- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discus...▲
- 4 Conclusions▲
- References
- Figure
- Fig. 1 DSC-TGA profile of Sn-Co-C composite at temperature ranges of 20–1 000 °C and heating rate of 5 °C /min
- Fig. 2 Schematic of formation of Sn-Co-C composite through carbothermal reduction.
- Fig. 3 Structures of carbon and Sn-Co-C composite:
- Fig. 4 Cyclic voltammogram curves of Sn-Co-C microcomposite in potential window of 0–1.2 V collected at scanning rate of 0.5 mV/s
- Fig. 5 Electrochemical impedance spectrum for electrode of Sn-Co-C microcomposite (a) and corresponding equivalent circuit (b)
- Fig. 6 First galvanostatic charge/discharge profile (a) and reversible charge capacities profiles (b) of carbonized phenolic resin at rate of 0.1C (Curve 1) and prepared Sn-Co-C microcomposites at rates of 0.1C (Curve 2) and 1C (Curve 3)
J. Cent. South Univ. (2013) 20: 326–331
DOI: 10.1007/s11771-013-1491-1
Layer by layer synthesis of Sn-Co-C microcomposites and their application in lithium ion batteries
ZHOU Xiang-yang(周向阳), ZOU You-lan(邹幽兰), Yang Juan(杨娟), XIE Jing(谢静), WANG Song-can(王松灿)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Abstract:
Alloy anodes were studied for pursuing Sn-based microcomposite synthesis, assembly and performance for lithium ion batteries. The self-assembled Sn-Co-C composites with nano-scaled microstructures were prepared via solution method and carbothermal technology. The morphology and physical structure were investigated with scanning electron microscope (SEM) and X-ray diffraction (XRD). The as-prepared materials were assembled to half cell coin for the purpose of discussing the galvanostatic cycling, cyclic voltammetry and rate-capability performance. Results reveal that nanoscaled CoSn2 alloys covered with Sn and C layer by layer are wrapped by cross-linked porous carbon network to form spherical microstructure. This distinguishing feature of Sn-Co-C composites provides a possible solution to the problems of Sn particle aggregation and poor electron transport, and has strong effect on improving electrochemical performance.
Key words:
1 Introduction
It is unsuspicious that lithium ion batteries will surely play an ever-growing role in our living and industrial application [1–2], owing to their potential advantages of the superior energy density and high cyclicity as energy storage and conversion devices [3–4]. Correspondingly, tin acting as anode for lithium ion batteries intrigues great research interest for two reasons: First, it possesses both higher theoretical capacities than carbon and conductivity outdistance silicon [5–8]; Second, it is not only effortless to form alloys with many other metals (Co [9], Cu [10], Ni [11], Sb [12]), in order to optimize the electrochemical properties of the tin-based anodes, but also easy to interact with lithium ions at lower voltage than other anodes [13–14]. However, the cycling performance of tin-based electrodes is still ungratified for industrialization owing to the drastic volume expansion and the aggregation tendency of nano-scaled tin [15–17]. To date, great efforts have already been put to ameliorate this problem through in-depth modification of tin-based electrodes [18–20]. CHEN et al [10] prepared core–shell Cu@Cu6Sn5 nanowires which used Cu[OH)2 nanorods as matrix, then formed Cu6Sn5 stable crystal structure after heating treatment so as to improve the cycling performance and superior rate capability. Aside from the enhancement of the cycling performance, CHANG et al [21] confirmed that the incorporation of graphene considerably improved the electron transfer of the FL-SnS2/G hybrid. Recently, nanoscaled tin dispersed in a carbon matrix or encapsulated into elastic hollow carbon spheres were reported as anodes. These studies showed that both coating tin with carbon layer and dispersing tin in carbon matrix are effective to improve their electrochemical properties in lithium ion batteries. This tin-based anode material has to be designed to own enough void volume to compensate the volume expansion during Li+ insertion, which is important to improve its cycle performance.
