Preparation of glass sample
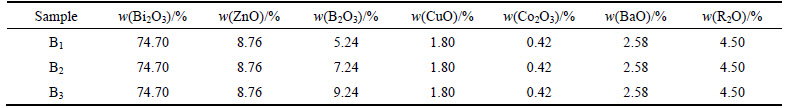
In order to obtiain a stable basic glass, glass network formers, such as Bi2O3 and B2O3, could be added into the glass. Meanwhile, the composition should also contain many oxides, such as ZnO, BaO, CuO, which could improve the forming range of the glasses. In addition, taking the glass-melting performance into account, Bi2O3 could reduce the melting temperature of the glasses. The composition of the samples is shown in Table 1.
Table 1 Composition of samples
The basic glasses materials were weighed, mixed and placed into corundum crucibles. The corundum crucible was then placed inside an electric furnace with a heating rate of 5-10 °C/min till the melting temperature (about 1000 °C) was reached, which took for 2 h. After that, the melt was poured into 80 mm×50 mm×20 mm graphite molds for cooling. The molds with the cooled melts were annealed in a muffle furnace at 320 °C for next 30 min to relieve residual stress in the glass samples. Then, the black and metallic luster glass samples were gained.
Massive glass samples were ground and sieved to size <75 μm. The glass power was mixed with appropriate amount of PVA for molding purpose. And then NYL-500-pressure testing machine was used to make “button” samples with d15 mm×5 mm shape under 40 kN. The bulk glasses were machined into a cylinder (d6 mm×30 mm) for thermal expansion measurement. “Button” samples were sintered at different temperatures with heating rate of 5 °C/min. The target temperatures were in the range of 380-500 °C for 30 min and the temperature interval was confirmed at 10 °C from 380 °C to 500 °C.
2.2 Characterization
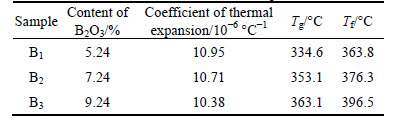
The thermal expansion curves of the samples were tested by the horizontal expansion instrument with a heating rate of 5°C /min. The test temperature range was from room temperature to 800 °C. The transition temperature (Tg) and the softening temperature (Tf) of the glasses are illustrated in Table 2. The diameter of samples was measured by a vernier caliper before and after sintering, and the sintering shrinkage rate of different samples was calculated.
“Button” samples were sintered at different temperatures with the heating rate of 5 °C/min. The target temperatures were from 380 °C to 520 °C for 30 min. Based on the sintering “button” experiment, the structure and fluidity of the glasses were studied.
Table 2 Coefficient of thermal expansion, Tg and Tf of samples
A Rheotronic III paralleled plate rheometer was used to measure the viscosity of the glass. The instrument was produced by USA THETA, which can be heated up to 900 °C with a high temperature sensitivity of 1 °C.
A high-temperature microscope (HTM Reetz GmbH) was used to describe the relationship between the cylinder samples profile and the heating temperature. The glass powder (<75 μm) was compressed to cylinder samples (about 4 mm in diameter and 6 mm in height) with using a pressing machine. A 439L stainless steel plate (12 mm×12 mm×1 mm) was used as the substrate. The measurements were taken in argon gas with the heating rate of 5 °C/min. A computerized image analysis system was used for samples’ geometry behaviors recording during the heating. Besides, the wetting process between cylinder samples and stainless steel was measured. And the shrinkage, the expansion, the sintering and the softening behaviour of the cylinder samples could be observed at elevated temperatures. The wetting process between cylinder samples and stainless steel was measured.
Scanning electron microscopy was used to study the microstructure of the sintered samples. The cross section of the samples was etched with HF solution (3% in volume fraction) for 20 s. Then the samples were rinsed immediately with distilled water and cleaned in ethanol for 3 min. The samples were gold-coated and then observed with a scanning electron microscope (JSM 600) operating at 25 kV.
3 Results and discussion
3.1 Viscosity of glasses
The melting property of the glasses could be described according to the relation between the glass viscosity and temperature. The cylinder glass sample has a size of about 8 mm in diameter and 8 mm in height. The materials of the paralleled plates are high-grade heat-resistant platinum. The measurement was performed firstly by holding the cylinder glass samples and sandwiched sample between the two paralleled platinum plates and under a fixed force. Then the sample compression as a function of temperature (or time) was recorded by raising the temperature at a rate of 5 °C/min under 0.588 N (60 g) load applied through the quartz glass tube. Finally, the viscosity is calculated through the following equation [13-15]:
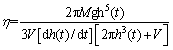 (1)
(1)
where η is the viscosity (Pa·s); M is the total applied load to the glass beam (g); g is the acceleration of gravity (980 cm/s2); h(t) is the sample height at the time t in cm; dh(t)/dt is the compression rate at the time t in cm/s; V is the sample volume in cm3.
