Trans. Nonferrous Met. Soc. China 23(2013) 253-259
Synthesis of Li+ adsorbent (H2TiO3) and its adsorption properties
Xi-chang SHI, Zhi-bing ZHANG, Ding-fang ZHOU, Li-fen ZHANG, Bai-zhen CHEN, Liang-liang YU
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 31 December 2011; accepted 4 May 2012
Abstract:
H2TiO3 was obtained from the acid-modified adsorbent precursor Li2TiO3, which was synthesized by a solid-phase reaction between TiO2 and Li2CO3. The extraction ratio of Li+ from Li2TiO3 was 98.86%, almost with no Ti4+ extracted. The effects of lithium titanium ratio, calcining temperature and time were investigated on the synthesis of Li2TiO3. Li2TiO3, H2TiO3 and the adsorbed Li+ adsorbent were characterized by XRD and SEM. The lithium adsorption properties were investigated by the adsorption kinetics and adsorption isotherm. The results indicate that H2TiO3 has an excellent adsorptive capacity for Li+. Two simplified kinetic models including the pseudo-first-order and pseudo-second-order equations were selected to follow the adsorption processes. The rate constants of adsorption for these kinetic models were calculated. The results show that the adsorption process can be described by the pseudo-second-order equation, and the process is proved to be a chemical adsorption. The adsorption process that H2TiO3 adsorbs Li+ in LiCl solution well fits the Langmuir equation with monolayer adsorption.
Key words:
Li+ adsorbent; Li2TiO3; adsorption property; kinetic models; monolayer adsorption; TiO2; Li2CO3;
1 Introduction
Traditional precipitation, solvent extraction and salting method [1,2] are not suitable for a small amounts of Li-containing sea water and salt lake brine on lithium extraction. A new separating technique [3,4] of alkali and alkaline metals with similar nature benefits from the inorganic separation material with ion function memory. At present, the study of lithium ionic sieve is mainly focused on manganese lithium ionic sieve [5-7], which exists in the disfigurement of few cyclic number and big dissolved loss etc. Titanium lithium ionic sieve has some remarkable advantages, such as less dissolved loss and stable structure. Li4Ti5O12 was synthesized by the sol-gel method [8,9] and the lithium ionic sieve was obtained after acid treatment. The pull-out rate of lithium reached 81.5%, while that of titanium ion was below 4.2%. The adsorptive capacity for lithium ion reached 42.30 mg/g. Li2TiO3 was obtained via the solid phase synthesis method [10-12] with mixed crystal titanium dioxide (anatase content was 83.1%) as raw material and lithium ionic sieve was acquired after pickling. Its saturated adsorptive capacity came up to 28.76 mg/g. Li2TiO3 had a special separation effect on lithium in the brine gas field with high calcium and magnesium but low lithium. It can be seen that the form of titanium dioxide used has a great influence on the adsorption performance of Li2TiO3, which was used as the lithium adsorbent precursor.
In this work, Li2TiO3 was synthesized by a solid- phase method with mixed crystal titanium dioxide. Then lithium adsorbent (H2TiO3) was obtained by pickling with hydrochloric acid. The structure characteristics and ion-exchange properties were studied by XRD, SEM, Li+ adsorption isotherm, kinetics and adsorption measurement, respectively.
2 Experimental
The experimental technology process is shown in Fig. 1. The lithium adsorbent precursor was synthesized by a solid-phase reaction between TiO2 and Li2CO3. Prior to calcination, the mixture of TiO2 and Li2CO3 was milled and blent with anhydrous alcohol as dispersant. Then it was dried at 100 °C for 2 h. Finally, the dried mixture was calcined at 850 °C for 24 h and pickled with hydrochloric acid. The adsorbent was used to adsorb lithium ion in solution, then it was regenerated with hydrochloric acid.

Fig. 1 Flow chart of experimental process
3 Results and discussion
3.1 Synthesis of lithium adsorbent precursor (Li2TiO3)
The XRD patterns of Li2TiO3 synthesized under different lithium titanium ratios (R=2.00, 2.04, 2.10, 2.16) with Li2CO3 and TiO2 are shown in Fig. 2. The results indicate the pure phase of Li2TiO3 can be obtained under all ratios. The intensities of diffraction peaks increase with increasing the lithium titanium ratio, which indicates that the crystal shape is more complete under a higher lithium to titanium ratio.