In this work, a simple and low cost preparation of Sn-Co-C microcomposites was reported by solution method employing alkali neutralization and carbothermal technology. We firstly attempt to combine doping with coating for the purpose of preventing the contact and expansion of tin. It is possible to exploit binary Sn-Co alloys with the merit that the volume expansion related to the formation of Sn–Li compounds is buffered by Co. The addition of Co component prevents the aggregation of the active Sn during cycling, further enhancing the cycling performance of the composites. However, only the alloying process of the active material is inadequate. Therefore, it is necessary to be extended to other materials. The present work refers to integrate Sn-Co-C composites to amorphous carbon with multi-branch network, and study the electrochemical performance of the integrated Sn-Co-C microcomposites. This application of Sn-Co-C microcomposite is probably compatible with large-volume manufacturing approaches.
2 Experimental
0.75 g CoO and 2.0 g phenolic resin were impregnated into 100 mL transparent aqueous solution of 10.53 g SnCl4·5H2O at 50 °C. During stirring, 0.4 mol/L NH3×H2O was dript to prepare Sn (OH)4 colloid. After 2 h, the mixed slurry was filtrated and washed with de-ionized water several times, followed by drying at 80 °C for 24 h. Finally, the dried precursor was ground and reduced at 800 °C in argon atmosphere. The sample was obtained after natural cooling. For comparison, the reference sample was prepared using the same method without CoO and SnCl4·5H2O addition.
The morphology and phase analysis of the as-received samples were examined by scanning electron microscope (SEM, JSM-6 360LV) and X-ray diffraction (XRD, Rigaku-TTRIII) with Cu Ka radiation.
The electrodes for electrochemical evaluation were prepared by mixing 80% alloy powders, 10% carbon black, and 10% polyvinylidene fluoride (PVDF) (mass fraction) dissolved in N-methylpyrrolidinone (NMP) to form slurry, followed by coating on a copper foil, pressing, and drying at 120 °C for 24 h. The processed foil was cut into 1 cm2 in area, and weighed. The coin cell was assembled in a glove box filled with pure argon. Metallic lithium was used as the negative electrode and counter electrode, 1 mol/L LiPF6/ethylene carbonate (EC)/diethyl carbonate (DMC)/ethyl methyl carbonate (EMC) (1:1:1, by volume) was used as electrolyte. Galvanostatic discharge/charge experiments were performed between 0 and 1.2 V (vs Li+/Li) using a LAND CT2 001A Battery Cycler (Wuhan, China). The process of lithium insertion into the alloy electrode was referred to discharge and extraction to charge.
3 Results and discussion
The physical change, polycondensation and solid state reaction of the Sn-Co-C precursor can be monitored by thermogravimetric analysis (DSC-TGA) (as seen in Fig.1), and the schematic of forming Sn-Co-C composite through carbothermal reduction is demonstrated in Fig. 2. It can be seen from the DSC-TGA profile that there exist three mass loss stages. The endothermic peak bellow 125 °C is in correspondence with the decomposition of Sn(OH)4·5H2O hydrate to Sn(OH)4, with respect to the ratio of 2.922%. At the temperature region from 250 to 450 °C, the mass loss ratio is as high as 38.6%, which is mainly caused by decomposition of Sn(OH)4 to SnO2 and pyrolysis of phenolic resin. The third region from 450 to 800 °C correlates to the carbothermal reduction of CoO and SnO2 to solid Co (Fig. 2(a)) and liquid Sn (Fig. 2(b)) by carbon obtained during the second region, respectively. The heating process at 800 °C for 2 h may have promoted the fluid to flow around the solid Co (Fig. 2(c)) to produce CoSn2 alloy. Surplus Sn was coated outside the surface of CoSn2 alloy to form nanoparticles for the low melting point of tin (Fig. 2(d)). As is known to all, nanoparticles or nanowires alone often appear with explosion risk. In-situ formation of CoSn2/Sn granule is easy to be resincoated for another advantage that phenolic resin has certain fluidity at molten condition. This prevents CoSn2/Sn nanoparticles from baring (Fig. 2(g)). Considering the processing technique of phenolic resin from the Targus Company in Wuhan, China [22], huge cross-linked resin network is generated during hardening process. After carbonization, phenolic resin is pyrolyzed to carbon but remains the cross-linked network. It is profitable to preserve the stability of the Sn-Co-C composites. Therefore, phenolic resin is often supposed to be one of the ideal precursors. After being coated, the Sn-Co-C nano-composites are assembled to multi-branch network (Fig. 2(g)), even spherical microstructures (Fig. 2(h)), depending on the morphology of the carbon with multi-branch channels (Fig. 2(e)). Finally, the microcomposites with multi- branch network described above are transformed into a dense solid after natural cooling process.
