
Double reverse flotation process of collophanite and regulating froth action
GE Ying-yong(葛英勇), GAN Shun-peng(甘顺鹏), ZENG Xiao-bo(曾小波), YU Yong-fu(余永富)
School of Resources and Environmental Engineering, Wuhan University of Technology, Wuhan 430070, China
Received 16 July 2007; accepted 8 November 2007
Abstract:
A new double reverse flotation process was used to beneficiate the Yichang region collophanite of Hubei Province, China. Its final concentrate yield is 67.37%, P2O5 grade is 32.17%, P2O5 recovery is 87.80%, and the main impurities MgO, Fe2O3 and Al2O3 are 0.95%, 1.04%, 1.36%, respectively. The difficult problem was successfully solved that plentiful froths are bought by the cationic collector reverse flotation in collophanite beneficiation by adding inorganic froth regulator CA to the pulp. The defrothing mechanism was studied through mensurating surface tension and Zeta potential of the pulp after adding CA. It is found that the changing of surface tension and hoisting of Zeta potential may be the main reasons that froths become friable and break up.
Key words:
collophanite ore; reverse flotation; defrothing;
1 Introduction
1.1 Beneficiation methods of phosphate ore
The phosphate deposits are quite abundant in China, which mainly distribute in Yunnan, Guizhou, Sichuan, Chongqing, Hubei and Hunan etc. However, their quality is very poor, because the average grade of P2O5 is only 16.75%, over 75% phosphate rock is medium or low grade collophanite[1], and MgO content is between 2% and 6%, with excessive silica and magnesium contents. These phosphates are difficult to beneficiate.
Flotation is one of the most important beneficiation methods. Today, more than half of the world’s marketable phosphate is upgraded by the flotation method[2]. The flotation methods of phosphate ore include direct flotation, reverse flotation, direct-reverse flotation, reverse-direct flotation and so on[3]. Siliceous phosphate ores are generally beneficiated by two stage flotation techniques using amine and fatty acids for silica based gangue and phosphate respectively [4]. The beneficiating effect of this kind of direct-reverse or reverse-direct flotation is good, but the pulp requires high temperature, generally above 25 ℃, and some phosphates even reach 40 ℃. Fatty acids and their salts are commonly used as collectors in flotation of phosphate ores. However, their use suffers from sensitivity to slimes and dissolved ions, higher temperature requirement, and relatively high consumption[5].
As for this phenomenon, we try to use a new double reverse flotation process for collophanite. It has some merits, for example, it needn’t heat the pulp, the dosage consumption is small, concentrate is easily filtrated, deleterious impurities are thorough removed. Through systematic experiment, we apply this double reverse flotation to float collophanite of Yichang region, and obtain very good beneficiation index: final concentrate yield 67.37%, P2O5 grade 32.17%, P2O5 recovery 87.80%, and main impurity MgO 0.95%.
1.2 Existent problem of cationic collector used in reverse floatation of collophanite ore
Cationic collector has good selectivity, low-temperature resistance, strong collecting strength and simple reagent system. But there are some problems such as sticky froths, big tenacity, bad fluidity, being difficult to defroth if the feed ores contain large amounts of clays and workers do not add defrother into the pulp or deslime feed, which will lead to a bad flotation index, and the back-sequent process such as scavenging tailings can not carry on. If it is too serious, beneficiation plant production will be stopped. For instance, dodecylamine reversely floats silica of the iron ore, siliceous froths are unfruitful and sticky, the selectivity is bad, and the dosage is inconvenient[6-7]. The Florida Institute of Phosphate Research(FIPR) applied cationic collector double reverse flotation process to float siliceous and magnesia phosphate ore, and found out that if the pulp hasn’t been deslimed before beneficiation, the consumption of reagent dramatically ascended and selectivity became worse[8].
1.3 Introduction of flotation defrothing research
There is not much about flotation defrothing research. German scientists first put forward that they used chemical method to defroth[9]. Japanese and American chemists singly researched defrothing problem during the Second World War. In 1950s, China began to study the defoamer of ferment and paper making industry[10].
Phosphate ores before beneficiation are subjected to different processes such as desliming, screening or classification, and the clay depressant is added to reduce the negative influence of flotation process by clay or slimes, so the cationic collector reverse flotation process has gained extensively application[11]. When using cationic collector to reversely float phosphate ore, because amine ions are very sensitive to clay and mud, deslimes, adding polymer to the flotation feed and/or water, and adding amine stagewise, are necessary in order that reverse flotation process is not influenced by clay[8]. Actually, lots of cationic reverse flotation of phosphate and hematite are all added in the process of deslimes before beneficiation[12-14]. GITERHOFF[15] indicated that cationic collector reversely floating minerals needed some organic depressant to make the surface of the wall rock (clay) become hydrophilic, so that clay can not adsorb the collector ions. The relation between the stability of flotation froths and recovery was revealed via measuring the thickness of flotation froths with and without Ca2+ ions and nonionpolymer[16].