Fig. 2 XRD patterns of Li2TiO3 synthesized with different titanium to lithium ratios
3.1.1 Lithium to titanium ratio
The effect of lithium to titanium ratio (R= n(Li)/n(Ti), 2.00, 2.04, 2.08, 2.10, 2.14, 2.16) on lithium extraction and titanium dissolved loss rate is shown in Fig. 3. Under the same pickling conditions (0.25 mol/L HCl, 60 °C), lithium drawn out reduces with the increasing of R. The extraction rate of lithium is 85.62% when R is 2, but it is reduced to 77.87% at R=2.16. In contrast, the dissolved loss rate of titanium increases slowly with the increasing of R, which is 0.17% when R is 2 and it is increased to 0.34% at R=2.16. Figure 4 shows the effect of R on the adsorptive capacity. As can be seen from Fig. 4 the adsorptive capacity decreases with an increase of R under the same pickling and adsorption conditions. The adsorptive capacity is 25 mg/g when R is 2 and decreases to 11.8 mg/g when R increases to 2.16. This indicates that the crystal structure of Li2TiO3 becomes more complete and stable with the increase of R, which is consistent with that in Fig. 2. According to lithium extraction, titanium dissolution loss rate and adsorptive capacity, the optimum chemical ratio is chosen as 2.

Fig. 3 Effects of lithium to titanium mole ratio on lithium extraction and titanium dissolved loss rate

Fig. 4 Effect of lithium to titanium mole ratio on adsorptive capacity
3.1.2 Synthesis temperature
Figure 5 shows the effects of temperature on the XRD patterns of lithium adsorbent precursor obtained under the conditions of lithium to titanium ratio of 2 and calcining time of 24 h. The pure phase of Li2TiO3 can be synthesized from 550 °C to 1050 °C. All diffraction peak intensities increases with the synthesis temperature increased from 550 °C to 850 °C, which illustrates that the crystal structure of Li2TiO3 becomes more complete with increasing the temperature. The results show that all the diffraction peak intensities become weak gradually when the temperature is higher than 850 °C. According to Refs. [13-15], the single inclined crystal of Li2TiO3 is converted into the cubic crystal when the temperature is higher than 1150 °C. It can be concluded that the structural change stage happened when the temperature is higher than 850 °C, which leads to a weaker diffraction peak intensity.

Fig. 5 XRD patterns of Li2TiO3 synthesized at different temperatures
Figure 6 shows the effect of synthesis temperature on lithium extraction and titanium dissolved loss rate. Lithium extraction rate decreases with the increase of synthesis temperature under the same pickling conditions (0.25 mol/L HCl, 60 °C). The rates of lithium elicited are 88.31%, 85.62% and 56.21% at synthesis temperatures of 550, 850 and 1050 °C, respectively. The titanium dissolved loss rate increases with the increase of synthesis temperature. The titanium dissolved loss rates are 0.15%, 0.17% and 1.16% at synthesis temperatures of 550, 850 and 1050 °C, respectively.
The lithium adsorbent adsorptive capacity shows little dependence on temperature in the range of 550-850 °C, as shown in Fig. 7. The adsorptive capacities are 26.2 and 25 mg/g at 550 °C and 850 °, respectively. When the temperature is 1050 °C, the adsorption capacity decreases sharply to 4 mg/g. It can be concluded that the suitable synthesis temperature is between 550 °C and 850 °C by considering the lithium extraction rate, titanium dissolved loss rate and lithium adsorptive capacity comprehensively.

Fig. 6 Effects of synthesis temperature on lithium extraction and titanium dissolved loss rate
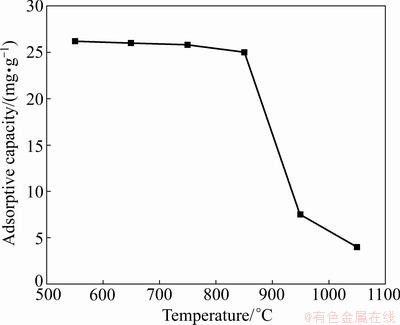
Fig. 7 Effect of synthesis temperature on lithium adsorptive capacity
3.2 Preparation and characterization of lithium adsorbent (H2TiO3)
Lithium adsorbent (H2TiO3) was obtained when the lithium adsorbent precursor was treated with hydrochloric acid. Figure 8 shows the relationship between the lithium extraction and the extracting time. As can be seen from Fig. 8, the rate of lithium extracted reached 83.64% when Li2TiO3 was treated by pickling for 24 h. Then it increased slowly with the extension of time, so it reached 98.86% after 96 h. While the content of the titanium ion in solution was almost zero through spectrophotometry method, which suggests that titanium is almost insoluble loss in the pickling process.

