
Reaction mechanism in high Nb containing TiAl alloy by elemental powder metallurgy
WANG Yan-hang(王衍行)1, LIN Jun-pin(林均品)1, HE Yue-hui(贺跃辉)2, WANG Yan-li(王艳丽)1, LIN Zhi(林 志)1, CHEN Guo-liang(陈国良)1
1. State Key Laboratory for Advanced Metals and Materials, University of Science and Technology Beijing,
Beijing 100083, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 15 September 2005; accepted 5 March 2006
Abstract:
High Nb containing TiAl alloy was fabricated in argon atmosphere by reactive hot pressing process. Reaction mechanism was investigated by means of microstructural analyses and thermodynamic calculations. The results show that it is feasible to prepare high Nb containing TiAl alloy with fine lamellar colonies by reactive hot pressing process. The reaction between Ti and Al powders is dominant in Ti-Al-Nb system. Nb powders dissolve into the Ti-Al matrix by diffusion. Pore nests are formed in situ after Nb powders diffusion. The hot pressing atmosphere is optimized by thermodynamic calculations. Vacuum or argon protective atmosphere should be adopted.
Key words:
TiAl based alloy; high Nb containing TiAl alloy; powder metallurgy; reaction mechanism; thermodynamics;
1 Introduction
High Nb containing TiAl alloys have attracted much attention owing to their low densities and potential applications at high-temperature environments[1,2]. It has been found that Nb is the essential and effective element improving their mechanical properties, especially, high-temperature strength[3]. However, high Nb addition enhances the difficulty of their preparation. At present, high Nb containing TiAl alloys are mostly fabricated by ingot metallurgy, but this process is usually encountered with macrosegregations[4]. Meanwhile, the inherent low-temperature ductility of high Nb containing TiAl alloys is poor[5]. Therefore, many advanced processing routes have been attempted to improve their microstructural homogeneity and workability, such as forging, hot isostatic pressing(HIP) and extrusion, but their microstructures are coarse[4, 6].
Recently, powder metallurgy technique is of special interest since high degrees of chemical homogeneities can be obtained and large scale segregations are avoided [7]. Among the powder metallurgy techniques, the elemental powder metallurgy(EPM) is an attractive technique due to economical reasons. The particular technique has been investigated extensively by DAHMS et al[8], KIM et al[9] as well as HWANG et al[10] for producing TiAl based alloys. However, little attention has been paid to the preparation of high Nb containing TiAl alloys through elemental powder metallurgy. Therefore, it is interesting to fabricate high Nb containing TiAl alloys with a fine and homogeneous microstructure to overcome the problem of poor workability by EPM. Reaction mechanism is important to improve the understanding of reaction process and optimize the preparation technique. In this study, reaction mechanism in high Nb containing TiAl alloy prepared by elemental powder metallurgy is discussed by microstructural analyses and thermodynamic calcula- tions.
2 ExperimentalElemental Ti, Al, Nb, W and B powders with mean particle sizes smaller than 25 μm were mixed in air to desired composition of Ti-45Al-9 (Nb, W, B) (mole frac-tion, %). The powder mixture was die-pressed under a pressure of 380 MPa to green compacts of 32 mm in diameter and 12 mm in height. The compacts were then put into a graphite die and hot pressed to form sintered billets for 1 h in an argon protective atmosphere under a pressure of 25 MPa at 670 ℃ (TAN1), 1 300 ℃ (TAN2) and 1 400℃(TAN3), respectively. Differential scanning calorimeter (DSC) was adopted to analyze the reaction process at heating rate of 20 K/min. Micro- structural observation was carried out by scanning electron microscopy(SEM) using back scattering electron imaging (BSE) and energy dispersive spectroscopy(EDS). X-ray diffraction(XRD) was conducted to characterize the constituent phases.
3 Results3.1 DSC test
Fig.1 shows the DSC curve of the blended Ti-45Al-9(Nb, W, B) powders. It can be seen that the curve contains one endothermic peak and one exothermic peak under 1 200 ℃, respectively. The endothermic peak (937 K) corresponds to the melting of elemental Al powders and the exothermic peak (983 K) accords with the reaction between elemental Ti and Al powders: Ti+3Al→TiAl3. The curve increases slowly below melting point of Al, which indicates that the exothermic reaction between solid Ti and Al powders has happened.
