DOI: 10.11817/j.ysxb.1004.0609.2020-39597
拜耳法溶出条件下含硫脱硅产物的形成和转化
李小斌1,席亚伟1,王一霖1, 2,齐天贵1,刘桂华1,周秋生1,彭志宏1
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 粉末冶金研究院,长沙 410083)
摘 要:
结合物相和成分分析结果,阐明了模拟拜耳法溶出条件下含硫脱硅产物(S-DSP)的形成和转化机制。结果表明:不同含硫离子参与形成的S-DSP中硫含量和脱硫率由大到小的顺序为 、
、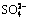 、
、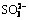 、S2-,且
、S2-,且 和
和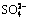 能够显著促进方钠石向钙霞石的转化。S-DSP中硫含量与钙霞石含量线性相关,即含硫离子嵌入过程发生在含硅物相溶解、成核、长大过程中。当溶液初始
能够显著促进方钠石向钙霞石的转化。S-DSP中硫含量与钙霞石含量线性相关,即含硫离子嵌入过程发生在含硅物相溶解、成核、长大过程中。当溶液初始 含量为2.4 g/L、w(S)/w(SiO2)=0.13时,S-DSP可以脱除铝酸钠溶液中63%的硫。
含量为2.4 g/L、w(S)/w(SiO2)=0.13时,S-DSP可以脱除铝酸钠溶液中63%的硫。
关键词:
文章编号:1004-0609(2020)-08-1906-09 中图分类号:TF802 文献标志码:A
拜耳法是目前氧化铝生产的主要方法,包括铝土矿溶出、赤泥沉降分离、过饱和铝酸钠溶液晶种分解和氢氧化铝焙烧等过程[1]。中国适用于现行拜耳法生产氧化铝的优质铝土矿资源逐渐短缺,合理利用储量巨大的高硫铝土矿(S含量大于1%)可进一步保障资源供给。铝土矿溶出过程(包含预脱硅和高温溶出)中,黄铁矿中硫主要以S2-的形式进入溶液并随循环累积[2],造成碱耗增大、设备腐蚀和产品铁含量超标[2-4]。针对高硫铝土矿的处理方法大致可分为两类:①预处理脱硫,减少进入拜耳法流程的总硫量,如焙烧[5-6]、选矿[7-8]、电解[9]等;②溶液脱硫,减少硫在拜耳法流程中的积累,如通过加入ZnO[10]、Fe(OH)2和 [11-12]等形成相应硫化物以脱除S2-,或将溶液中低价硫离子氧化为
[11-12]等形成相应硫化物以脱除S2-,或将溶液中低价硫离子氧化为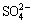 后再加入含钡化合物[13]、石灰[14]等形成相应硫酸盐沉淀。这些方法在一定程度上减轻了硫对拜耳法过程的影响,但存在成本高,或脱硫不彻底的问题,致使至今仍无法直接通过拜耳法短流程处理高硫铝土矿。
后再加入含钡化合物[13]、石灰[14]等形成相应硫酸盐沉淀。这些方法在一定程度上减轻了硫对拜耳法过程的影响,但存在成本高,或脱硫不彻底的问题,致使至今仍无法直接通过拜耳法短流程处理高硫铝土矿。
需要指出的是,除上述脱硫剂外,铝土矿中的含硅矿物本身也具有一定的脱硫能力。以高岭土(Al2O3·2SiO2·2H2O)为例,在高温铝酸钠溶液中,其首先溶解形成硅酸根和铝酸根(见式(1)),然后析出为脱硅产物(DSP,Na6(Al6Si6O24)·Na2X·yH2O)(见式(2))[15],DSP中X2-可以是2Cl-、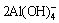 、2OH-、S2-、
、2OH-、S2-、 、
、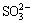 、
、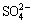 、
、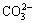 等阴离子,部分阴离子嵌入方钠石能力由大到小的顺序为
等阴离子,部分阴离子嵌入方钠石能力由大到小的顺序为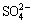 >>
>> 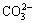 ≥Cl->
≥Cl->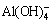 >>OH-[16]。
>>OH-[16]。
Al2O3·2SiO2·2H2O+6OH-→2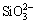 +2
+2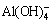 +H2O (1)
+H2O (1)
6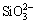 +6
+6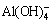 +8Na++X2-+6(y-6)H2O→Na6(Al6Si6O24)·Na2X·yH2O+12OH- (2)