Fig. 8 Variation of Li+ extraction rate with reaction time

Fig. 9 XRD patterns of Li2TiO3 (a), H2TiO3 before (b) and after (c) adsorbing Li+
Figure 9 shows XRD patterns of Li2TiO3, H2TiO3 before and after adsorbing Li+. Figure 9(a) shows XRD patterns of pure monoclinic crystal of Li2TiO3 (the lattice constant: a=0.5069 nm, b=0.8799 nm, c=0.9759 nm, JCPDS 33—831). The pattern in Fig. 9(b) shows that the diffraction peak shifts to right and the strength weakens significantly compared with the pattern in Fig. 9(a). The diffraction peaks of pattern become wide. All of the changes indicate that hydrogen ions (radius is about 0.0012 nm) exchange with lithium atoms (radius is about 0.076 nm) when Li2TiO3 is treated with acid. The crystal interplanar spacing becomes narrow and the grains become small after treatment. It can be seen that the XRD pattern in Fig. 9(c) is alike with that in Fig. 9(b), which suggests that the structure does not change much after absorption.
Figure 10 shows the SEM images of Li2TiO3, H2TiO3 before and after adsorbing Li+. It can be seen from Fig. 10, Li2TiO3 obtained by solid-phase synthesis method is comparatively uniform, with the particle size of 1-2 μm. Figures 10(b) and (c) show the particles with some irregular crackles.
3.3 Adsorptive properties
3.3.1 Saturated adsorptive capacity
Figure 11 shows the adsorptive capacity of lithium adsorbent (H2TiO3) in LiOH solution of 694.1 mg/L Li+, LiCl solution of 282.5 mg/L Li+ and 87.5 mg/L Li+. The results show that the adsorptive capacity increases with the increase of time in LiOH or LiCl solution. The adsorptive capacity increases quickly in the first 24 h and then increases slowly with prolonging time. Finally, it reaches a plateau after 192 h. The adsorptive capacities are 39.8, 30.9 and 28.63 mg/g in deferent solutions described above, respectively. The results indicate that the absorbent has strong adsorption ability to lithium in the alkaline solution. The adsorption reaction is as follows: H2TiO3+Li+→Li2TiO3+H+. H+ was released and OH- was consumed constantly when lithium ion in the solution was adsorbed constantly. The adsorptive capacity increased slowly until the adsorption balance reached with the extension of time. Thus the adsorptive capacity in LiOH solution was more than that in LiCl solution, which was also concluded by AYDIN et al [16] and MARTIN et al [17].