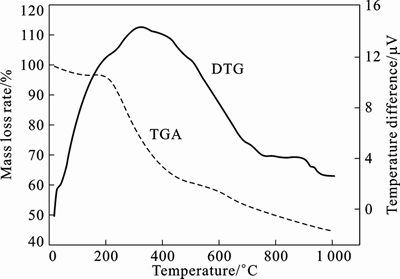
Fig. 1 DSC-TGA profile of Sn-Co-C composite at temperature ranges of 20–1 000 °C and heating rate of 5 °C /min
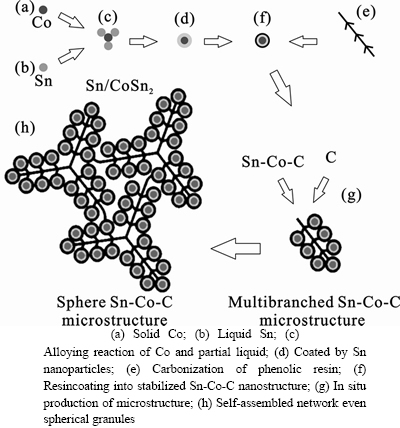
Fig. 2 Schematic of formation of Sn-Co-C composite through carbothermal reduction.
Scanning electron microscopy (SEM) shows the morphologies of amorphous carbon obtained from reference sample (Fig. 3(a)) and spherical Sn-Co-C microcomposites (Figs. 3(b) and (c)). It can be seen clearly that treelike channels with a wide range of pore distribution (Fig. 3(a)) appear on the surface of carbon. Benzene ring structure in phenolic resin possesses permanent bonding energy and molecular cohesion. After decomposition, carbon maintains its end-function and provides multiple accessible sites as a binder. Liquid CoSn2/Sn alloy was firmly coated and then cohered into treelike channels to form multi-branch Sn-Co-C sphere microparticles. The diameter of microparticles ranges from 5 to 10 μm from macroscopic perspective (Fig.3(b)) and shows much smaller Sn-Co-C particle size with an average diameter of about 800 nm under higher magnification (Fig. 3(c)). The particle size distribution is controlled by stirring rate during hydrolysis and heating temperature during carbothermal reduction. Sn and Co with molar ratio of 3:1 form the solid CoSn2 alloys by peritectic reaction from liquid Sn at 525 °C, while the formation of CoSn and Co3Sn2 occurs at 936 °C and 1 170 °C, respectively, much higher than 800 °C [23]. Surplus liquid Sn is coated outside the solid CoSn2 alloys to form CoSn2/Sn granule. In-situ formation of CoSn2/Sn granule is easy to be resincoated to Sn-Co-C nanoparticles, further to binder and re-resincoat to the Sn-Co-C microparticles that show a general whole surface (from Fig. 3(b)). X-ray diffraction (XRD) analysis of the produced samples verifies that as-prepared composite is composed of Sn and CoSn2 without observation of amorphous carbon in contrast with the pure carbon patterns (Fig. 3(d)). However, from EDS, the containing carbon element is detected (Fig. 3(e)). This is mainly due to the high intensity peaks of crystalline Sn and CoSn2 alloys that overlap the relative low intensity peaks of amorphous carbon and transfer it into unobvious background peaks. No impurities have been detected in the sample from both XRD and EDS patterns. Cyclic voltammogram curves reveal broad lithium ion insertion peak at 0.61 and 0.12 V, and narrower lithium ion extraction peaks at 0.53 and 0.75 V (Fig. 4). Peaks at 0.61 and 0.53 V are pointed to the metal Sn insertion into and extraction from Li, respectively. The peaks at 0.12 and 0.75 V are correlated to the reversible reaction of CoSn2 with Li. The pair of cathodic/anodic peaks appearing around 0.005 V, which is associated with the insertion/extraction processes of carbon, is inconspicuous, as a result of the very small contribution of carbon to the overall electrode capacity. The increase of the lithium ion extraction during cycling indicates improvement in extraction kinetics. It can be seen that the initial discharge profile differs from that of subsequent discharges, which indicates the drastic structural variation, and morphological change upon the potential change in the first cycle and cannot be recovered afterwards. But the following two curves seem to be basically overlaid and become more stable when cycle number continues to increase, suggesting that the synthesized electrode has a stable electrochemical performance.