+8Na++X2-+6(y-6)H2O→Na6(Al6Si6O24)·Na2X·yH2O+12OH- (2)
充分利用脱硅产物和含硫离子的相互作用有望大幅度脱除铝酸钠溶液中的硫,并进一步实现高硫铝土矿的拜耳法直接利用。前期工作已证明S2-能够与含硅矿物反应形成含硫钠硅渣,但有石灰存在时,钠硅渣向钙硅渣的转化将导致硫元素返回溶液[17]。为此,我们提出采用非钙溶出添加剂取代石灰以避免水合铝硅酸钙的形成[18],从而最大限度利用脱硅产物(添加非钙添加剂时主要为水合铝硅酸钠,钠硅渣)除硫。但即便如此,由于拜耳法溶出过程中含硫离子和脱硅产物形态复杂,不同含硫离子和脱硅产物间的作用关系并不明晰,导致含硫脱硅产物的形成与转化机制缺乏理论支撑。
铝酸钠溶液中S2-可被氧化为 ,
,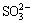 和
和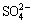 [19-20],而脱硅产物可能以沸石(ZEO,1.08Na2O·Al2O3·1.68SiO2·1.8H2O,立方晶系)[21]、方钠石(SOD,Na6(Al6Si6O24)·2NaOH·2H2O,立方晶系)和钙霞石(CAN,Na6(Al6Si6O24)·Na2CO3·2H2O,六方晶系)等多种形态存在[22]。因此,本文拟研究模拟拜耳法溶出条件下S2-、
[19-20],而脱硅产物可能以沸石(ZEO,1.08Na2O·Al2O3·1.68SiO2·1.8H2O,立方晶系)[21]、方钠石(SOD,Na6(Al6Si6O24)·2NaOH·2H2O,立方晶系)和钙霞石(CAN,Na6(Al6Si6O24)·Na2CO3·2H2O,六方晶系)等多种形态存在[22]。因此,本文拟研究模拟拜耳法溶出条件下S2-、 、
、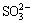 和
和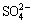 四种含硫离子与含硅物质的反应能力,并探究含硫离子对脱硅物相转化(方钠石→钙霞石)的影响,以期明确含硅矿物脱除含硫离子的机理和调控措施,从而为开发短流程经济处理高硫铝土矿技术提供理论依据。
四种含硫离子与含硅物质的反应能力,并探究含硫离子对脱硅物相转化(方钠石→钙霞石)的影响,以期明确含硅矿物脱除含硫离子的机理和调控措施,从而为开发短流程经济处理高硫铝土矿技术提供理论依据。
1 实验
1.1 实验原料
为排除杂质矿物的影响,所用含硫化合物(硫化钠、硫酸钠、亚硫酸钠和硫代硫酸钠)和含硅物质(硅酸钠、方钠石和钙霞石)均为分析纯或实验室合成。铝酸钠溶液(Na2Ok 350 g/L,Al2O3 192 g/L)由分析纯氢氧化铝、氢氧化钠和去离子水配制,其中Na2Ok为苛性碱浓度。
1.2 实验过程
低温(≤150 ℃)和高温实验(≥180 ℃)分别在甘油和熔盐介质加热的低压、高压反应群釜(DY-8/XYF-6,中南大学机械厂)中进行,控温精度均为±1 ℃。
实验步骤:向不锈钢反应釜中加入一定体积的铝酸钠溶液含硅物和含硫化合物,然后加入去离子水使体系总体积为100 mL,即调整体系Na2Ok为230 g/L。其中,Na2S2O3、Na2SO4和Na2SO3以固体形式加入,Na2S则是溶解于去离子水中标定后以溶液形式加入。含硫化合物添加量以S元素的含量计,表示为ρ0(S) (g/L)。再向反应釜中加入2×d 18 mm和2×d 8 mm共计4粒钢球以加强搅拌,将反应釜密封后浸入到已加热至设定温度的加热介质中,持续转动(转速为150 r/min)至反应时间后取出反应釜并浸入冷水中使其冷却至室温,反应浆液过滤并用沸水洗涤滤饼,收集滤液并将滤饼放入烘箱于85 ℃烘干,用于成分和物相分析。
1.3 样品表征
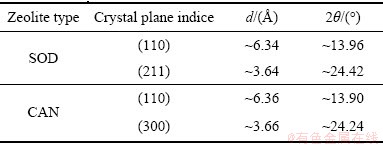
钠硅渣中硫含量(以S元素的质量分数计,表示为w(S)(%))采用测硫仪(HDS3000,湖南华德电子有限公司,中国)测定,测试温度为1150 ℃,误差为±0.01%。滤液中硅含量用电感耦合等离子光谱发生仪(ICP,ICAP7400 Radial,赛默飞世尔科技公司,美国)测定,并换算为SiO2含量,表示为ρ(SiO2) (g/L)。钠硅渣的物相分析采用X射线衍射分析仪(铜靶,D/MAX2500X,理学株式会社,日本),扫描速率为10 (°)/min。衍射图谱通过jade 6.5软件解析。如表1所示,方钠石的XRD谱中(211)晶面对应的最强峰和(110)晶面对应的次强峰分别与钙霞石的XRD谱中(300)和(110)晶面对应的衍射峰基本重合,难以运用内标法[23]、RIR法[24-25]等方法计算方钠石、钙霞石的质量分数。同时,在方钠石转化为钙霞石的过程中,随着钙霞石含量的增大,其(101)和(211)晶面对应衍射峰的强度不断增强。基于此变化规律,Barnes将不同质量的方钠石和钙霞石混合,对混合物进行XRD分析,计算各衍射图中C211/ (C110+S110)、C211/(C300+S211)、C101/(C110+S110)和C101/ (C300+S211)的数值。结果表明,上述四个变量均与钙霞石质量分数呈正比例关系,因此可以通过四个变量的线性组合来计算钙霞石的质量分数,表示为w(CAN),具体参数如式(3)~(7)所示。钙霞石含量较低时,该计算方法更加敏感。当质量分数在10%以内,误差为±5%;当质量分数在20%以上,误差为±8%。该方法的计算下限为钙霞石含量2.5%[26]。