Fig. 10 SEM images of Li2TiO3 (a), H2TiO3 before (b) and after (c) adsorbing Li+
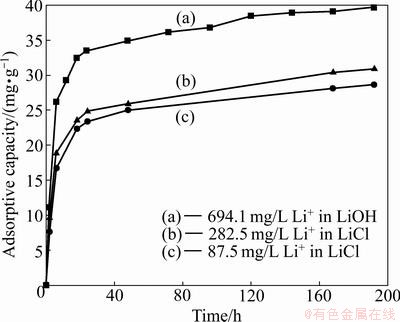
Fig. 11 Variation of adsorptive capacity with time
3.3.2 Adsorption dynamics
The lithium generally exists in the form of LiCl in saline brine, so the adsorption dynamics that the adsorbent adsorbed in LiCl solution with different concentrations was investigated. There were many reports about the adsorption dynamics research of adsorbent for metal ions. At present, the pseudo-first- order (equation 1) and pseudo-second-order (equation 2) kinetic models are widely used to study the adsorption mechanism and determine the rate constant of adsorption process [18-20]:
lg(qe-qt)=lgqe- ×t (1)
×t (1)
 =
= +
+ ×t (2)
×t (2)
where qe and qt are the absorbent adsorptive capacities to Li+ at adsorption equilibrium and any time t, respectively; kad and k are the rate constants of the pseudo-first-order and pseudo-second-order kinetic models, respectively.
The curves b and c in Fig. 11 are made a linear fitting with the pseudo-first-order and pseudo-second- order dynamic equations, and the results are shown in Fig. 12 and Table 1. As shown in Fig. 12 and Table 1, the linear correlation coefficient of the pseudo-second-order dynamic equation is 0.999, which is greater than that of the pseudo-first-order dynamic equation. Thus it can be concluded that the adsorption dynamics in LiCl solution is conformed to the pseudo-second-order kinetic model. Some important kinetic parameters can be calculated by the pseudo-second-order dynamic equation. For example, the rate constants at the two concentrations are 0.00574 and 0.0051 g/(mg·h), respectively. The equilibrium adsorptive capacities are 29.32 and 31.63 mg/g. The adsorptive capacities are fairly near 28.63, 30.9 mg/g according to Fig. 11, respectively. This further confirms the conclusion that the adsorption in LiCl solution conforms to the pseudo-second-order dynamic equation and the adsorption process is mainly a chemical adsorption [21].
3.3.3 Adsorption isotherm
The experimental data were analyzed by the Langmuir and Freundlich models [22], the equations of which can be expressed as
 =
= ρe+
ρe+ (3)
(3)
lg qe = lg ρe +lg KF (4)
lg ρe +lg KF (4)
where ρe is the equilibrium concentration of Li+ solution; qe is the equilibrium adsorptive capacity of Li+; qm is the theoretical maximum adsorptive capacity of Li+; KL is the Langmuir empirical constant; n and KF are the Freundlich constants indicating relative adsorptive capacity and adsorption rate, respectively.

Fig. 12 Pseudo-first-order (a) and pseudo-second-order (b) kinetic plots for Li+ adsorption at different Li+ concentrations
Table 1 Kinetic parameters for Li+ adsorption at different concentrations

The equilibrium concentration of Li+ and adsorptive capacity of adsorbent in LiCl solution of different concentrations were fitted linearly with Langmuir and Freundlich models. The results are shown in Fig. 13 and Table 2. The linear correlation coefficient of Langmuir model reaches 0.9984, which is much greater than that of Freundlich model. This indicates the adsorption of absorbent for Li+ conforms to Langmuir model. It is a monolayer adsorption and the maximum absorption capacity is 37.01 mg/g.

Fig. 13 Langmuir (a) and Freundlich (b) isotherms in LiCl solution of different concentrations
Table 2 Type and parameters of isothermal adsorption of Li+-adsorbent