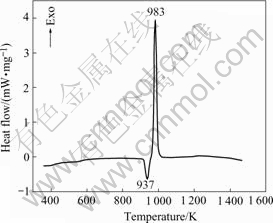
Fig.1 DSC curve of Ti-45Al-9 (Nb, W, B) alloy
3.2 Microstructure analyses
The XRD patterns in Fig.2 illustrate the phase composition of samples after reactive hot pressing. TAN1 mainly consists of Ti, Al, Nb and TiAl3 phases; while TAN 2 is composed of TiAl, Ti3Al, and a small amount of Nb powders. However, the diffraction peaks of element Nb disappear in TAN 3, and TiAl, Ti3Al and β(B2) phases are observed. It is deduced that, therefore, with the increase of hot pressing temperature, the reaction between Ti and Al powders becomes more sufficient, and the content of remnant Nb powders is smaller. The diffraction peaks of TiAl and Ti3Al phases in TAN 3 are relatively sharp, indicating that their degree of crystallization in TAN 3 is better.
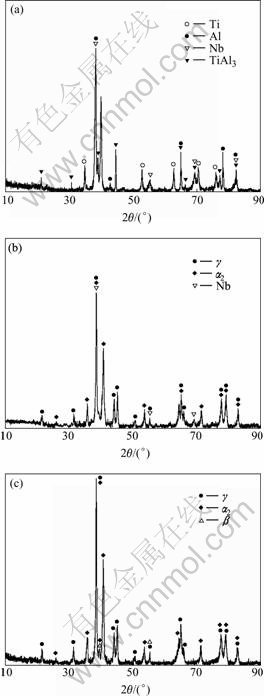
Fig.2 XRD patterns of samples hot pressed at different temperatures: (a) TAN 1; (b) TAN 2; (c) TAN 3
Fig.3(a) shows the BSE microstructure of TAN 1. EDS analyses results of Fig.3(a) are shown in Table 1, which suggests that TiAl3 phase forms around Ti powders through the reaction between Ti and Al powders.

Fig.3 BSE microstructures of samples hot pressed at different temperatures: (a) TAN1; (b) TAN2; (c) TAN3
Table 1 EDS analyses of Fig.3(a) (mole fraction, %)
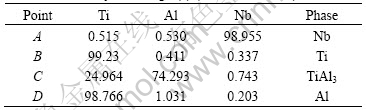
Table 2 EDS analyses of Fig.3(b) (mole fraction, %)
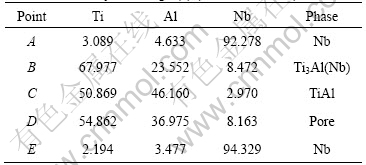
Fig.3(b) and Table 2 show that the large Nb powders are crashed into some small particles under the applied force in TAN 2. Lots of pores (also called pore nests) form around the crashed Nb powders. However, some Nb powders with smaller sizes do not get crashed. The inhomogeneity is related to applied unilateral force in the hot pressing process. Furthermore, the micro- structure of TAN 2 is inhomogeneous. The matrix is mainly TiAl phase; while the particulate substances are composed of island-like Ti3Al phase and Nb powders.
Fig.3(c) shows the BSE microstructure of TAN 3. It indicates that pore nests also form, and compression deformation as well as agglomeration phenomena of pores are observed in some pore nests. Similarly, A typical nearly lamellar (NL) microstructure containing fine lamellar colonies (α2/γ) within 30-50 μm and a few equiaxed γ grains distributing around lamellar colonies form. Furthermore, a small amount of lumpish β (B2) phase and acicular borides appear in Ti-Al matrix[6].
3.3 Thermodynamic calculation
Free energy variations of the reactions between the elements (Ti, Al and Nb) and O2 were calculated to optimize hot pressing atmosphere[11]. The calculated results indicate that all the oxidation reactions can perform spontaneously within 2 000 K (Fig.4).