Figure 1 shows the relationship between T and viscosity η of the ZnO-Bi2O3-B2O3 glass. Figure 2 shows the relationship between 10000/T and lnη of the glasses. The symbols represent the experimental data measured by the paralleled plate method. It shows clearly in Fig. 1 that the viscosity of the glasses decreased when the temperature (400-470 °C) was increased. The increasing of B2O3 content in B1-B3 between 400 °C and470 °C leads to the increase of the viscosity. B2O3 is the main network forming oxide in the samples, the network is improved by increasing the B2O3 content. It can be considered that the increasing of B2O3 content increases all the activation energy for viscous flow and the melting temperature.
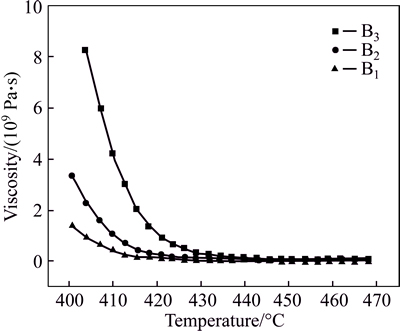
Fig. 1 Relationship between T and viscosity of ZnO-Bi2O3- B2O3 glasses
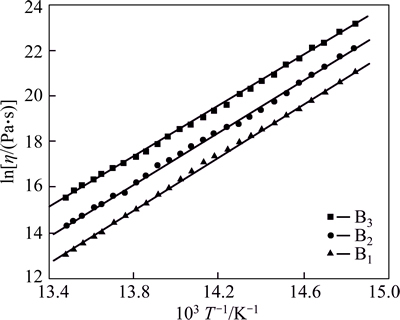
Fig. 2 Relationship between T-1 and lnη
The viscosity data in Fig. 2 show the nearly linear trends which can be fitted over these restricted temperature ranges using the approximation of Arrhenian behaviour [16-17]:
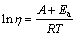 (2)
(2)
where η is the viscosity (Pa·s); A is the pre-exponential term; Ea is the activation energy of viscous flow; R is the gas constant (8.3145 J/Kmol); T is the thermodynamic temperature.
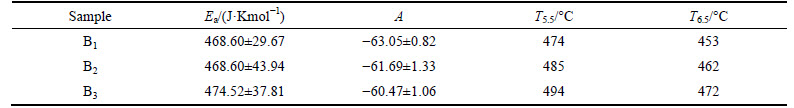
Table 3 summarizes the values of Ea, A, and the temperature corresponding to 105.5 Pa·s and 106.5 Pa·s, which are referred to as the glass’s suitable sealing temperature range (T5.5-T6.5) of the investigated ZnO-Bi2O3-B2O3 glass. The value of pre-exponential term A and activation energy Ea show less difference and no-obvious regularity as the content of B2O3 increases. The values (calculated by using Eq. (1)) of T5.5 and T6.5 increase as the content of B2O3 increases, respectively; Otherwise, the temperature difference between T5.5 and T6.5 is 21-23 °C for each glass composition. It can be found that the sealing temperature range which accords with the optimal viscosity range and the appropriate sealing temperature range is 450-490 °C. The sealing temperature range is greatly influenced by the composition and structure of the glass samples. The structure of the investigated ZnO-Bi2O3-B2O3 glass is relatively simple. B2O3 is the only main glass network in the glasses, and its content will determine the connected extent of the glass. When the content of Bi2O3 is invariant, the viscosity and surface tension of the glass samples are influenced by the content of B2O3. When increasing the content of B2O3 in B1-B3 samples, the connected extent of the glass may be enhanced, leading to the increase of the viscous resistance of the material particles as well. The appropriate sealing temperature of B1-B3 samples is confirmed increasing form 450 °C to 490 °C gradually.
Table 3 Pre-exponential term A, activation energy Ea, temperature corresponding to 105.5 Pa·s, T5.5 and 106.5 Pa·s, T6.5 calculated using Eq. (2).