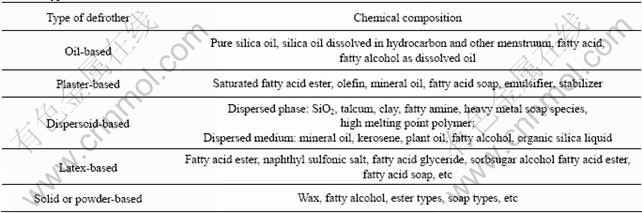
As for the problem of froths which are generated by cationic collector flotation, scholars in China chiefly study the deslimes of ore and adding chemical reagent [17-20]. At present, practical and feasible defrothing method mainly consists of adding inhomogeneous defrother, changing pH value, changing the solubility of frother with conjunct ion function and salting out action, adding the substance which reacts with frother, adding opposite surfactant and so on. The types of defrother is demonstrated in Table 1[21].
Table 1 Types of defrother/defoamer
2 Results and discussion
2.1 Collophanite ore
Experimental phosphate ores, came from Yichang region of Hubei Province, China, are of sedimentary phosphate rock. This collophanite ore is the accrete ore with quartz, feldspar, dolomite, calcite and so on. The useful mineral is phosphate, and the main impurity minerals are SiO2, MgO, Al2O3, Fe2O3, CaCO3 etc. Phosphorite distributes in oolite and clitellum tubercular shape, or forms particle with impurity minerals mutually, with disseminated grain size of about 0.10 mm. Calcicolous and magnesian accrete ore exist in dolomite [CaO?MgO?(CO2)2] and calcite [CaCO3], silica and aluminum exist in calcicolous feldspar [CaAl2SiO8] and sheet mica, and Fe2O3 exists in the concomitant ore. The chemical multi-element analysis results of crude ore are demonstrated in Table 2.
Table 2 Chemical multi-element analysis results of crude ore (mass fraction, %)
![]()
2.2 Double reverse flotation process
Fig.1 shows the double reverse flotation process of this experiment. Rougher flotation was used to wipe off magnesium minerals. The rough tailing is scavenged one time and concentrate Ⅱ is attained. The rough concentrate is cleaned one time to wipe off silica and concentrate Ⅰ is attained. The cleaner tailing is also scavenged one time, then this scavenging concentrate returns to the cleaner flotation.
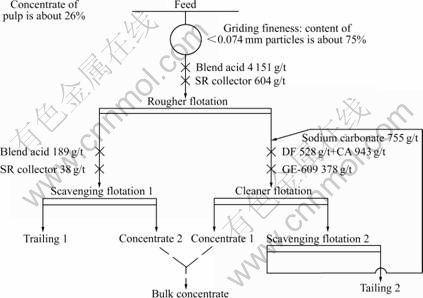
Fig. 1 Flowsheet of double reverse flotation process
Table 3 lists the closed circuit results of this double reverse flotation process. It is shown that the index of final concentrate is satisfactory. Its yield is 67.37%, P2O5 grade is 32.17% and recovery is 87.80%. The content of deleterious impurity in the concentrate is low, and the ratio of the total content of MgO, Fe2O3 and Al2O3 to P2O5 content is 10.40%, which completely satisfies the need (12%) of producing phosphatic acid deammonium.
Table 3 Closed circuit results
![]()
2.3 Defrothing
Although the froth performance of GE-609 is better than dodecylamine, it hasn’t thoroughly resolved the obstacle about flotation froth of cationic collector. Through long-term tentative experiment in the laboratory, we finally find out two kinds of defrothers which are effective and cheap: one is organic froth regulator DF, and the other is inorganic froth regulator CA. DF is a kind of metamorphic starch, and CA is a kind of metal oxide. Three parallel experiments were curried out without adding defrother, only adding DF and only adding CA, respectively. The index of evaluating defrothing effect is the residual amount of froth. The residual amount of froth is the amount of froth which doesn’t break up in the vessel after finishing flotation. Volumes of measured vessels are 5 L and 2 L, respectively. Fig.2 shows the results of defrothing experiment.
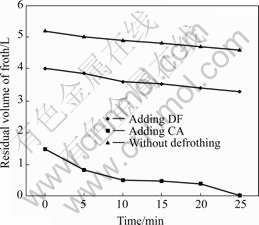
Fig.2 Residual volume of froth for different defrothing experiments
We find out that DF has little defrothing effect from Fig.2, but the effect of CA is more obvious. The froth of CA almost vanishes at 10 min after flotation. Actually, the amount of froth is greatly decreased for only adding CA. But adding DF at the same time can remarkably improve the fluidity of froth, so CA and DF are used in experiment.
