
Thermodynamic description of Au-Ag-Si ternary system
WANG Jiang(王 江), LIU Hua-shan(刘华山), LIU Li-bin(刘立斌), JIN Zhan-peng(金展鹏)
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Based on the available experimental information, the Ag-Si binary system was thermodynamically assessed using the CALPHAD method. The solution phases, including liquid, fcc-A1 and diamond-A4, were modeled as substitutional solutions, of which the excess Gibbs energies were expressed by Redlich-Kister polynomial functions. Combined with previous assessment of the Ag-Au and Au-Si binary systems, thermodynamic description of the Au-Ag-Si ternary system was performed to reproduce the reported phase equilibria. Thermodynamic properties of liquid alloys, liquidus projection and several vertical and isothermal sections of this ternary system were calculated, which are in reasonable agreement with the reported experimental data.
Key words:
thermodynamics; phase diagram; CALPHAD; Ag-Si binary system; Au-Ag-Si ternary system;
1 Introduction
Continuous search for suitable materials for interconnect and contact in very large-scale integrated circuits has been conducted because the packing density, speed, etc. are usually limited by packaging technology[1]. The bonding materials in electronic/ optoelectronic packaging serve one or all of the following three major functions: electrical connection, mechanical support and heat dissipation. According to the melting temperature, these materials are classified as soft solders and hard solder[2]. Soft solders, such as Sn-based alloys, have low melting temperature and are widely used to solder the metallization[3-4]. Hard solders, including Au-rich Au-Sn, Au-Si and Au-Ge alloys, are useful for bonding devices that are sensitive to high processing temperature but need good creep resistance, such as GaAs or large Si die on alumina[5-6]. In order to meet the requirements of high electrical conductivity and good adhesion during the fabrication and subsequent service of electronic devices, thermo- dynamic properties and phase relations of the related metal systems are indispensable for alloy design and processing. The purpose of the present work was to evaluate Au-Ag-Si ternary system and to obtain precise thermodynamic description of this ternary system.
Among three binary systems, Ag-Au and Au-Si binary systems have been well assessed thermodynamically by HASSAM et al[7] and MENG et al[8], respectively. Fig.1 and Fig.2 show the calculated phase diagrams of Ag-Au and Au-Si binary systems, respectively. Good agreement was realized between the calculated thermodynamic properties and experimental data. Therefore, Gibbs energies of various phases in Ag-Au and Au-Si binary systems were directly adopted in the present work. Ag-Si binary system was previously optimized respectively by CHEVALIER[9] and OLESINSKI et al[10], which all reproduced most experimental information on thermodynamic properties and phase boundaries. However, the lattice stabilities of elements adopted are different from those proposed by DINSDALE[11]. In order to achieve the compatibility of thermodynamic database in the multi-component systems, thermodynamic parameters of various phases in the Ag-Si binary system were reassessed in the present work. Based on the readily assessed Ag-Au and Au-Si binary systems, the Au-Ag-Si ternary system was optimized using the CALPHAD method[12] and Thermo-calc software package[13].
2 Experimental
2.1 Ag-Si binary system
The Ag-Si binary system is a simple eutectic system without an intermediate compound. The liquidus has

Fig.1 Calculated phase diagram of Ag-Au binary system
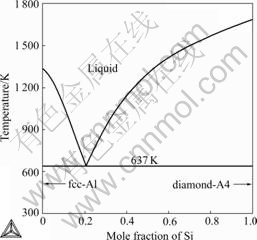
Fig.2 Calculated phase diagram of Au-Si binary system
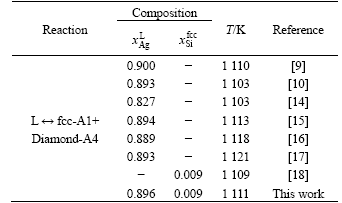
been determined using thermal analysis technique by ARRIVAUT[14], HAGER[15], MOURON and VUILLARD[16], and PREDEL and BANKSTAHL[17]. Experimental data reported are generally consistent with each other and adopted during the optimization. The solubility of Ag in diamond-A4(Si) was not reported and thus not taken into account in the present work. However, the equilibrium solubility of Si in fcc-A1(Ag) was measured recently by WEBER[18] using electron microprobe analysis, which was used in the present optimization. The temperature and composition of the eutectic reaction in this binary system were measured in Refs.[14-18], as listed in Table 1.