表1 方钠石和钙霞石的特征衍射峰
Table 1 Characteristic X-ray diffraction peaks of sodalite and cancrinite
C1=[C211/(C110+S110)]/0.024804 (3)
C2=[C211/(C300+S211)]/0.017963 (4)
C3=[C101/(C110+S110)]/0.017895 (5)
C4=[C101/(C300+S211)]/0.012961 (6)
w(CAN)=(C1+C2+C3+C4)/4 (7)
式中:C211、C101为钙霞石(211)、(101)晶面对应衍射峰的强度;C110+S110为钙霞石(110)与方钠石(110)重合衍射峰的强度;C300+S211为钙霞石(300)与方钠石(211)重合衍射峰的强度。
2 结果与讨论
2.1 预脱硅过程中含硫离子的行为
在预脱硅条件下,部分高活性硅矿物(高岭石和蛋白石)即可与铝酸钠溶液反应形成脱硅产物。为明确预脱硅条件下含硫离子的形态对其嵌入钠硅渣行为的影响,将不同的含硫化合物(Na2S2O3、Na2SO4、Na2SO3和Na2S)、硅酸钠溶液(SiO2 18 g/L)和铝酸钠溶液混合后在100 ℃反应8 h,脱硅产物中硫含量和脱硫率的变化如图1所示。
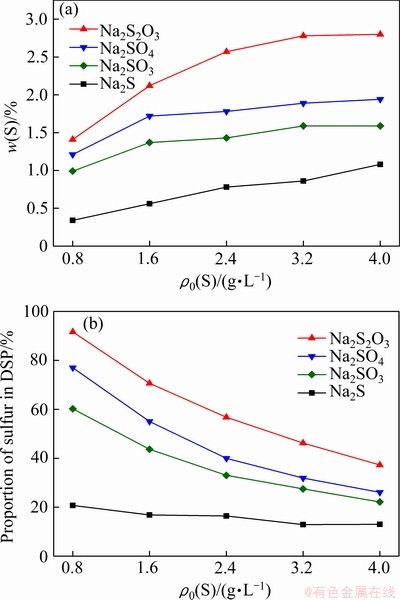
图1 含硫离子种类对低温脱硅产物硫含量和脱硫率的影响(100 ℃, 8 h)
Fig. 1 Effect of sulfur ion species on sulfur content of S-DSP (a) and sulfur removal rate (b) (100 ℃, 8 h)
由图1(a)可知,钠硅渣含硫率随初始硫含量增加逐步上升,初始硫含量相同时,含硫离子参与形成的钠硅渣中硫含量由大到小的顺序为 、
、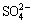 、
、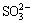 、S2-。添加
、S2-。添加 时所得钠硅渣硫含量约为2.8% (ρ0(S)=4.0 g/L),显著高于其他三种含硫离子,因此调控溶液中含硫离子形态至
时所得钠硅渣硫含量约为2.8% (ρ0(S)=4.0 g/L),显著高于其他三种含硫离子,因此调控溶液中含硫离子形态至 有利于硫的脱除。此外,从图1(b)中还可以看出,当含硫离子主要以
有利于硫的脱除。此外,从图1(b)中还可以看出,当含硫离子主要以 存在时,通过含硫脱硅产物的形成可以脱除溶液中92%的含硫离子(ρ0(S)=0.8 g/L),但升高初始硫浓度导致脱硫率下降,表明含硫脱硅产物中硫的容量是有限度的。
存在时,通过含硫脱硅产物的形成可以脱除溶液中92%的含硫离子(ρ0(S)=0.8 g/L),但升高初始硫浓度导致脱硫率下降,表明含硫脱硅产物中硫的容量是有限度的。
对图1中样品进行物相分析,结果如图2所示。空白试验中钠硅渣主要物相为方钠石和沸石,在17°~39°可观察到驼峰状突起,表明钠硅渣含有非结晶相[27]。从图2(a)和(b)可知,当 、
、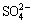 的初始含量分别为0.8 g/L和1.6 g/L时,钠硅渣的XRD在2θ为18.86°和27.43°处出现新的衍射峰,分别对应于钙霞石的(101)和(211)晶面,即部分方钠石转化为钙霞石,通过式(3)~(7)计算得到钙霞石含量分别约为7%和3%,其余物相为沸石、方钠石和无定形相;当其初始含量分别增大至1.6 g/L和2.4 g/L时,(101)和(211)的晶面强度增大,钙霞石的含量分别约为23%和9%,此后随着其添加量增大,钙霞石的含量未继续升高。从图2(c)和(d)可知,随着S2-和
的初始含量分别为0.8 g/L和1.6 g/L时,钠硅渣的XRD在2θ为18.86°和27.43°处出现新的衍射峰,分别对应于钙霞石的(101)和(211)晶面,即部分方钠石转化为钙霞石,通过式(3)~(7)计算得到钙霞石含量分别约为7%和3%,其余物相为沸石、方钠石和无定形相;当其初始含量分别增大至1.6 g/L和2.4 g/L时,(101)和(211)的晶面强度增大,钙霞石的含量分别约为23%和9%,此后随着其添加量增大,钙霞石的含量未继续升高。从图2(c)和(d)可知,随着S2-和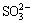 添加量的增大,沸石(311)晶面对应衍射峰逐渐消失,同时XRD中的驼峰逐渐消失,说明沸石逐渐转化为方钠石,非结晶相含量减小,但并未出现钙霞石的特征峰。因此,在