In order to describe theoretically the relationship between viscosity and temperature for glass melts, several mathematical expressions, such as the Arrhenius equation [18], the Vogele Fulchere Tamman equation [19] and Avramove Milchev equation [20], are commonly used to express the temperature dependence of the viscosity of glass forming melts. Over the temperature interval between the glass transition temperature and the glass melting temperature, the viscosity spans 12 orders of magnitude. Most importantly, three important points between viscosity and temperature for glass processing could be gained. The first corresponds to the glass transition viscosity of η=1012 Pa·s. The second one is the sealing viscosity range of η=105.5-106.5 Pa·s. The third point is the glass melting, fining, and conditioning viscosity range at which is η=100-103 Pa·s. The sealing viscosity range is just in the middle of the glass transition viscosity and the glass melting viscosity.
3.2 Sintering properties
From the analysis of thermal expansion, Tg and Tf of glass samples were gained, and the results are shown in Table 3. As can be seen from the table, with the content of B2O3 increasing, the Tg and Tf of the samples were increased. The reason is that B2O3 is the forming composition of glass network [21] and each sample has enough free oxygen to make boron as [BO4] in the form of glass structure. The level of glass network connection became more strongly as the content of B2O3 increases due to the high bonding energy between the oxides that B2O3 has the largest single bond energy which is hard to be overcome in the form of molten glass process and, possibly, the network forming. Therefore, as the relative content of B2O3 increases, it needs more energy to form liquid phase. Tg and Tf can indicate the opposite temperature of glass powder sintering indirectly. It has been reported that Tg is related to the density of cross-linking, tightness of the network formers, as well as the coordination number of the network-forming atoms, etc [22]. According to the free volume theory, a glass system creates free volume at a higher temperature than Tg in the liquid stage. The change of thermal parameters can be explained by the structural change in ternary ZnO-Bi2O3-B2O3 glass system. The increase in Tg with increasing content of B2O3 corresponds to higher binding force. This will lead to a higher potential energy barrier, which has to be overcome to initiate glass transition by removing at least one of the ions. The fluidity and densification increase with the glass component as well, which concur with the results for the B2O3 content shown in Fig. 1 and Fig. 2.
“Button” experiment can be used for expressing the sintering properties directly [23]. The “button” samples were sintered in an electric resistance furnace under different schedules. The curves of sintering shrinkage rate are shown in Fig. 3. As shown in Fig. 3, the curves could be roughly divided into three stages. The first stage (stage 1) was the beginning of sintering that the sample had a low sintering shrinkage. With the temperature increasing, the second stage (stage 2) can be found that the sintering shrinkage increased rapidly and 80% of the sample sintering shrinkage had completed. Besides, during this stage, a lot of liquid phase could form especially for the low-melting temperature glasses. When the temperature continued to rise, “tardiness” phenomenon would show. In this stage (stage 3), the sintering of the sample came to an end almost, and the diameter of the samples became larger due to the appearance of a large quantity of liquid phase. As a result of this, the samples would reach a maximum rating of sintering shrinkage. Liquid phase, appearing inside the glass powders or at the interface of the glass powders, could bond glass powders together. And the gaps between the glass powders could be filled or occupied by the liquid phase. With the liquid phase increasing, the densification and sintering shrinkage rate of the glass samples became more effectively. The viscous flow of the “button” samples was controlled by the composition and temperature of liquid phase forming.
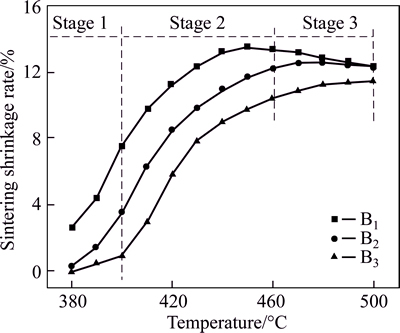
Fig. 3 Sintering shrinkage curve of samples
It could be found from Fig. 3 that the sintering shrinkage rate of the sample decreased with the increasing of B2O3. This could mainly caused by the reason that B2O3 was the forming composition of glass network and the glass network connection became more strongly when the content of B2O3 was increased. As a result of this, the migration of the glass powder became harder that more energy was required to be overcome and the temperature for liquid phase forming became even higher as well. By analyzing Table 2 and Fig. 3, we could infer that when the sintering temperature was higher than Tf, the sintering shrinkage rate of the glass sample would increase rapidly that a large amount of viscous liquid could be formed, the particles could migrate effectively and the sintering shrinkage could be observed significantly. When the “button” samples sintered at 460 °C, very bright luster can be observed on the surface of the samples, which proves the appearance of the liquid phase and the formation of the continuous glass phase. With the content of B2O3 increasing at the same sintered temperature, the surface luster of the samples reduced, which shows that the higher B2O3 content leads to lower amount of liquid phase at the given temperature.