Enthalpies of mixing of the Ag-Si liquid alloys have been measured by HASSAM et al[19] at 1 423 K and 1 550 K through the calorimetry method. CASTANET [20] has also determined enthalpies of formation of the Ag-Si alloys at 1 348 K by means of the direct reaction calorimetry (drop method) using a high temperature calorimeter. In light of the experimental data reported by HASSAM et al[19], the temperature dependence of mixing enthalpy for the Ag-Si liquid alloys is not obvious, and thus was not considered.
Table 1 Invariant reaction in Ag-Si binary system
By using the electromotive force method, the activities of Si in the Ag-Si liquid alloys referred to diamond-A4(Si) have been determined at 1 473 K by SAKAO and ELLIOTT[21]. ROBINSON and TARBY[22] have employed partial pressure method to measure the activities of Ag and Si in the Ag-Si liquid alloys in the temperature range of 1 723-1 873 K. However, their results are very different from the data measured by SAKAO and ELLIOTT[21]. Considering the reliability of experimental method, experimental data determined by SAKAO and ELLIOTT[21] were employed in the present work.
2.2 Au-Ag-Si ternary system
Thermodynamic properties and phase diagram of the Au-Ag-Si ternary system were experimentally investigated by several authors[7,19,23-24]. On the base of these experimental results, PRINCE et al[25] reviewed this ternary system when compiling phase diagrams of Au-based alloys. No stable ternary compound was found.
So far, activities of Au, Ag and Si in the ternary alloys were not investigated in published literatures. However, using calorimetry, HASSAM et al[19] measured the enthalpies of mixing of liquid ternary alloys at 1 423 K for the following sections: Ag0.30Au0.70- Si, Ag0.50Au0.50-Si, Au0.50Si0.50-Ag, Au0.60Si0.40-Ag, Au0.80Si0.20-Ag and Ag0.90Si0.10-Au. The measured data by HASSAM et al[19] were adopted in the present optimization.
HASSAM et al[7] have employed differential thermal analysis to determine the vertical sections as follows: Ag0.50Au0.50-Si, Ag0.30Au0.70-Si, Ag0.75Au0.25-Si and Au0.50Si0.50-Ag. In addition, HASSAM et al[23] have also determined the vertical section of 20% Ag by means of differential thermal analysis. KUPINA[24] investigated many ternary alloys with 10%, 20%, 30%, 40%, 50% and 60% Si by thermal analysis and metallographic observation. According to the experimental results, the vertical sections at different Si contents were constructed. However, PRINCE et al[25] suggested the data reported by KUPINA[24] are not accepted after compared with the data measured by HASSAM et al[7,23]. Therefore, the data determined by HASSAM et al[7,23] were taken into account.
3 Thermodynamic modelingIn the Au-Ag-Si ternary system, no ternary compound is stable and there are three solution phases, including liquid, fcc-A1 and diamond-A4. Gibbs energies of the solution phases are modeled as follows:
![]() (1)
(1)
where xi is the mole fraction of component i, and ![]() is the molar Gibbs energy of pure element i in the structural state of solution phase
is the molar Gibbs energy of pure element i in the structural state of solution phase![]() .
. ![]() is the excess Gibbs energy, which is formulated as
is the excess Gibbs energy, which is formulated as
 (2)
(2)
where ![]()
![]() and
and ![]() are binary interaction parameters.
are binary interaction parameters. ![]() and
and ![]() are taken
are taken
directly from the Ag-Au and Au-Si systems assessed by HASSAM et al[7] and MENG et al[8], respectively.
However, ![]() is to optimize in the present work
is to optimize in the present work
according to the available experimental information of the Ag-Si binary system. The ternary interaction
parameter![]() is formulated with Redlich-Kister-
is formulated with Redlich-Kister-
Muggianu expression as follows:
![]() (3)
(3)
where ![]() are parameters to be optimized based
are parameters to be optimized based
on the available experimental data of the Au-Ag-Si ternary system in the present work.
4 Results and discussionUsing the lattice stabilities of Ag, Au and Si in different states assessed by DINSDALE[11], phase diagram of Au-Ag-Si ternary system was optimized within PARROT module of Thermo-Calc software package[13]. Thermodynamic parameters for all condensed phases in Au-Ag-Si ternary system were obtained.