添加量的增大,沸石(311)晶面对应衍射峰逐渐消失,同时XRD中的驼峰逐渐消失,说明沸石逐渐转化为方钠石,非结晶相含量减小,但并未出现钙霞石的特征峰。因此,在 和
和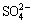 的作用下,初始生成的沸石和方钠石在100 ℃能够部分转化为钙霞石,
的作用下,初始生成的沸石和方钠石在100 ℃能够部分转化为钙霞石, 的促进效果更加显著,结合
的促进效果更加显著,结合 脱除率高的结果可以推测出硫离子的脱除可能与钠硅渣物相的转化存在关系,而当溶液中存在
脱除率高的结果可以推测出硫离子的脱除可能与钠硅渣物相的转化存在关系,而当溶液中存在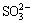 时,反应则直接生成方钠石,即表明方钠石和钙霞石晶体结构的差异同样可能是影响含硫离子脱除的原因之一。
时,反应则直接生成方钠石,即表明方钠石和钙霞石晶体结构的差异同样可能是影响含硫离子脱除的原因之一。
2.2 含硅物相高温转化过程中含硫离子的行为
预脱硅条件下形成的钠硅渣在高温溶出过程中会逐渐转化为钙霞石,为明确含硫离子在这一转化过程中的行为,将硅酸钠溶液、铝酸钠溶液和含硫化合物在100 ℃反应8 h后转移至260 ℃继续反应2 h。高温过程中钠硅渣硫含量和脱硫率随含硫化合物添加量的变化如图3所示。
对于Na2S、Na2SO3和Na2SO4,钠硅渣硫含量和脱硫率随初始硫含量变化的规律与2.1节中结果一致。而对于Na2S2O3,初始含量为0.8 g/L时高温转化后钠硅渣硫含量较低,这可能是由于高温转化过程中钠硅渣中的 伴随着脱硅产物部分溶解重新进入溶液,在高温部分分解转变为S2-、
伴随着脱硅产物部分溶解重新进入溶液,在高温部分分解转变为S2-、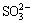 [10],使嵌入钠硅渣中
[10],使嵌入钠硅渣中 的相对含量降低,钠硅渣硫含量也随之降低。而随着添加量的增大,这一影响逐渐减弱,由此也可以看出,在与钠硅渣的结合过程中
的相对含量降低,钠硅渣硫含量也随之降低。而随着添加量的增大,这一影响逐渐减弱,由此也可以看出,在与钠硅渣的结合过程中 比S2-和
比S2-和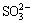 更占优势。
更占优势。
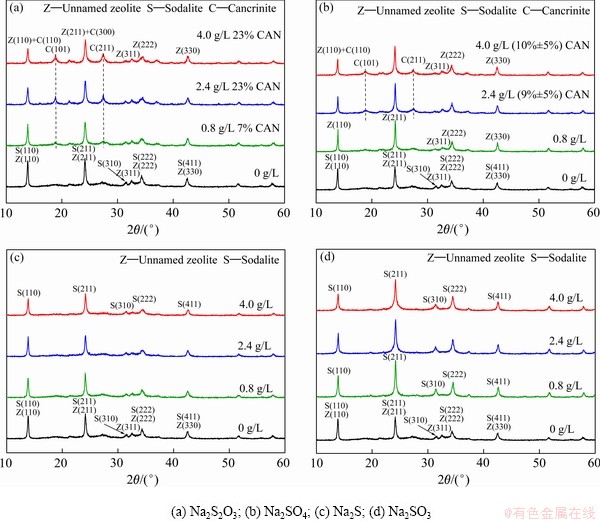
图2 预脱硅条件下S-DSP的XRD谱(100 ℃,8 h)
Fig. 2 XRD patterns of S-DSP formed under pre-desilication conditions (Na2Ok 230 g/L, αk 3.0, SiO2 18 g/L, 100 ℃, 8 h)
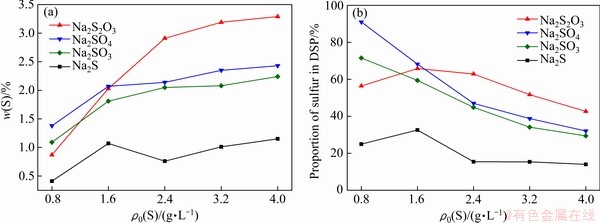
图3 含硫离子种类对高温脱硅产物硫含量和脱硫率的影响(260 ℃, 2 h)
Fig. 3 Effect of sulfur ion species on sulfur content of S-DSP (a) and sulfur removal rate (b) (260 ℃, 2 h)
含硫化合物添加量对高温钠硅渣钙霞石含量的影响如图4所示。Na2S并不能促使低温形成的钠硅渣转化为钙霞石,结合硫含量也基本不变的事实,再次证明了含硫离子嵌入钠硅渣的过程与物相转化过程存在关联。Na2SO3添加量大于2.4 g/L时才可以观察到方钠石向钙霞石的转化,而少量的Na2S2O3和Na2SO4就能显著促进钙霞石的形成。
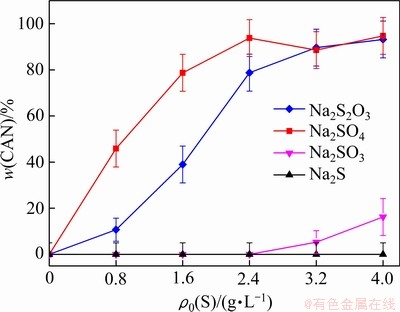
图4 含硫化合物初始含量对高温脱硅产物中钙霞石含量的影响(260 ℃, 2 h)
Fig. 4 Effect of initial sulfur concentration on mass fraction of CAN in S-DSP (260 ℃, 2 h)
考虑到相同硫质量浓度条件下,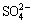 的初始摩尔浓度是
的初始摩尔浓度是 的2倍,因此,
的2倍,因此, 与
与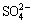 对于转化过程的促进作用需要进一步分析。为此,采用硅酸钠溶液与铝酸钠溶液在100 ℃反应24 h预先合成方钠石,并称取5 g方钠石在140 ℃分别与含有0.125 mol/L Na2S2O3、0.125 mol/L Na2SO4的铝酸钠溶液进行反应。转化时间包括将反应体系由常温升温至140 ℃的时间,约5 min。图5所示分别为添加Na2S2O3和Na2SO4时转化产物中钙霞石含量变化。