High-temperature microscope was used to record the sample geometry and the start sealing temperature during heating. It is clear that the morphology of cylinder samples changed as the temperature was increased (see Fig. 4). When the wetting angle is just less than 90°, the temperature can be defined as the start sealing temperature, and the temperature can be used for materials sealing. The shape of the cylinder sample changed due to the viscosity change of the glasses. Figure 4 shows the selected images derived from the high-temperature microscopy test. The selection criterion of the images is temperature, which is correspondent with the viscosity [13]. Table 4 shows the relation between wetting angle and the temperature. Since the selection criterion of the images is temperature, the relationship between the geometry and the viscosity can be obtained.
As shown in Table 4, with the content of B2O3 increasing, the wetting angle between the glasses samples and stainless steel increased. B2O3 was the forming composition of glass network and the glass network connection became more strongly when the content of B2O3 increased. This phenomenon could increase the viscosity and the surface tension of the glasses.
Figure 5 shows the SEM photographs of glass samples, it could be found that, when the sintering temperature was 420 °C, glass particles in sample B1 became very smooth that no obvious edges and corners could be found, and most of the particles were fused together, leading to the dramatical reduction of the glass porosity. When the sintering temperature reached 440 °C, the glass particles in sample B1 were melted together completely, leaving some closed pore in it. Besides, the edges and corners of B2 glass particles became smooth and liquid phase was formed, which helps particles bonding together. When the sintering temperature was 460 °C, the particles of sample B2 would be melted together. Although some closed pores were formed in the sample, the porosity would be reduced dramatically. Similar to what happened at 440 °C of the sample B2,the corners of glass particles in sample B3 became rounded, and liquid phase would be formed. When the sintering temperature was 480 °C, the particles of B3 melted together with reduced dramatically porosity. With glass composition changing, each sample had its appropriate sintering temperature to allow glass phase forming. At the same time, the interfaces among the particles disappeared and the particles were melted together.
Table 4 Relationship between wetting angle and temperature
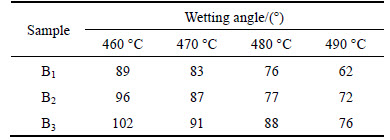
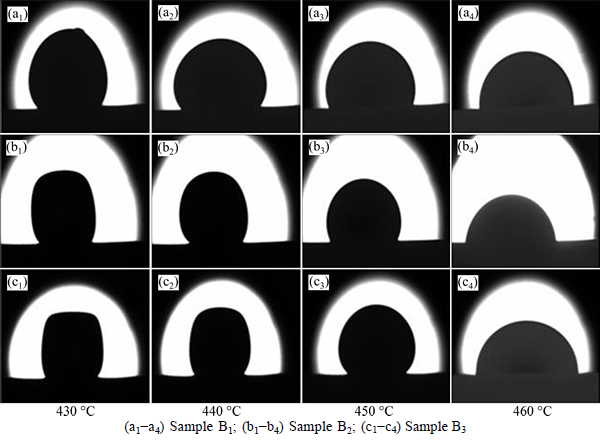
Fig. 4 Shape changes of samples as a function of temperature (heating rate of 5 °C/min):
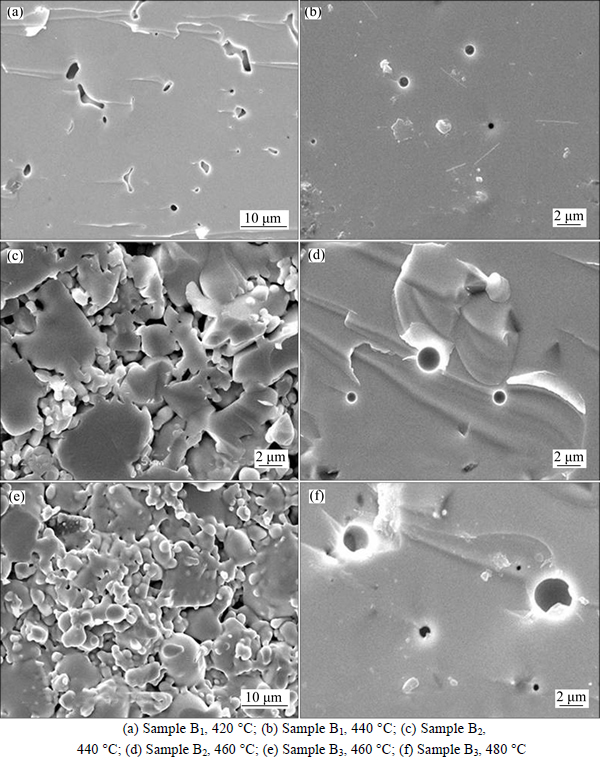
Fig. 5 SEM photographs of samples sintered at different temperature:
4 Conclusions
1) The increasing of the content of B2O3 in samples B1-B3 at the same temperature range leads to the increasing of the viscosity.