The closed circuit result of double reverse flotation with and without CA is listed in Table 4.
Table 4 Closed circuit result of double reverse flotation with and without adding CA

Table 4 shows that the yield, P2O5 grade, recovery, chief impurity content of concentrate with and without CA have no obvious differences. P2O5 grade and recovery of adding CA are little less than those without adding CA. The froth problem has been solved, which also proves that CA is a kind of favorable foam adjustor of cationic collector.
3 Defrothing mechanism
3.1 Surface tension
In experiment, we measured the surface tension of solution (got from upper clear liquor of pulp which had ground and had not been added with any reagent) in different dosage of CA in order to discuss the defrothing mechanism of CA, as shown in Fig.3.

Fig.3 Surface tension of solution in different dosage of CA
From equations γSG=γSL+γLG?cosθ and -ΔG=γSG+ γLG-γSL=WSL, we can speculate the wetting function WSL=γLG(1+cosθ). After adding defrother CA, γLG minishes, WSL follows to minish, which makes the solid/liquid interface combination force diminish, so the concentration of collector on the film of bubbles is decreased, the hydrophobicity of mineral particle is weakened, and the stability of bubbles goes down.
3.2 Zeta potential
Zeta potential of pure quartz and quartz with adding CA in different pH values is shown in Fig.4.
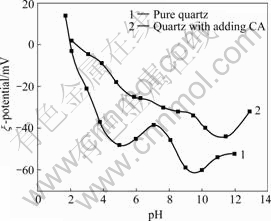
Fig.4 Comparison of zeta potential of pure quartz and quartz with adding CA (180 mg/L)
Fig.4 shows that the zero point of charge of quartz turns from pH 2.1 to 2.5 because of adding CA. Under the same pH value, the Zeta potential of quartz with adding CA rises a little. This is because CA forms Mn+ and M(OH)(n-1)+ ions in water. These two kinds of ions adsorb onto the surface of quartz. They counteract the negative charge of the surface of quartz partially. Thereby the Zeta potential of quartz rises, the zero point of charge of quartz moves a little.
Furthermore, 100 mg/L of GE-609, 180 mg/L of CA and 100 mg/L of GE-609 were respectively added into distilled water of quartz, then the Zeta potential of quartz under different pH was measured.
Fig.5 shows that the Zeta potential of quartz declines at first and climbs later after adding GE-609. It presents degressive trend below pH=4.5 and climbing trend above pH=4.5. When pH value is about 8, the Zeta potential of quartz becomes positive. This is because the surface of quartz charges negative charge originally. With the concentration of positive amine ions increasing, more and more amine ions are adsorbed onto the surface of quartz, consequently Zeta potential of the surface of quartz continuously rises. GE-609 molecule forms R—NH3+ in water through ionization, —NH3+ ions are adsorbed onto the surface of quartz, and R— easily adheres to the air bubbles because of its hydrophobicity, which makes the quartz particles float with bubbles. As CA and GE-609 are successively added into the same distilled water of quartz, CA forms Mn+ and M(OH)(n-1)+ in water by ionization. They compete to adsorb on the surface of quartz with amine ions, so the Zeta potential of quartz becomes higher and higher, as described in Fig.5. Because the three kinds of ions all charge positive charge, the competitive adsorption on the surface of quartz takes place. And the same charges exclude each other, and Mn+ and M(OH)(n-1)+ make the amount of amine ions on the surface of quartz decrease a little, consequently the hydrophobicity generated by quartz adsorbing collector function group R—NH3+ slows down, and the intensity that quartz particles adsorbed on bubbles diminishes a little, that is to say, the flotation froth becomes friable, and the defrothing is more easily.
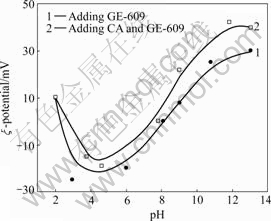
Fig.5 Curves of Zeta potential of quartz for adsorbing different reagents
4 Conclusions
1) The collophanite with P2O5 grade of crude ore of 23.52% was beneficiated by double reverse flotation. Adding inorganic defoaming adjustor CA can attain the excellent concentrate. The yield is 67.37%, P2O5 grade is 32.17% and recovery is 87.80%, and the impurity contents of MgO, Fe2O3, Al2O3 are 0.95%, 1.04%, 1.36%, respectively.
2) Through adding organic defrothing regulator DF and inorganic defrothing CA, the surface tension of the pulp is reduced and Zeta potential is increased. The superposition of all kinds of factors makes the froths become friable and evanesce, which effectively solves the difficult problem of cationic collector GE-609. It is possible to apply the cationic collector to the industrial beneficiation of collophanite.
3) Flotation reagent special collector is the key of the double reverse flotation. The SR collector for wiping off magnesia minerals and GE-609 for wiping off silica possess good selectivity and strong collecting force. This double reverse flotation can be applied to the beneficiation industry of collophanite after the industrial experiment. The beneficiated problem of medium and low grade collophanite will be solved.