3.3.4 Peculiarity of selective adsorbent to lithium ion
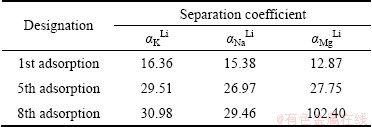
The lithium absorbent was placed in the salt lake brine to make a cycle experiment and carry a study on the separation properties of absorbent for the main metal ions in it. Table 3 lists the separation coefficients of lithium ion to several main metal ions in brine. As can be seen from Table 3, the adsorbent has better separation properties of Li+ to Na+, K+ and Mg2+, but the separation coefficient is not very large, which is related with the adsorption time and the nature of brine. In the brine, the content of Li+ is low, while that of Na+, K+ or Mg2+ is very high. The separation coefficient becomes more and more with the cycle carrying. This indicates that the increase of cycle times is beneficial to the separation of Li+ to K+, Na+ and Mg2+ in salt lake brine. The separation coefficient of lithium to magnesium reaches 102.40 at the 8th adsorption, which can be a good separation of lithium and magnesium ions in salt lake brine.
Table 3 Separation coefficients of lithium ion to several main metal ions in brine
4 Conclusions
1) A single crystal phase of Li2TiO3 can be synthesized by solid method using titanium dioxide and lithium carbonate as raw materials. Optimization preparation conditions of metatitanate lithium precursor are: lithium to titanium ratio of 2, synthesis temperature of 850 °C and synthesis time of 24 h.
2) Lithium adsorbent (H2TiO3) can be obtained after pickling Li2TiO3 with hydrochloric acid. The extraction ratio of Li+ from Li2TiO3 reached 98.86%, and the rate of titanium dissolved loss was only 0.17%.
3) Lithium adsorbent (H2TiO3) has an excellent adsorption ability to Li+. The equilibrium adsorptive capacity in LiOH solution containing 694.1 mg/L Li+ reached 39.8 mg/g.
4) The adsorption process that H2TiO3 adsorbs Li+ in LiCl solution conformed to the pseudo-second-order dynamic equation, which shows that the adsorption process is mainly chemical adsorption. What’s more, the adsorption process also conformed to Langmuir model, which shows that the adsorption process is monolayer absorption.
5) The separation coefficient of lithium to magnesium reaches 102.4 at the 8th adsorption, which can be a good separation of lithium and magnesium ions in salt lake brine.
References
[1] VAN G S W, TANG Y, RITTMANN B E. Impact of precipitation on the treatment of real ion-exchange brine using the H2-based membrane biofilm reactor [J]. Water Sci Technol, 2011, 63(7): 1453-1458.
[2] HAMZAOUI A H, HAMMI H, MNIF A . Operating conditions for lithium recovery from natural brines [J]. Russ J Inorg Chem, 2007, 52(12): 1859-1863.
[3] YANG S T, KIM J, AHN W S. CO2 adsorption over ion-exchanged zeolite beta with alkali and alkaline earth metal ions [J]. Microporous Mesoporous Mat, 2010, 135(1-3): 90-94.
[4] BRUzzoniti M C, CARlO R M D, Fiorilli S, ONIDA B, SARZANINI C. Functionalized SBA-15 mesoporous silica in ion chromatography of alkali, alkaline earths, ammonium and transition metalions [J]. J Chromatogr A, 2009, 1216(29): 5540-5547.
[5] Wang L, Meng C G, Ma W. Study on Li+ uptake by lithium ion-sieve via the pH technique [J]. Colloid Surf A, 2009, 334(1-3): 34-39.
[6] Zhang Q H, Sun S Y, Li S P, Jiang H, Yu J G. Adsorption of lithium ions on novel nanocrystal MnO2 [J]. Chem Eng Sci, 2007, 62(18-20): 4869-4874.
[7] Wang L, Ma W, Liu R, Li H Y, Meng C G. Correlation between Li+ adsorption capacity and the preparation conditions of spinel lithium manganese precursor [J]. Solid State Ionics, 2006, 177(17-18): 1421-1428.
[8] Xiang H F, Tian B B, Lian P C, Li Z, Wang H H. Sol–gel synthesis and electrochemical performance of Li4Ti5O12/graphene composite anode for lithium-ion batteries [J]. J Alloy Compd, 2011, 509(26): 7205-7209.
[9] Wang D, Ding N, Song X H, CHEN C H. A simple gel route to synthesize nano-Li4Ti5O12 as a high-performance anode material for Li-ion batteries [J]. J Mater Sci, 2009, 44(1): 198-203.
[10] Sinha A, Nair S R and Sinha P K. Single step synthesis of Li2TiO3 powder [J]. J Nucl Mater, 2010, 399(2-3): 162-166.