对于转化过程的促进作用需要进一步分析。为此,采用硅酸钠溶液与铝酸钠溶液在100 ℃反应24 h预先合成方钠石,并称取5 g方钠石在140 ℃分别与含有0.125 mol/L Na2S2O3、0.125 mol/L Na2SO4的铝酸钠溶液进行反应。转化时间包括将反应体系由常温升温至140 ℃的时间,约5 min。图5所示分别为添加Na2S2O3和Na2SO4时转化产物中钙霞石含量变化。
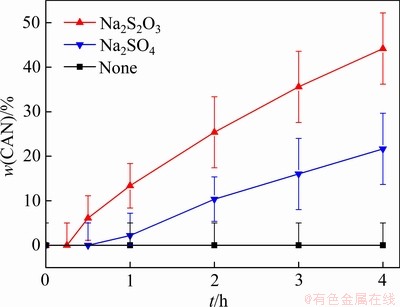
图5 转化时间对脱硅产物中钙霞石含量的影响
Fig. 5 Effect of transformation time on mass fraction of CAN in S-DSP
随着反应时间的延长,空白试验中方钠石未转化为钙霞石,添加等摩尔量的Na2S2O3、Na2SO4时,方钠石迅速转化为钙霞石,且添加Na2S2O3时转化速率达到添加Na2SO4的2倍以上。同时,添加Na2S2O3时运用XRD检测到产物中钙霞石的衍射峰的反应时间在15~30 min,而添加Na2SO4时此反应时间在30~60 min,若以XRD的检出时长来衡量钙霞石晶粒诱导成核时长,则 参与转化时诱导成核时长显著缩短。KUMAR等[28]研究了不同含氧酸根(
参与转化时诱导成核时长显著缩短。KUMAR等[28]研究了不同含氧酸根(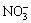 、
、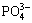 、
、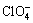 、
、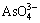 等)对沸石水热合成过程的影响,发现含氧酸根能够极化硅酸根单元周围形成的疏水水化球体,促进硅酸根相互靠近并缩合,加速硅酸根低聚物(主要是四聚体)的形成,从而缩短成核时间,加速晶粒长大,且不同含氧酸根的促进能力正比于含氧酸根的极化能力。因此,转化过程的速率控制步骤可能是铝硅酸根的缩聚过程。由此推测,
等)对沸石水热合成过程的影响,发现含氧酸根能够极化硅酸根单元周围形成的疏水水化球体,促进硅酸根相互靠近并缩合,加速硅酸根低聚物(主要是四聚体)的形成,从而缩短成核时间,加速晶粒长大,且不同含氧酸根的促进能力正比于含氧酸根的极化能力。因此,转化过程的速率控制步骤可能是铝硅酸根的缩聚过程。由此推测, 和
和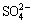 参与反应,加速了铝硅酸根的缩聚,进而促进了方钠石的转化,且
参与反应,加速了铝硅酸根的缩聚,进而促进了方钠石的转化,且 的极化能力强于
的极化能力强于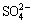 的。因此,相同摩尔含量时,不同含硫离子对于方钠石向钙霞石转化的促进能力由大到小的顺序为
的。因此,相同摩尔含量时,不同含硫离子对于方钠石向钙霞石转化的促进能力由大到小的顺序为 、
、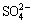 、
、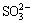 、S2-,即在拜耳法流程中,通过调控含硫离子的形态可以实现含硫离子的进一步脱除。
、S2-,即在拜耳法流程中,通过调控含硫离子的形态可以实现含硫离子的进一步脱除。
2.3 含硫离子嵌入与含硅物相转化的线性关系
表2 DSP-Ⅰ转化产物中硫含量和溶液SiO2含量(100 ℃)
Table 2 Sulfur content of DSP-Ⅰ transformation product and SiO2concentration in solution (100 ℃)
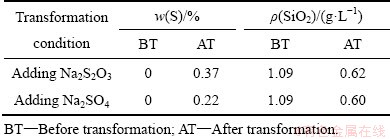
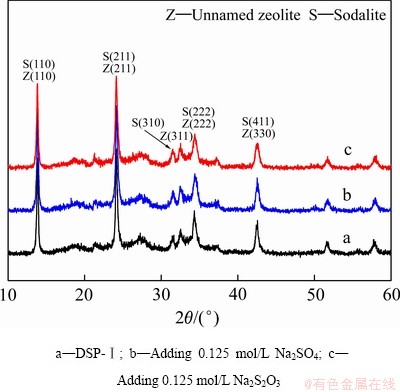
图6 DSP-Ⅰ及其在100 ℃转化产物的XRD谱
Fig. 6 XRD patterns of DSP-Ⅰ and its conversion products (100 ℃)
为明确含硫离子嵌入钠硅渣与含硅物相转化的内在联系,采用硅酸钠溶液与铝酸钠溶液在100 ℃反应8 h生成不含硫钠硅渣(以DSP-Ⅰ代指),再向体系中加入0.125 mol/L Na2S2O3或Na2SO4,然后分别在100、140、180、220和260 ℃转化反应2 h。表2所列为DSP-Ⅰ在100 ℃转化后产物硫含量和溶液SiO2含量。当分别加入Na2S2O3、Na2SO4后,转化后产物的硫含量分别增大0.37%、0.22%,溶液SiO2分别由1.09 g/L分别减小至0.62 g/L、0.6 g/L。图6所示为DSP-Ⅰ及其在100 ℃转化产物的XRD谱,揭示了硫含量和溶液SiO2含量的原因。添加Na2S2O3或Na2SO4后转化产物的物相与DSP-Ⅰ的基本相同,均由方钠石、沸石和非晶相组成,但谱线b和c中在17°~39°处的驼峰较谱线a中的略有降低,钠硅渣的结晶度增高。结合表2可知,转化过程中少量的非结晶相转化为结晶相,钠硅渣变得更加稳定,溶液中SiO2含量降低。在此转化过程中,少量 、