References
[1] LIU Yi-hua. My country and the world phosphate resources & exploitation actuality (continuation) [J]. Phosphate & Compound Fertilizer. 2005, 20(6): 9-12. (in Chinese)
[2] SIS A H, CHANDER S. Reagents used in the flotation of phosphate ores: A critical review [J]. Minerals Engineering, 2003, 16(7): 577-585.
[3] LUO Zhao-jun, QIAN Xin, WANG Wen-qian. Progress of phosphate ore processing [J]. China Mining Magazine, 1999, 8(4): 50-53. (in Chinese)
[4] ZAFAR IQBAL ZAFAR, ANWAR M M, PRITCHARD D W. A new route for the beneficiation of low grade calcareous phosphate rocks [J]. Fertilizer Research, 1996, 44: 133-142.
[5] SIS A H, CHANDER S. Improving froth characteristics and flotation recovery of phosphate ores with nonionic surfactants [J]. Minerals Engineering, 2003, 16(7): 587-595.
[6] ZHANG Hong-ru. Study on reverse flotation collector for removing silicate [J]. Industrial Minerals & Processing, 1998(4): 10-11. (in Chinese)
[7] LIU Jing, ZHANG Jian-qiang, LIU Jiong-tian. Status of iron ores flotation reagent [J]. China Mining Magazine, 2007, 16(2): 106-108.
[8] Zhang p, Yu y, Bogan m. Challenging the “Crago” double float process II amine-fatty acid flotation of siliceous phosphate [J]. Minerals Engineering, 1997, 10(9): 983-994.
[9] YANG Jun-ling. Foam and defoaming technology [J]. Textile Quxiliaries, 1995(4): 29-32. (in Chinese)
[10] FENG Qi-ming, MU Xiao, ZHANG Guo-fan. Foaming and defoaming technology in flotation processes [J]. Conservation and Utilization of Mineral Resources, 2005(4): 31-35. (in Chinese).
[11] ZAFAR I Z, ANWAR M M. PRITCHARD D W. Innovations in beneficiation technology for low grade phosphate rocks [J]. Nutrient Cycling in Agroecosystems, 1996, 46: 135-151.
[12] HERNAINZ F, CALERO M, BLAZQUEZ G. Flotation of low-grade phosphate ore [J]. Advanced Powder Technology, 2004, 15(4): 421-433.
[13] AWADALLAH R M, MOHAMED A E, EL HAZEK N T, HASSAN M Y. Beneficiation of west Sibaiya phosphate ores by flotation in alkaline media [J]. Metallurgical and Materials Transactions B, 1998, 29B: 1149-1156.
[14] ARUJIOM A C. Flotation reagent of iron ore [J]. Metallic Ore Dressing Abroad, 2006(2): 4-7. (in Chinese)
[15] GITHOFF S. The study of activation of frother and organic depressant in cation flotation process [J]. Metallic Ore Dressing Abroad, 2004(8): 19-23. (in Chinese).
[16] LU S, SUN K. Development of phosphate flotation reagents in China [C]// ZHANG P, EI-SHALL H, WIEGEL R. Beneficiation of Phosphates: Advances in Research and Practice. Littleton: SME Inc, 1999: 21-26.
[17] QIU Guan-zhou, WU Xi-qing, WANG Yu-hua, FENG Qi-ming, HU Yue-hua. Advance in flotation in recent years [J]. Metal Mine, 2006(1): 41-52. (in Chinese).
[18] GAO Lin-zhang, WANG Yi-da, MA Hou-hui. Research of improving iron grade and reducing SiO2 content of concentrate [J]. Metal Mine, 2004(3): 17-19. (in Chinese)
[19] GE Ying-yong, YU Yong-fu, CHEN Da, ZHANG Ming. Flotation performance of low-temperature-resistant cationic collector GE-609 using in separating SiO2 [J]. Journal of Wuhan University of Technology, 2005, 27(8): 17-19. (in Chinese)
[20] FENG Qi-ming, MU Xiao, ZHANG Guo-fan, LU Yi-ping, OU Le-ming, SHAO Yan-hai, CHEN Yun. Investigation on antifoaming of flotation bauxite [J]. Journal of Central South University of Technology(Natural Science), 2005, 35(6): 956-959. (in Chinese)
[21] ZENG Xiao-bo. Study on two-stage reverse flotation for glue phosphorous ores and foam behavior [D]. Wuhan: Wuhan University of Technology, 2007.
Foundation item: Project(2006 AA107A01) supported by Science and Technology Key Program of Hubei Province, China
Corresponding author: GE Ying-yong; Tel: +86-27-62506493; E-mail: geyy@mail.whut.edu.cn