2) When the amount of B2O3 increases from 5.24% to 9.24%, the coefficient of thermal expansion of glass sample decreases from 10.94×10-6 to 10.71×10-6 and 10.38×10-6 °C-1, respectively.
3) With the content of B2O3 increasing, the wetting angle between the glasses samples and stainless steel would also increase, and the resulting appropriate sealing temperature range is 460-490 °C in ZnO-Bi2O3- B2O3 system low-melting glass.
Acknowledgements
The authors thank Material Research and Test Center of Wuhan University of Technology for technical help. This work has been supported by Key Laboratory of Silicate Materials for Architectures. Many thanks to the laboratory members for technical support and fruitful discussions.
References
[1] HONG Jian-he, ZHAO De-sen, GAO Jin-fei, HE Ming-zhong, LI Hai-feng, HE Gang. Lead-free low-melting point sealing glass in SnO-CaO-P2O5 system [J]. Journal of Non-Crystalline Solids, 2010, 356: 1400-1403.
[2] REIS S T, PASCUAL M J, BROW R K, RAY C S, ZHANG T. Crystallization and processing of SOFc sealing glass [J]. Journal of Non-Crystalline Solids, 2010, 356: 3009-3012.
[3] ARDELEAN I, CORA S, RUSU D. EPR and FT-IR spectroscopic studies of Bi2O3-B2O3-CuO glasses [J]. Physics B, 2008, 403: 3682-3685.
[4] SHASHIDHAR BALE, SYED RAHMAN, AWASTHI A M, SATHE V. Role of Bi2O3 content on physical, optical and vibrational studies in Bi2O3-ZnO-B2O3 glasses [J]. Journal of Alloys Compdounds, 2008, 460: 699-703.
[5] KIM B S, LIM E S, LEE J H, KIM J J. Effect of Bi2O3 content on sintering and crystallization behavior of low-temperature firing Bi2O3-ZnO-B2O3 glasses [J]. Journal of Europe Ceramics Society, 2007, 27: 819-824.
[6] HE Feng, CHENG Jin-shu, DENG Da-wei, WANG Jun. Structure of Bi2O3-ZnO-B2O3 system low-melting sealing glass [J]. Journal of Central South University Technology, 2010, 17(2): 257-262.
[7] CHENG Y, XIAO H N, GUO W M, GUO W M. Structure and crystallization kinetics of Bi2O3-B2O3 glasses [J]. Thermochimica Acta, 2006, 444: 173-178.
[8] FAN Hui-yan, WANG Guo-nian, HU Li-li. HYPERLINK infrared, Raman and XPS spectroscopic studies of Bi2O3–B2O3–Ga2O3 glasses [J]. SolidStateSciences,2009,11(12):2065-2070.
[9] BAI L C, STEFAN R, KIEFER W, POPP J, SIMON S. Structural investigations of copper doped B2O3-Bi2O3 glasses with high bismuth oxide content [J]. Journal of Non-Crystalline Solids, 2002, 303(3): 379-386.
[10] SHASHIDHAR BALE N, RAO S, RAHMAN S. Spectroscopic studies of Bi2O3-Li2O-ZnO-B2O3 glasses [J]. Solid State Science, 2008, 10: 326-331.
[11] KIRDSIRI K, KAEWKHAO J, CHANTHIMA N, LIMSUWAN P. Comparative study of silicate glasses containing Bi2O3, PbO and BaO: Radiation shielding and optical properties [J]. Annals of Nuclear Energy, 2011, 38(6): 1438-1441.