、 嵌入钠硅渣。
嵌入钠硅渣。
图7所示为DSP-Ⅰ在不同温度转化2 h后产物的硫含量和钙霞石含量。由图7可知,随着转化温度不断升高,钠硅渣硫含量和钙霞石含量不断增大,且硫含量的变化规律与钙霞石含量的变化基本一致。对于不同温度条件下转化产物的钙霞石含量和含硫率的线性拟合结果如图8所示。钠硅渣硫含量与钙霞石含量线性相关。因此可以认为,只有当钠硅渣发生物相变化时,初始物相溶解,发生新的成核、长大过程,含硫离子才能嵌入并共同生成钠硅渣,且当溶液中含硫离子充分过量时,含硫离子的嵌入量正比于新物相形成量,这一结果也揭示了为生产过程中强化硫硅矿物的交互作用提供理论依据。
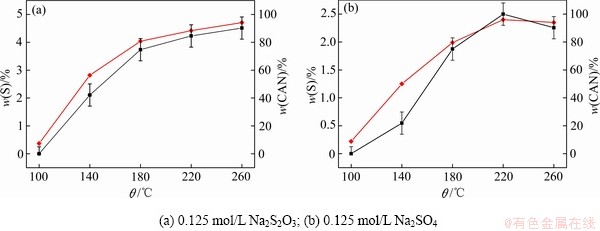
图7 转化温度对脱硅产物中钙霞石与硫含量的影响
Fig. 7 Effects of transformation temperatures on CAN and sulfur contents in S-DSP
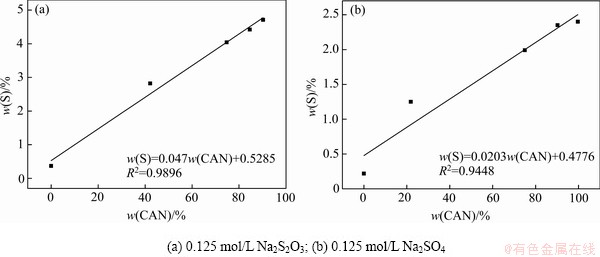
图8 产物的钙霞石含量与硫含量的相关性
Fig. 8 Linear correlation between CAN and sulfur contents in S-DSP
3 结论
1) 拜耳法溶出过程中,不同含硫离子均可与含硅矿物反应形成含硫脱硅产物。溶液中硫含量相同时,不同含硫离子参与形成的S-DSP硫含量和脱硫率由大到小的顺序均为 、
、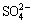 、
、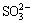 、S2-,且
、S2-,且 和
和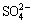 能够显著促进方钠石向钙霞石的转化。
能够显著促进方钠石向钙霞石的转化。
2) S-DSP中硫含量与钙霞石含量线性相关,正比于新物相形成量,这一嵌入过程主要发生在含硅物相的溶解、成核、长大过程中。
3) 当溶液ρ0(S)=2.4 g/L、ρ0(SiO2)=18 g/L,体系初始w(S)/w(SiO2)=0.13,接近于实际矿石硫硅比,调整含硫离子形态为 时,高温转化后钠硅渣能够脱除铝酸钠溶液中63%的硫。
时,高温转化后钠硅渣能够脱除铝酸钠溶液中63%的硫。
4) 研究结果为拜耳法无钙溶出处理高硫铝土矿提供理论依据,但对于高温高碱条件下含硫离子的调控仍需进一步研究。
REFERENCES
[1] SMITH P. The processing of high silica bauxites - Review of existing and potential processes[J]. Hydrometallurgy, 2009, 98(1/2): 162-176.
[2] 李小斌, 李重洋, 齐天贵, 周秋生, 刘桂华, 彭志宏. 拜耳法高温溶出条件下黄铁矿的反应行为[J]. 中国有色金属学报, 2013, 23(3): 829-835.
LI Xiao-bin, LI Chong-yang, QI Tian-gui, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong. Reaction behavior of pyrite during Bayer digestion at high temperature[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(3): 829-835.
[3] HINDA A R, BHARGAVAA S K, GROCOTT S C. The surface chemistry of Bayer process solids: A review[J]. Colloids and Surfaces A, 1999, 146(1/3): 359-374.
[4] XIE Qiao-ling, CHEN Wen-mi. Corrosion behavior of 16Mn low alloy steel in sulfide-containing Bayer solutions[J]. Corrosion Science, 2014, 86: 252-260.
[5] 胡小莲, 陈文汨, 谢巧玲. 高硫铝土矿氧化钙焙烧脱硫研究[J]. 轻金属, 2010(1): 9-14.
HU Xiao-lian, CHEN Wen-mi, XIE Qiao-ling. Study on desulfuration of high sulfur bauxite by calcium oxide roasting[J]. Light Metals, 2010(1): 9-14.