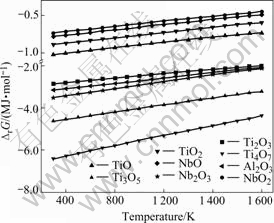
Fig.4 Relationship between free energy of oxidation and tem- perature
4 Discussion
Reaction mechanism is usually investigated from the point of view of crystallography (microstructure), thermodynamics and kinetics. Microstructural analyses and thermodynamic calculations are conducted in this study. It can be seen that the reaction between Ti and Al powders is more complete, and microstructure is more homogeneous with rising of hot pressing temperature from microstructural analyses. TiAl3 phase is the only production at low reactive temperature (such as 670 ℃). Lots of particulate substances containing Ti3Al phase linked to island-like and Nb powders are dispersed in TiAl phase matrix at 1 300 ℃. However, nearly lamellar microstructure with fine lamellar colonies forms at 1 400 ℃.
Thermodynamic assessment and calculation of the ternary Ti-Al-Nb system has been studied extensively [12-14]. Most calculations of Ti-Al-Nb system were based on the accepted calculations of the binary systems and available experimental data. KATTNER et al[15,16] considered that the free energy of formation (?Gf) of intermetallics in binary systems (Ti-Al and Nb-Al) was a linear function of temperature(T), namely
?Gf =A+BT
Fig.5 shows the relationship between free energy of formation of intermetallics in Ti-Al-Nb system and temperature. It can be seen that ?Gf of NbAl3 is the lowest, which indicates NbAl3 phase should be the first production. However, NbAl3 intermetallic is not found in the DSC curve and microstructural analyses. In fact, thermodynamic calculations are not in contradiction with the experimental results. The main reason is that, thermodynamic calculations are based on the binary systems; while the investigated alloy is Ti-Al-Nb ternary system. A ternary system cannot be simply divided into three binary systems, for the reaction in every binary system is affected by the third element. Generally, Ti seems to be the fastest specie, Al having a mobility close to that of Ti, and Nb being the lowest mover in Ti-Al-Nb system at higher temperature (such as 1 200 ℃)[17]. But the diffusivity of Al is faster than that of Ti at low temperature[7]. Ti powders can react with Al powders by solid diffusion below the melting point of Al because their diffusivities are higher. With increasing sintering temperature, the diffusivities of Ti and Al and their reaction velocity increase, which makes Al powder be consumed completely in a short time. Nb2Al and Nb3Al phases cannot form at elevated temperatures even if their free energies of formation are lower than those of TiAl and Ti3Al. It can be deduced that, therefore, the reaction ability between Ti and Al powders is dominant in Ti-Al-Nb system, which restrains the reaction between Nb and Al powders. At last, only titanium aluminides form, while elemental Nb powders dissolve into Ti-Al matrix by diffusion. Pore nests formed in situ after Nb powders diffusion.
Similarly, the free energies of formation of TiAl2 and Ti2Al5 are the lowest in Ti-Al binary system as shown in Fig.5. However, they are not formed initially, for the formation of these transient phases is based on the existence of TiAl phase[18]. Therefore, TiAl3 should be the first produce, and then the TiAl and Ti3Al phases are obtained by a series of reactions. This is in accordance with the previous research results on reaction mechanism of Ti-Al system[14,18].
To optimize the sintering atmosphere, the thermo- dynamic calculation of the oxidation reactions were carried out. It can be seen that these elements (Ti, Al and Nb) will be oxidized if oxygen (O2) exists in hot pressing process. Therefore, the sintering process should be performed in vacuum or protective atmosphere for the existence of their oxides are harmful to mechanical property. In general, the protective atmospheres include nitrogen (N2) and argon (Ar). However, Al and Nb will react with N2. In conclusion, hot pressing process in Ar atmosphere is feasible by view of thermodynamics, which is also proved in the present experiment.
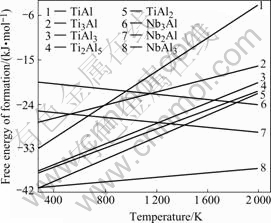
Fig.5 Relationship between free energy of formation of Ti-Al and Nb-Al intermetallics and temperature
5 Conclusions
1) It is suitable to fabricate high Nb containing TiAl alloys with fine lamellar colonies by reactive hot pressing process.
2) Reaction between Ti and Al powders is dominant in the Ti-Al-Nb system. Elemental Nb powders dissolve into the Ti-Al matrix by diffusion. Pore nests will be formed in situ after Nb powders diffusion.