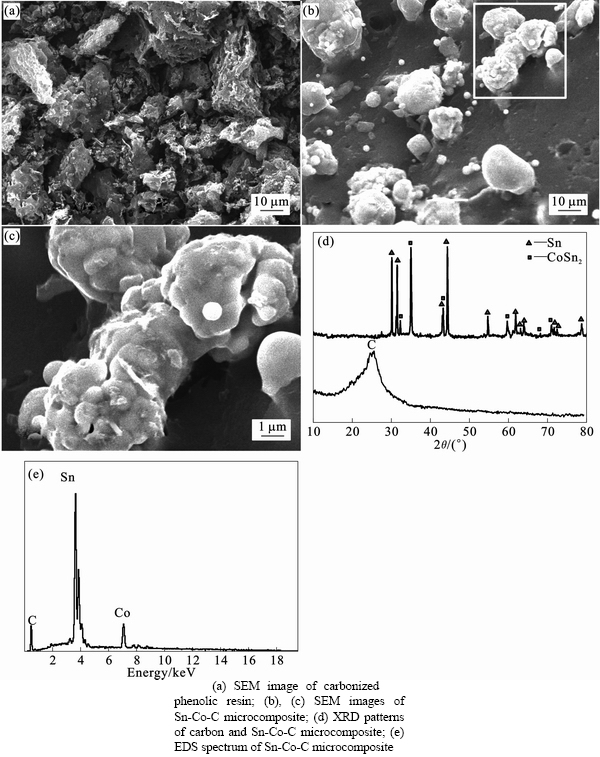
Fig. 3 Structures of carbon and Sn-Co-C composite:
To further investigate electrode kinetics, the activation energy of the microcomposite is calculated from electrochemical spectrum data. The Nyquist complex plane plot of the microcomposite after 40 cycles is presented in Fig. 5(a). The impedance spectrum can be divided into two parts: one depressed semicircle at intermediate frequencies which is attributed to the kinetics barriers caused by lithium migration and the charge transfer, and a straight line at low frequencies accordant with the lithium ion diffusion into the composite. The electrochemical spectrum for the composite electrode can be imitated by an equivalent circuit, as presented in Fig. 5(b). R1 value of 3.672 Ω is the electrolyte resistance, R2 value of 74.45 Ω is the charge-transfer resistance, CPE is the double-layer capacitance and Zw is the Warburg impedance related to the diffusion of lithium ions into the bulk of the electrode material. Fitting the experimental impedance plot to the equivalent circuit will provide important information about the changes in the polarization resistance due to the composition and construction transformation. The exchange current (i0) can be calculated by the following equation:
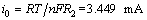 (1)
(1)
 (2)
(2)
where R is the gas constant; T is the absolute temperature; n is the number of transferred electrons; F is Faraday constant; A is the temperature-independent coefficient.