4.1 Ag-Si binary system
The calculated phase diagram of the Ag-Si binary system was compared with experimental data, as shown in Fig.3. The calculated liquidus is in good agreement with most experimental data measured by different sources[14-17]. Moreover, the calculated solubility of Si in fcc-A1(Ag) is satisfactorily consistent with experimental data reported by WEBER[18]. In combination with Table 1, it is evident that reasonable agreement has been realized between the calculated results and experimental data.
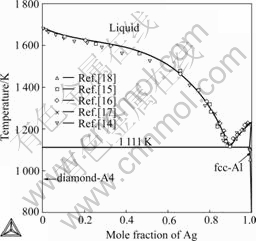
Fig.3 Calculated phase diagram of Ag-Si binary system compared with experimental data
Fig.4 presents the calculated enthalpies of mixing of the liquid Ag-Si alloys with experimental data[19] at 1 423 K. The calculated enthalpies of formation of the Ag-Si alloys at 1 348 K with experimental data[20] referred to liquid Ag and diamond-A4(Si) are compared in Fig.5. The calculated values agree with experimental data measured by HASSAM et al[19] and CASTANET [20] within experimental error (about 8%).
Fig.6 shows the comparison of the calculated activities of Si in liquid Ag-Si alloys with experimental data[21] referred to diamond-A4(Si). As it can be seen that the calculated values are consistent with experimental data.
4.2 Au-Ag-Si ternary system
Combining the Ag-Si binary system assessed in the present work with the Ag-Au and Au-Si binary systems optimized previously, the Au-Ag-Si ternary system has been further optimized based on available experimental information. Thermodynamic properties of liquid alloys, liquidus projection and several vertical and isothermal sections are also calculated and compared with experimental information in Figs.7-11.

Fig.4 Comparison of calculated enthalpy of mixing with experimental data referred to liquid Ag and liquid Si
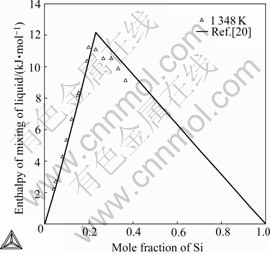
Fig.5 Comparison of calculated enthalpy of formation with experimental data referred to liquid Ag and diamond-A4 Si
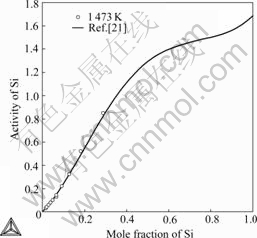
Fig.6 Comparison of calculated activities of Si in liquid alloys with experimental data referred to solid Si
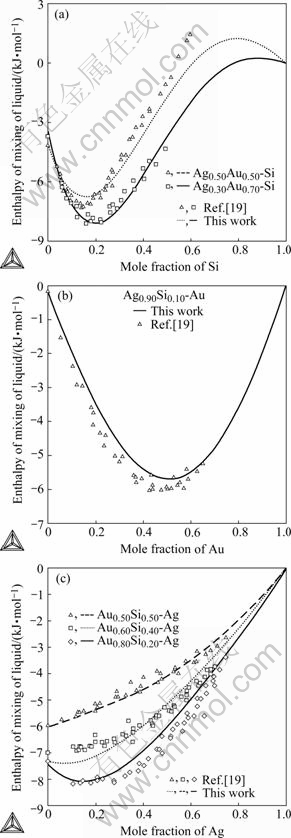
Fig.7 Comparison of calculated enthalpies of mixing of ternary liquid alloys with experimental data at 1 423 K: (a) Ag0.30- Au0.70-Si and Ag0.50Au0.50-Si; (b) Ag0.90Si0.10-Au; (c) Au0.50- Si0.50-Ag, Au0.60Si0.40-Ag and Au0.80Si0.20-Ag

Fig.8 Calculated liquidus projection of Au-Ag-Si ternary system in this work
Enthalpies of mixing of the ternary liquid alloys along different across sections are calculated in comparisons with experimental data measured by HASSAM et al[19], as shown in Fig.7. It is obvious that the calculated values show a slight deviation from experimental data. However, the calculated results in the present work are still reasonable and acceptable if one considers the experimental error (about 8%) of the data reported by HASSAM et al[19].