[11] Zhong Hui. Property of H2TiO3 type ion-exchangers and extraction of lithium from brine of natural gas wells [J]. Chinese Journal of Applied Chemistry, 2000, 17(3): 307-309. (in Chinese)
[12] Deptula A, Olczak T, Lada W, Sartowska B, Chmielewski A G, Alvani C, Carconi P L, Bartolomeo A D, Pierdominici F, Casadio S. Fabrication of Li2TiO3 spherical microparticles from TiCl4 by a classical, inorganic sol-gel route; Characteristics and tritium release properties [J]. J Mater Sci, 2002, 37(12): 2549-2556.
[13] Kataoka K, Takahashi Y, Kijima N,Nagai H, Akimoto J,Idemoto Y,Ohshima K. Crystal growth and structure refinement of monoclinic Li2TiO3 [J]. Mater Res Bull, 2009, 44(1): 168-172.
[14] KLEYKAMP H. Heat capacity and enthalpy of transformation of Li2TiO3 [J]. J Nucl Mater, 2001, 295(2-3): 244-248.
[15] Kleykamp H. Phase equilibria in the Li-Ti-O system and physical properties of Li2TiO3 [J]. Fusion Eng Des, 2002, 61-62: 361-366.
[16] Aydin F, Yasar F, Aydin I, Guzel F. Determination of lead separated selectively with ion exchange method from solution onto BCW in Sirnak, East Anatolia of Turkey [J]. Microchem J, 2011, 98(2): 246-253.
[17] Martin M H, Lasia A. Study of the hydrogen absorption in Pd in alkaline solution [J]. Electrochim Acta, 2008, 53(22): 6317-6322.
[18] Zhou L M, Wang Y P, Liu Z R , Huang Q W. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres [J]. Hazardous Materials, 2009, 161(2): 995-1002.
[19] Benhammou A, Yaacoubi A, Nibou L, Tanouti B. Adsorption of metal ions onto Moroccan stevensite: Kinetic and isotherm studies [J]. J Colloid Interface Sci, 2005, 282(2): 320-326.
[20] Barkat M, Nibou D, Chegrouche S, Mellah A. Kinetics and thermodynamics studies of chromium (VI) ions adsorption onto activated carbon from aqueous solutions [J]. Chem Eng Process, 2009, 48(1): 38-47.
[21] Naiya T K, Bhattacharya A K, Das S K. Removal of Cd(II) from aqueous solutionsusing clarified sludge [J]. J Colloid Interface Sci, 2008, 325(1): 48-56.
[22] Wang L, Meng C G, Han M, Ma W. Lithium uptake in fixed-pH solution by ion sieves [J]. J Colloid Interface Sci, 2008, 325: 31-40.
锂离子吸附剂(H2TiO3)的合成及吸附性能
石西昌,张志兵,周定方,张丽芬,陈白珍,余亮良
中南大学 冶金科学与工程学院,长沙 410083
摘 要:选取TiO2为钛源、Li2CO3为锂源,采用高温固相法合成锂吸附剂前躯体Li2TiO3,并探讨锂钛比、焙烧温度、焙烧时间等因素对合成Li2TiO3性质的影响。将Li2TiO3用一定浓度的盐酸酸洗脱锂后制得偏钛酸型锂吸附剂H2TiO3,酸洗过程中锂的抽出率达到98.86%,而离子筛中的钛溶损却很小。采用XRD和SEM等分析手段对TiO2、Li2TiO3和H2TiO3及其吸附锂后的样品进行表征。最后应用伪一级和伪二级动力学方程对H2TiO3的吸附性能进行研究,并对吸附过程进行拟合从而计算相应的速率常数。结果表明:H2TiO3对锂离子具有较大的吸附能力,吸附过程符合伪二级动力学方程,表明吸附过程主要为化学吸附;在LiCl溶液中的吸附平衡数据符合Langmuir等温吸附方程,表明吸附过程为单层吸附。
关键词:锂离子吸附剂;Li2TiO3;吸附性能;动力学模型;单层吸附;TiO2;Li2CO3
(Edited by Hua YANG)
Foundation item: Project (2008BAB35B04) supported by the National Key Technologies R&D Program of China; Project (2010QZZD003) supported by Central South University Advanced Research Program, China
Corresponding author: Xi-chang SHI; Tel/Fax: +86-731-88877352; E-mail: xichang.shi@gmail.com
DOI: 10.1016/S1003-6326(13)62453-X
Abstract: H2TiO3 was obtained from the acid-modified adsorbent precursor Li2TiO3, which was synthesized by a solid-phase reaction between TiO2 and Li2CO3. The extraction ratio of Li+ from Li2TiO3 was 98.86%, almost with no Ti4+ extracted. The effects of lithium titanium ratio, calcining temperature and time were investigated on the synthesis of Li2TiO3. Li2TiO3, H2TiO3 and the adsorbed Li+ adsorbent were characterized by XRD and SEM. The lithium adsorption properties were investigated by the adsorption kinetics and adsorption isotherm. The results indicate that H2TiO3 has an excellent adsorptive capacity for Li+. Two simplified kinetic models including the pseudo-first-order and pseudo-second-order equations were selected to follow the adsorption processes. The rate constants of adsorption for these kinetic models were calculated. The results show that the adsorption process can be described by the pseudo-second-order equation, and the process is proved to be a chemical adsorption. The adsorption process that H2TiO3 adsorbs Li+ in LiCl solution well fits the Langmuir equation with monolayer adsorption.