Fig. 4 Cyclic voltammogram curves of Sn-Co-C microcomposite in potential window of 0–1.2 V collected at scanning rate of 0.5 mV/s
According to Eq. (2), the apparent activation energy Ea is 3.07 kJ/mol. The high exchange current density and low activation energy of as-prepared composites indicate facilitated lithium ion interaction with tin. This improved electrode kinetics is probably due to the fact that treelike channels with wide range pore distribution and long symmetrical split shorten the diffusion route of lithium ions. Sn is probably protected by means of alloying with cobalt and coating by carbon. Moreover, Sn-Co-C composites are embedded into carbon network to alleviate the aggregation of formed Sn nanoparticles.
The capacity-rate characteristic of Sn-Co-C microcomposite and amorphous carbon are investigated in Fig. 6. Figure 6(a) demonstrates the initial charge/discharge curves of Sn-Co-C microcomposite and amorphous carbon. From the discharge curves, it can be found that curve 2 exhibits two plateaus of 0.9 V and 0.3 V, which divide this curve into three slopes. The steep slope from beginning to 0.9 V demonstrates the initial potential change of the coin cell. The mild slop from 0.9 to 0.3 V is correlated to the formation of solid electrolyte interphase (SEI) film and the lithium ion insertion into metal Sn. The other mild slop from 0.3 to 0 V is resulted from the reaction of lithium ions with CoSn2. This pattern of capacity storage is compatible with curve 3 and in accordance with cyclic voltammogram curves in Fig. 4. In contrast to carbon electrode, the potential drops rapidly to the unique plateau of 0.6 V and then decreases gradually to 0.005 V, which illustrates the different dynamic characteristics to Sn-Co-C electrode. From Fig. 6(b), the reversible charge capacities of Sn-Co-C composite at the charge rate of 0.1C and 1C are 746 and 565 mA×h/g, respectively. The average charge capacity of the Sn-Co-C composite after 40 cycles reaches nearly 3 times higher than that of carbonized phenolic resin at 0.1C, although the overall carbon contribution for the alloy electrode is estimated to be 230 mA×h/g, which is identical with lithium ion reaction with carbon at 0.005 V in Fig. 5. With the increase of the current density, the capacity of Sn-Co-C composite appreciably decreases because of the hysteresis kinetics. It is interesting to note that Sn-Co-C electrode shows a higher capacity retention rate at high current value, according to capacity retention of 80.6% and 87.8% at 0.1C and 1C, respectively, which is mainly due to the fast lithium ion transportation and less attached side reaction. When increasing the charge/discharge rate, the total working time at the same cycles gets shorter, leading to diminishing of the self-discharging and also improvement of the capacity retention.

Fig. 5 Electrochemical impedance spectrum for electrode of Sn-Co-C microcomposite (a) and corresponding equivalent circuit (b)
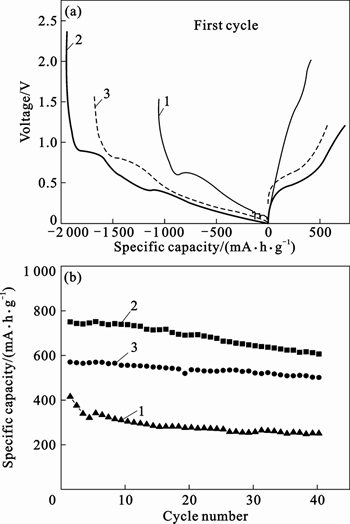
Fig. 6 First galvanostatic charge/discharge profile (a) and reversible charge capacities profiles (b) of carbonized phenolic resin at rate of 0.1C (Curve 1) and prepared Sn-Co-C microcomposites at rates of 0.1C (Curve 2) and 1C (Curve 3)
4 Conclusions
1) Sn-Co-C microcomposites are simply prepared by solution method and carbothermal technology, and the application of Sn-Co-C microcomposite is probably compatible with large-volume manufacturing approaches.
2) CoSn2 nanoparticles are coated by surplus liquid Sn and C layer by layer to form about 800 nm Sn-Co-C nanocomposites, then, bind to 5–10 μm sphere microstructures, further to be re-coated by carbon. This design provides layer by layer protection of the active metal Sn from expansion and aggregation during cycling and is beneficial to lithium ion transfer.