In the Au-Ag-Si ternary system, invariant reaction associated with liquid phase is a ternary monovariant eutectic one. Fig.8 illustrates the calculated liquidus projection of this ternary system. The monovariant curve e1e2 runs smoothly from the Ag-Si binary eutectic point e1 to the Au-Si binary eutectic point e2.
The calculated vertical sections of the Au-Ag-Si ternary system compared with experimental data[7,23] are given in Fig.9. The vertical section at 20% Ag (molar fraction) was presented in Fig.10. The calculated phase relations and phase boundaries are in accordance with experimental data measured by HASSAM et al[7,23]. Fig.11 illustrates the calculated isothermal sections of the Au-Ag-Si ternary system at 673 K and 1073 K in the present work. The phase relations and phase boundaries of isothermal sections are consistent with the results reviewed by PRINCE et al[25].
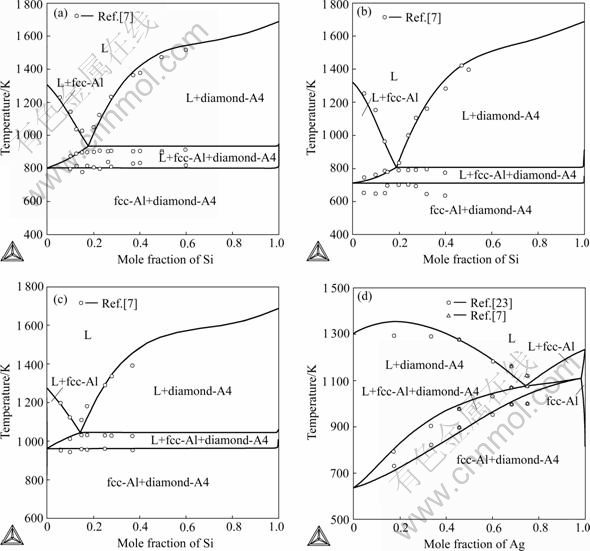
Fig.9 Calculated vertical sections of Au-Ag-Si ternary system compared with experimental data: (a) Ag0.50Au0.50-Si; (b) Ag0.30Au0.70- Si; (c) Ag0.75Au0.25-Si; (d) Au0.50Si0.50-Ag
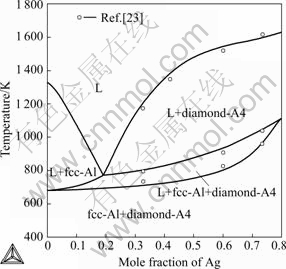
Fig.10 Calculated vertical section at 20% Ag compared with experimental data
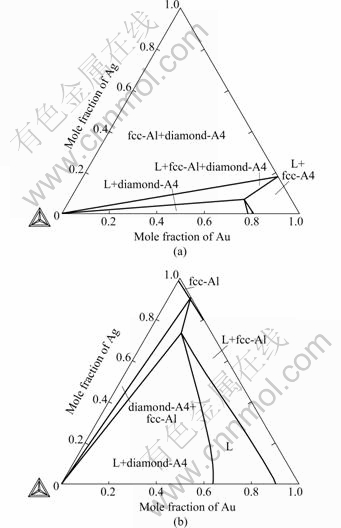
Fig.11 Calculated isothermal sections of Au-Ag-Si ternary system: (a) 673 K; (b) 1 073 K
5 Conclusions
The Ag-Si binary system was reassessed using the CALPHAD method. A set of self-consistent parameters for the Ag-Si binary system was obtained, which can be used to reproduce most experimental data, including thermodynamic properties and phase diagrams. Thermodynamic description of the Au-Ag-Si ternary system was performed by combining the previous assessment of the Ag-Au and Au-Sn binary systems with available experimental information on this ternary system. Thermodynamic properties of liquid alloys, liquidus projection and several vertical and isothermal sections were calculated. The calculated results are in good agreement with the reported experimental data.
References
[1] SOM T, AYYUB P, KABIRAJ D, KULKARNI N, KULKARNI V N, AVASTHI D K. Formation of Au0.6Ge0.4 alloy induced by Au-ion irradiation of Au/Ge bilayer [J]. J Appl Phys, 2003, 93(2): 903-906.
[2] TSAI J Y, CHANG C W, SHIEH Y C, HU Y C, KAO C R. Controlling the microstructures from the gold-tin reaction [J]. J Electron Mater, 2005, 34(2): 182-187.
[3] ABTEW M, SELVADURAY G. Lead-free solders in microelectronics [J]. Mater Sci Eng R, 2000, 27: 95-141.