3) Hot pressing atmosphere is optimized by thermodynamic calculations. Vacuum or argon protective atmosphere should be adopted.
References[1] PARANSKY E, GUTMANAS E Y, GOTMAN I, et al. Pressure- assisted reactive synthesis of titanium aluminides from dense 50Al-50Ti elemental powder blends [J]. Metall Mater Trans A, 1996, 27: 2130-2139.
[2] CHEN G, SUN Z, ZHOU X. Oxidation of intermetallic alloys in Nb-Ti-Al ternary system [J]. Corrosion, 1992, 48(11): 939-946.
[3] ZHANG W, CHEN G, WANG Y. Oxidation of ternary Ti18Nb48Al and Ti10Nb45Al [J]. Scripta Materialia, 1993, 28(5): 563-567.
[4] YAN Yun-qi, ZHANG Zhen-qi, LUO Guo-zhen, et al. Microstructures observation and hot compressing tests of TiAl based alloy containing high Nb [J]. Materials Science and Engineering A, 2000, 280: 187-191.
[5] LIU Z C, LIN J P, LI S J, et al. Effects of Nb and Al on the microstructures and mechanical properties of high Nb containing TiAl base alloys [J]. Intermetallics, 2002, 10: 653-659.
[6] XU X J, XU L H, LIN J P, et al. Pilot processing and microstructure control of high Nb containing TiAl alloy [J]. Intermetallics, 2005, 13: 337-341.
[7] WANG G X, DAHMS M. An overview: TiAl-based alloys prepared by elemental powder metallurgy [J]. PMI, 1992, 24(4): 219-225.
[8] YONGCHAN K, NACK J K, TAESIK YOON, et al. Densification behavior of PIMed TiAl parts [J]. Journal of the Japan Society of Powder and Powder Metallurgy, 1999, 46(8): 882-886.
[9] JONATHAN C B, WILLIAM W, MICHAEL C M. Densification of γ-TiAl powder by hot isostatic pressing [J]. The International Journal of Powder Metallurgy, 1992, 28(3): 313-325.
[10] LIU Yong, HUANG Bai-yun, HE Yue-hui, et al. Densification abnormality in reactive hot pressing of Ti and Al elemental powders [J]. Trans Nonferrous Met Soc China, 2000, 10(4): 453-455.
[11] KNACLEE O, KUBASCHEWSKI O, HESSELMANN K. Thermo- chemical Properties of Inorganic Substances [M]. Berlin: Springer- Verlag, 1991: 19-70, 1388-1408, 2057-2099.
[12] SERVANT C, ANSARA I. Thermodynamic assessment of the Al-Nb-Ti system [J]. Physics Chemistry, 1998, 102(9): 1189-1205.
[13] ECKERT M, KATH D, HILPERT K. Thermodynamic activities in the alloys of the Ti-Al-Nb system [J]. Metallurgical and Materials Transactions A, 1999, 30(5): 1315-1326.
[14] KATTNER U R, BOETTINGER W J. Thermodynamic calculation of the ternary Ti-Al-Nb system [J]. Mater Sci Eng A, 1992, A152: 9-17.
[15] OHNUMA I, FUJITA Y, MITSUI H, et al. Phase equilibria in the Ti-Al binary system [J]. Acta Materialia, 2000, 48: 3113-3123.
[16] COLINET C, PASTUREA L, NGUYEN D, et al. Phase-stability study of the Al-Nb system [J]. Physical Review B, 1997, 56(2): 552-565.
[17] EBRAHIMI F, RUIZ-APARICIO J G L. Diffusivity in the Nb-Ti-Al ternary solid solution [J]. Journal of Alloys and Compounds, 1996, 245: 1-9.
[18] SUJATA M, BHARGAVA S, SANGAL S. On the formation of TiAl3 during reaction between solid Ti and liquid Al [J]. Journal of Materials Science Letters, 1996, 16: 1175-1178.
(Edited by LONG Huai-zhong)
Foundation item: Project(704008) supported by the Key Grant Project of Chinese Ministry of Education; Project(NCET-04-01017) supported by the Program for New Century Excellent Talents in University, China
Corresponding author: LIN Jun-pin; Tel: +86-10-62332192; E-mail: linjunpin@skl.ustb.edu.cn