3) The specific capacities of this Sn-Co-C electrode reach as high as 745 and 565 mA×h/g at rates of 0.1C and 1C, respectively.
4) Further research to optimize the material composition and microstructures, particularly to enhance the rate performance, is urgent to be in hand.
References
[1] ANTTI V, JUSTIN S. Lithium ion battery production [J]. Journal of Chemical Thermodynamics, 2012, 46(1): 80–85.
[2] MENKIN S, GOLDNITSKY D, PELED E. Artificial solid-electrolyte interphase (SEI) for improved cycleability and safety of lithium-ion cells for EV applications [J]. Electrochemistry Communications, 2009, 11(9): 1789–1791.
[3] WANG Cen, DU Gao-hui Du, Kenny S, HUANG Hai-xiao, ZHONG Yi-jun, JIANG J Z. Ultrathin SnO2 Nanosheets: Oriented attachment mechanism,nonstoichiometric defects, and enhanced lithium-ion battery performances [J]. Journal of Physical Chemstry C, 2012, 116(6): 4000–4011.
[4] MAI Li-qiang, XU Xu, HAN Chun-hua, LUO Yan-zhu, XU Lin, WU Yi-min ZHAO Yun-long. Rational synthesis of silver vanadium oxides/polyaniline triaxial nanowires with enhanced electrochemical property[J]. Nano Letters, 2011, 11(11): 4992–4996.
[5] YANG Juan, ZHOU Xiang-yang, ZOU You-lan, TANG Jing-jing. A hierarchical porous carbon material for high power lithium ion batteries [J]. Electrochimica Acta, 2011, 56(24): 8576–8581.
[6] LIN Yu-sheng, GUH Jenq-gong, HUANG Min-hsiu. Shell-by-shell synthesis and applications of carbon-coated SnO2 hollow nanospheres in lithium-ion battery [J]. Journal of Physical Chemistry C, 2010, 114(30): 13136–13141.
[7] VIDHYA C, RUSLI F, MICHEAL A F. Quaternary ammonium ionic liquid electrolyte for a silicon nanowire-based lithium ion battery [J]. Journal of Physical Chemistry C, 2011, 115(44): 22048–22053.
[8] WANG Wei, RIGVED E, PRASHANT N K. Vertically aligned silicon/carbon nanotube (VASCNT) arrays: Hierarchical anodes for lithium-ion battery [J]. Electrochemistry Communication, 2011, 13(5): 429–432.
[9] FERGUSON P P, MARTINE M L, GEORGE A E, DAHN J R. Studies of tin–transition metal–carbon and tin–cobalt–transition metal–carbon negative electrode materials prepared by mechanical attrition [J]. Journal of Power Sources, 2009, 194(2): 794–800.
[10] CHEN Ji-zhang, YANG Li, FANG Shao-hua, HIRANO Shin-ichi, KAZUHIRO T. Three-dimensional core–shell Cu@Cu6Sn5 nanowires as the anode material for lithium ion batteries[J]. Journal of Power Sources, 2012, 199(1): 341–345.
[11] WANG Shuang-yin, JIANG San-ping, WHITE T J, GUO Jun, WANG Xin. Electrocatalytic activity and interconnectivity of Pt nanoparticles on multiwalled carbon nanotubes for fuel cells [J]. Journal of Physical Chemistry C, 2009, 113(43): 18953–18961.
[12] PARK C, SOHN H J. A mechano-and electrochemically controlled SnSb/C nanocomposite for rechargeable Li-ion batteries [J]. Electrochimica Acta, 2009, 54(26): 6367–6373.
[13] ZHANG Dong-feng, SUN Ling-dong, JIA Chun-jiang, YAN Zheng-guang, YOU Li-ping, YAN Chun-hua. Hierarchical assembly of SnO2 nanorod arrays on -Fe2O3 nanotubes: A case of interfacial lattice compatibility [J]. Journal of American Chemistry Society, 2005, 127(39): 13492–13493.