[4] ZENG K, TU K N. Six cases of reliability study of Pb-free solder joints in electronic packaging technology [J]. Mater Sci Eng R, 2002, 38: 55-105.
[5] LIU X S, HU M H, NGUYEN H K, CANEAU C G, RASMUSSEN M H. Comparison between epi-down and epi-up bonded high-power single-mode 980 nm semiconductor lasers [J]. IEEE Trans Adv Packag, 2004, 27(4): 640-646.
[6] TEW J W R, SHI X Q, YUAN S. Au/Sn solder for face-down bonding of AlGaAs/GaAs ridge waveguide laser diodes [J]. Mater Lett, 2004, 58(21): 2695-2699.
[7] HASSAM S, ?GREN J, GAUNE-ESCARD M, BROS J P. The Ag-Au-Si system: Experimental and calculated phase diagram [J]. Metall Trans A, 1990, 21: 1877-1884.
[8] MENG F G, LIU H S, LIU L B, JIN Z P. Thermodynamic description of the Au-Si-Sn system [J]. J Alloys Compds, 2007, 431: 292-297.
[9] CHEVALIER P Y. Thermodynamic evaluation of the Ag-Si system [J]. Thermochi Acta, 1988, 130: 33-41.
[10] OLESINSKI R W, GOKHALE A B, ABBASCHIAN G J. The Ag-Si system [J]. Bull Alloy Phase Diagrams, 1989, 10(6): 635-640.
[11] DINSDALE A T. SGTE data for pure elements [J]. CALPHAD, 1991, 15: 317-425.
[12] KAUFMAN L, BERNSTEIN H. Computer calculation of phase diagrams [M]. New York: Academic Press, 1970.
[13] SUNDMAN B, JANSSON B, ANDERSSON J O. The program for optimization [J]. CALPHAD, 1985, 9: 153-190.
[14] ARRIVAUT G. On the alloys of silicon and silver [J]. C R Hebd Séances Acad Sci, 1908, 147: 859-861.
[15] HAGER J P. Thermodynamic properties of the silver-silicon system [J]. Trans Metal Soc AIME, 1963, 227: 1000-1002.
[16] MOURON P, VUILLARD G. On the crystallization of the eutectic alloys silver-silcon [J]. C R Hebd Séances Acad Sci Ser C, 1969, 269: 595-598.
[17] PREDEL B, BANKSTAHL H. Thermodynamic properties of liquid silver-germanium, silver-silicon, gold-germanium and gold-silicon [J]. J Less-Common Metals, 1975, 43: 191-203.
[18] WEBER L. Equilibrium solid solubility of silicon in silver [J]. Metall Mater Trans A, 2002, 33(4): 1145-1150.
[19] HASSAM S, GAUNE-ESCARD M. Enthalpies of formation of Ag-Si, Au-Si and Ag-Au-Si liquid alloys at 1423 K [J]. Ber Bunsenges Phys Chem, 1983, 87: 785-792.
[20] CASTANET R. Enthalpy of formation of Cu-Ag-Si and Cu-Ag-Ge liquid alloys [J]. Z Metallkd, 1984, 75(1): 41-45.
[21] SAKAO H, ELLIOTT J F. Thermodynamic properties of liquid Ag-Si alloys [J]. Metall Trans, 1974, 5: 2063-2067.
[22] ROBINSON V S, TARBY S K. The thermodynamic properties of liquid Ag-Si alloys [J]. Metall Trans, 1971, 2: 1347-1352.
[23] HASSAM S, GAUNE-ESCARD M, BROS J P. Phase diagram and heats of formation of the gold-silver-silicon system at 1 400 K and 1 550 K [J]. J Calorim Anal Therm, 1983, 14: 166-175.
[24] KUPRINA V V. Equilibrium diagram of the Ag-Au-Si system [J]. Russian J Inorg Chem, 1962, 7: 833-834.
[25] PRINCE A, RAYNOR G V, EVANS D S. Phase diagrams of ternary gold alloys [M]. London: The Institute of Metals, 1990.
(Edited by YANG Bing)
Foundation item: Projects(50371104; 50671122) supported by the National Natural Science Foundation of China
Corresponding author: JIN Zhan-peng; Tel: +86-731-8836209; E-mail: jin@mail.csu.edu.cn