[12] BAO R Q, BUSTA C M, SU X F, TOMOZAWA M, CHRISEY D B. Microstructures, phases, and properties of low melting BaO-B2O3-ZnO glass films prepared by pulsed laser deposition [J]. Journal of Non-Crystalline Solids, 2013, 371-372: 28-32.
[13] HE Feng, WANG Jun, DENG Da-wei. Effect of Bi2O3 on structure and wetting studies of Bi2O3-ZnO-B2O3 glasses [J]. Journal of Alloys Compounds, 2011, 509: 6332-6336.
[14] WANG Mi-tang, CHENG Jin-shu. Viscosity and thermal expansion of rare earth containing soda–lime–silicate glass [J]. Journal of Alloys Compounds, 2010, 504: 273-276.
[15] GRYLORD S, TINCHER B, PETIT L, RICHAEDSON K. Viscosity properties of sodium borophosphate glasses [J]. Materials Research Bulletin, 2009, 44: 1031-1035.
[16] STRIEPE S, DEUBENER J. Viscosity and kinetic fragility of alkaline earth zinc phosphate glasses [J]. Journal of Non-Crystalline Solids, 2012, 358: 1480-1485.
[17] HRMA P, HANS S. Effect of glass composition on activation energy of viscosity in glass-melting-temperature range [J]. Journal of Non-Crystalline Solids, 2012, 358: 1818-1829.
[18] HRMA P. Glass viscosity as a function of temperature and composition: A model based on Adam–Gibbs equatio [J]. Journal of Non-Crystalline Solids, 2008, 354(18): 1962-1968.
[19] TAKEUCHI A, KATO H, INOUE A. Vogel–Fulcher–Tammann plot for viscosity scaled with temperature interval between actual and ideal glass transitions for metallic glasses in liquid and supercooled liquid states [J]. Intermetallics, 2010, 18(4): 406-411.
[20] AVRAMOV I, MILCHEV A. Effect of disorder on diffusion and viscosity in condensed systems [J]. Journal of Non-Crystalline Solids, 1988, 104 (2/3): 253-260.
[21] SHIH P Y, CHIN T S. Preparation of lead-free phosphate glasses with low Tg and excellent chemical durability [J]. Journal of Materials Science Letter, 2001, 20: 1811-1813.
[22] ZHAO Guo-ying, TIAN Ying, FAN Hui-yan, ZHANG Jun-jie, HU Li-li. Properties and structures of Bi2O3-B2O3-TeO2 glass [J]. Journal of Materials Science Technology, 2013, 29: 209-214.
[23] HE Feng, DENG Da-wei, WANG Jun. Effect of Bi2O3 Contents on sintering property of Bi2O3-ZnO-B2O3 system low-melting electronic sealing glass [J]. Journal of Wuhan University of Technology, 2009, 31(22): 33-35. (in Chinese)
(Edited by YANG Hua)
Foundation item: Project(2012BAA08B04) supported by the National “Twelfth Five-Year” Plan for Science & Technology Support of China
Received date: 2015-06-30; Accepted date: 2015-12-10
Corresponding author: HE Feng, PhD, Professor; Tel: +86-27-87871643; E-mail: He-Feng2002@163.com
Abstract: Glasses based on ZnO-Bi2O3-B2O3 system are expected to be a new kind of sealing glasses because of their low melting temperature and other properties. In order to reveal the effect of B2O3 on the rheological behavior of ZnO-Bi2O3-B2O3 system glass melt, the properties of viscosity, thermal expansion, fluxion property and wetting process between cylinder samples and stainless steel were investigated with the rotating crucible viscometer, dilatometer and high-temperature microscope. The structure of sintered glass samples was investigated with scanning electron microscope. The results show that the B2O3 content increasing in B1-B3 at the given temperature between 400 °C and 500 °C leads to the increasing of the sample viscosity. When the amount of B2O3 increases from 5.24% to 9.24% (mass fraction), the coefficients of thermal expansion of glass samples decrease smoothly from 10.94×10-6 to 10.71×10-6 and 10.38×10-6 °C-1 respectively. In the case of sealing temperature, its value increases from 453 °C to 494 °C. ZnO-Bi2O3-B2O3 system low-melting glass powder sintering was with viscous liquid to participate, which could make the densification of glass sample more effective and more efficient. With the content of B2O3 increasing, the wetting angle between the glasses samples and stainless steel could also increase, and the resulting appropriate sealing temperature range is 460-490 °C.