[14] SUN Xiao-lei, WANG Xing-hui, QIN Yan-li, LI Xiu-wan, QIAO Li, FENG Na, HU Duo-kai, HE De-yan. Synthesis of novel pompon-like porous SnO2 and its application in lithium-ion battery [J]. Materials Letters, 2012, 66(1): 193–195.
[15] LIANG Shu-zhao, ZHU Xue-feng, LIAN Pei-chao, YANG Wei-shen, WANG Hai-hui. Superior cycle performance of Sn@C/graphene nanocomposite as an anode material for lithium-ion batteries [J]. Journal of Solid State Chemistry, 2011, 184(6): 1400–1404.
[16] HARUNA H, ITOH S, HORIBA T, SEKI E, KOHNO K. Large-format lithium-ion batteries for electric power storage [J]. Journal of Power Sources, 2011, 196(16): 7002–7005.
[17] HE Ze-qiang,XIONG Li-zhi,LIU Wen-ping, WU Xian-ming, CHEN Shang, HUANG Ke-long. Synthesis and electrochemical properties of SnO2-polyaniline composite [J]. Journal of Central South University of Technology, 2008, 15(2): 214–217.
[18] DU Zhi-jia, ZHANG Shi-chao. Enhanced electrochemical performance of Sn-Co nanoarchitectured electrode for lithium ion batteries [J]. Journal of Physical Chemistry C, 2011, 115(47): 23603–23609.
[19] ZAI Jiang-tao, WANG Kai-xue, SU Yue-zeng, QIAN Xue-feng, CHEN Jie-sheng. High stability and superior rate capability of three-dimensional hierarchical SnS2 microspheres as anode material in lithium ion batteries [J]. Journal of Power Sources, 2011, 196(7): 3650–3654.
[20] TIAN Miao, WANG Wei, LEE Se-hee, LEE Yung-cheng, YANG Rong-gui. Enhancing Ni–Sn nanowire lithium-ion anode performance by tailoring active/inactive material interfaces [J]. Journal of Power Sources, 2011, 196(23):10207–10212.
[21] CHANG Kun, WANG Zhen, HUANG Guo-chuang, LI He, LEE Jim-yang. Few-layer SnS2/graphene hybrid with exceptional electrochemical performance as lithium-ion battery anode [J]. Journal of Power Sources, 2012, 201(1): 259–266.
[22] YE Xiao-chuan. The heat moulding process of the phenolic resin with super fine and heat-resisting performance[EB/OL].[2012–2–04]. http://www.whtigers.com/article/details.asp?id=9/. (in chinese)
[23] XU Yan. Measurement of Gd-Co-Sn ternary alloy isothermal section and CoSn-GdCoSn Variable temperature section [D]. Guangxi: Guangxi University, 2007. (in Chinese)
(Edited by HE Yun-bin)
Foundation item: Projects(51074185, 51274240) supported by the National Natural Science Foundation of China; Project supported by the Fundamental Research Funds for the Central Universities
Received date: 2012–04–23; Accepted date: 2012–07–30
Corresponding author: Yang Juan, PhD; Tel: +86–731–88836329; E-mail: 15874853145@139.com
Abstract: Alloy anodes were studied for pursuing Sn-based microcomposite synthesis, assembly and performance for lithium ion batteries. The self-assembled Sn-Co-C composites with nano-scaled microstructures were prepared via solution method and carbothermal technology. The morphology and physical structure were investigated with scanning electron microscope (SEM) and X-ray diffraction (XRD). The as-prepared materials were assembled to half cell coin for the purpose of discussing the galvanostatic cycling, cyclic voltammetry and rate-capability performance. Results reveal that nanoscaled CoSn2 alloys covered with Sn and C layer by layer are wrapped by cross-linked porous carbon network to form spherical microstructure. This distinguishing feature of Sn-Co-C composites provides a possible solution to the problems of Sn particle aggregation and poor electron transport, and has strong effect on improving electrochemical performance.
- Layer by layer synthesis of Sn-Co-C microcomposites and their application in lithium ion batteries