[6] LOU Zhen-ning, XIONG Ying, FENG Xiao-dong, SHAN Wei-jun, ZHAI Yu-chun. Study on the roasting and leaching behavior of high-sulfur bauxite using ammonium bisulfate[J]. 2016, 165: 306-311.
[7] 李长凯, 孙 伟, 张 刚, 孟祥丽, 周苏阳. 调整剂对高硫铝土矿浮选脱硫行为的影响[J]. 有色金属(选矿部分), 2011(1): 56-59.
LI Chang-kai, SUN Wei, ZHANG Gang, MENG Xiang-li, ZHOU Su-yang. An experimental study on the effects of regulators on desulfurization of high-sulfur bauxite[J]. Nonferrous Metals (Mineral Processing Section), 2011(1): 56-59.
[8] 陈文汨, 谢巧玲, 胡小莲, 彭秋燕. 高硫铝土矿反浮选除硫试验研究[J]. 矿冶工程, 2008, 28(3): 34-37.
CHEN Wen-mi, XIE Qiao-lin, HU Xiao-lian, PENG Qiu-yan. Experimental study on reverse flotation technique for desulfurizing of high-sulfur bauxite[J]. Mining and Metallurgical Engineering, 2008, 28(3): 34-37.
[9] GONG Xu-zhong, GE Lan, WANG Zhi, ZHUANG Si-yuan, WANG Yu-hua, REN Li-hui, WANG Ming-yong. Desulfurization from bauxite water slurry (BWS) electrolysis[J]. Metallurgical and Materials Transactions B, 2016, 47(1): 649-656.
[10] 郑立聪, 谢克强, 刘战伟, 马文会. 一水硬铝石型高硫铝土矿脱硫研究进展[J]. 材料导报A, 2017, 31(3): 84-93.
ZHENG Li-cong, XIE Ke-qiang, LIU Zhan-wei, MA Wen-hui. Review on desulfurization of high-sulfur bauxite[J]. Materials Reports A, 2017, 31(3): 84-93.
[11] LI Xiao-bin, LI Chong-yang, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng, QI Tian-gui. Interaction of sulfur with iron compounds in sodium aluminate solutions[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(2): 608-614.
[12] LI Xiao-bin, NIU Fei, TAN Jie, LIU Gui-hua, QI Tian-gui, PENG Zhi-hong, ZHOU Qiu-sheng. Removal of S2- ion from sodium aluminate solutions with sodium ferrite[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(5): 1419-1424.
[13] ZHANG Nian-bing, LI Zhi-ying, GUO Pei-li. Desulfurization mechanism and application of high sulfur bauxite[J]. Metalurgia International, 2013, 18(3): 29-32.
[14] 罗玉长, 叶长龙. 铝酸钠溶液脱硫的研究[J]. 轻金属, 2003(4): 9-11.
LUO Yu-chang, YE Chang-long. A study of desulphurization of sodium aluminate solution[J]. Light Metals, 2003(4): 9-11.
[15] RADOMIROVICA T, SMITH P, SOUTHAM D, TASHI S, JONES F. Crystallization of sodalite particles under Bayer-type conditions[J]. Hydrometallurgy, 2013, 137: 84-91.
[16] PENG Hong, DING Meng-liu, VAUGHAN J. The anion effect on zeolite Linde type A to sodalite phase transformation[J]. Industrial and Engineering Chemistry Research, 2018, 57(31): 10292-10302.
[17] LI Xiao-bin, LI Chong-yang, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong. Interaction of sodium sulfide with silica-containing minerals in sodium aluminate solution[J]. International Journal of Mineral Processing, 2015, 137: 9-14.
[18] LI Xiao-bin, WANG Yi-lin, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong, WANG Hong-yang. Transformation of hematite in diasporic bauxite during reductive Bayer digestion and recovery of iron[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(12): 2715-2726.
[19] ABIKENOVA G K, KOVZALENKO V A, AMBARNIKOVA G A, IBRAGIMOV A T. Investigation of the effect and behavior of sulfur compounds on the technological cycle of alumina production[J]. Russian Journal of Non-Ferrous Metals, 2008, 49(2): 28-33.
[20] LIU Zhan-wei, LI Wang-xing, MA Wen-hui, YIN Zhong-lin, WU Guo-bao. Conversion of sulfur by wet oxidation in the Bayer process[J]. Metallurgical and Materials Transactions B, 2015, 46(4): 1702-1708.
[21] PAN Xiao-lin, YU Hai-yan, TU Gan-feng, BI Shi-wen. Effects of precipitation activity of desilication products (DSPs) on stability of sodium aluminate solution[J]. Hydrometallurgy, 2016, 165: 261-269.
[22] WHITTINGTON B I, FLETCHER B L, TALBOT C. The effect of reaction conditions on the composition of desilication product DSP/formed under simulated Bayer conditions[J]. Hydrometallurgy, 1998, 49(1/2): 1-22.
[23] ALEXANDER L, KLU H P. Basic aspects of X-ray absorption in quantitative diffraction analysis of powder mixtures[J]. Analytical Chemistry, 1948, 20(10): 886-889.
[24] CHUNG F H. Quantitative interpretation of X-ray diffraction patterns of mixtures.Ⅰ. Matrix-flushing method for quantitative multicomponent analysis[J]. Journal of Applied Crystallography, 1974, 7: 519-525.
[25] CHUNG F H. Quantitative interpretation of X-ray diffraction patterns of mixtures. Ⅱ. Adiabatic principle of X-ray diffraction analysis of mixtures[J]. Journal of Applied Crystallography, 1974, 7: 526-531.
[26] BARNES M C, ADDAI-MENSAH J, GERSON A R. A methodology for quantifying sodalite and cancrinite phase mixtures and the kinetics of the sodalite to cancrinite phase transformation[J]. Microporous and Mesoporous Materials, 1999, 31(3): 303-319.
[27] BOSNAR S, ANTONIC-JELIC T, BRONIC J, KRZNARIC I, SUBOTIC B. Influence of anions on the kinetics of zeolite A crystallization: A population balance analysis[J]. Journal of Crystal Growth, 2004, 267(1/2): 270-282.
[28] KUMAR R, BHAUMIK A, AHEDI R K, GANAPATHY S. Promoter-induced enhancement of the crystallization rate of zeolites and related molecular sieves[J]. Nature, 1996, 381: 298-299.
Formation and transformation mechanism of sulfur-containing desiliconization products under simulated Bayer digestion conditions
LI Xiao-bin1, 2, XI Ya-wei1, 2, WANG Yi-lin1, 2, QI Tian-gui1, 2, LIU Gui-hua1, 2, ZHOU Qiu-sheng1, 2, PENG Zhi-hong1, 2
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Powder Metallurgy Research Institute, Central South University, Changsha, 410083, China)
Abstract: The formation and transformation mechanisms of sulfur-containing desiliconization products (S-DSP) were studied under simulated Bayer digestion conditions by analyzing the phase and composition changes. The results show that the sulfur content and desulfurization rate of S-DSP decrease following the order of  ,
, 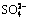 ,
, 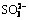 and S2-. Meanwhile,
and S2-. Meanwhile,  and
and 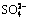 markedly promote the transformation of sodalite to cancrinite. The sulfur content in S-DSP is linearly correlated with the cancrinite transformation rate, indicating that the S-DSP forms through a nucleation and growth process. 63% of the total sulfur is removed from a simulated
markedly promote the transformation of sodalite to cancrinite. The sulfur content in S-DSP is linearly correlated with the cancrinite transformation rate, indicating that the S-DSP forms through a nucleation and growth process. 63% of the total sulfur is removed from a simulated  -bearing Bayer liquor (ρ0(S)=2.4 g/L, w(S)/w(SiO2)=0.13) by incorporating sulfur-bearing ions into desiliconization products. This progress provides a theoretical basis for the treatment of high-sulfur bauxite by lime-free Bayer digestion process.
-bearing Bayer liquor (ρ0(S)=2.4 g/L, w(S)/w(SiO2)=0.13) by incorporating sulfur-bearing ions into desiliconization products. This progress provides a theoretical basis for the treatment of high-sulfur bauxite by lime-free Bayer digestion process.
Key words: Bayer process; high-sulfur bauxite; sulfur-containing ion; sodalite; cancrinite
Foundation item: Projects(51604309, 51804142) supported by the National Natural Science Foundation of China; Project(2019M652799) supported by the China Postdoctoral Science Foundation
Received date: 2019-09-19; Accepted date: 2019-12-10
Corresponding author: WANG Yi-lin; Tel: +86-731-88877830; E-mail: wang.yi.lin@outlook.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51604309,51804142);中国博士后科学基金资助项目(2019M652799)
收稿日期:2019-09-19;修订日期:2019-12-10
通信作者:王一霖,博士;电话:0731-88877830;E-mail:wang.yi.lin@outlook.com
摘 要:结合物相和成分分析结果,阐明了模拟拜耳法溶出条件下含硫脱硅产物(S-DSP)的形成和转化机制。结果表明:不同含硫离子参与形成的S-DSP中硫含量和脱硫率由大到小的顺序为 、
、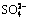 、
、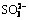 、S2-,且
、S2-,且 和
和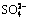 能够显著促进方钠石向钙霞石的转化。S-DSP中硫含量与钙霞石含量线性相关,即含硫离子嵌入过程发生在含硅物相溶解、成核、长大过程中。当溶液初始
能够显著促进方钠石向钙霞石的转化。S-DSP中硫含量与钙霞石含量线性相关,即含硫离子嵌入过程发生在含硅物相溶解、成核、长大过程中。当溶液初始 含量为2.4 g/L、w(S)/w(SiO2)=0.13时,S-DSP可以脱除铝酸钠溶液中63%的硫。
含量为2.4 g/L、w(S)/w(SiO2)=0.13时,S-DSP可以脱除铝酸钠溶液中63%的硫。
[2] 李小斌, 李重洋, 齐天贵, 周秋生, 刘桂华, 彭志宏. 拜耳法高温溶出条件下黄铁矿的反应行为[J]. 中国有色金属学报, 2013, 23(3): 829-835.
[5] 胡小莲, 陈文汨, 谢巧玲. 高硫铝土矿氧化钙焙烧脱硫研究[J]. 轻金属, 2010(1): 9-14.
[7] 李长凯, 孙 伟, 张 刚, 孟祥丽, 周苏阳. 调整剂对高硫铝土矿浮选脱硫行为的影响[J]. 有色金属(选矿部分), 2011(1): 56-59.
[8] 陈文汨, 谢巧玲, 胡小莲, 彭秋燕. 高硫铝土矿反浮选除硫试验研究[J]. 矿冶工程, 2008, 28(3): 34-37.
[10] 郑立聪, 谢克强, 刘战伟, 马文会. 一水硬铝石型高硫铝土矿脱硫研究进展[J]. 材料导报A, 2017, 31(3): 84-93.


