Trans. Nonferrous Met. Soc. China 23(2013) 849-854
Kinetics of rare earth leaching from roasted ore of bastnaesite with sulfuric acid
Xing-liang FENG1,2, Zhi-qi LONG1,2, Da-li CUI1,2, Liang-shi WANG1,2, Xiao-wei HUANG1,2, Guo-cheng ZHANG1,2
1. National Engineering Research Center for Rare Earth Materials, General Research Institute for Nonferrous Metals, Beijing 100088, China;
2. GRIREM Advanced Materials Co. Ltd., Beijing 100088, China
Received 31 March 2012; accepted 16 October 2012
Abstract:
Sulfuric acid leaching process was applied to extracting rare earth (RE) from roasted ore of Dechang bastnaesite in Sichuan, China. The effect of particle size, stirring speed, sulfuric acid concentration and leaching temperature on RE extraction efficiency was investigated, and the leaching kinetics of RE was analyzed. Under selected leaching conditions, including particle size (0.074-0.100 mm), sulfuric acid concentration 1.50 mol/L, mass ratio of liquid to solid 8 and stirring speed 500 r/min, the leaching kinetics analysis shows that the reaction rate of leaching process is controlled by diffusion through the product/ash layer which can be described by the shrinking-core model, and the calculated activation energy of 9.977 kJ/mol is characteristic for a diffusion-controlled process.
Key words:
bastnaesite; rare earth; roasting ore; leaching; kinetics;
1 Introduction
Numerous studies on the leaching of rare earth ore have been reported. BIAN et al [1] studied the leaching kinetics of bastnaesite concentrate from the Mianning, Sichuan deposit, in HCl solution. It was found that the dissolution kinetics can be represented by shrinking-core model with diffusion through a product layer as the rate controlling step; and the activation energies for RE2(CO3)3 and REF3 leached from bastnaesite concentrate were calculated to be 59.39 kJ/mol and 66.13 kJ/mol, respectively. TIAN et al [2] investigated the leaching kinetics of RE from the weathered crust elution-deposited rare earth ore. The result demonstrated that the leaching kinetics of the weathered crust elution-deposited rare earth ore can be described by the shrinking core model. And the leaching kinetics is controlled by the diffusion of porous solid layer. The apparent activation energies is 9.24 kJ/mol. GE and CHI [3] also studied the leaching kinetics of rare earth from weathered mud located in Southwestern China in hydrochloric acid solution. It was found that the leaching process is controlled by an inner diffusion. The apparent activation energy is 10.5 kJ/mol. The leaching kinetics of RE from phosphoric ore by nitric acid was studied by WANG et al [4]. The leaching process was controlled by the interracial chemical reaction. The apparent activation energy was calculated to be 70.6 kJ/mol, and the apparent reaction order was 0.83. TIAN and YIN [5] chose ammonium sulphate solution as the lixiviant to study the kinetics of leaching a south China heavier rare earth ore. The results showed that the leaching process could be demonstrated with the shrinking-core model. The leaching rate could be controlled by the diffusion rate of reacting reagents in a porous solid layer. The apparent activation energy was calculated to be 5.9 kJ/mol.
Dechang bastnaesite in Sichuan, China was found in 1990s. Rare earth mineral of Dechang bastnaesite in Sichuan is associated with strontium (Sr) and barium (Ba). The rare earth reserve of Dechang bastnaesite in Sichuan has been estimated as 730 kt [6]. And the strontium reserve has been estimated as 2759.3 kt [7-9].
In recent years, more and more attentions have been focused on Dechang bastnaesite deposit. The mineral composition is different between Dechang and Mianning rare earth deposit, and the ore dressing recovery is as low as 30%-40% [6]. But the ThO2 content of Dechang bastnaesite is only 0.10%-0.16%. Dechang bastnaesite concentrate must have a strong market competitiveness because of its lower radioactivity [8,10-12].
Up to now, there are no reports on the leaching kinetics of roasting ore from Dechang bastnaesite [13-19]. Sulfuric acid solution was chosen as the lixiviant in the present work and the leaching kinetics was studied in detail. Though many researchers have done a lot of studies on the leaching kinetics of rare earth ore, the materials used in those researches usually presented in one main single phase such as bastnaesite. The characteristics of those materials are quite different from the rare earth ore of Dechang bastnaesite deposit and it is very necessary to investigate the kinetics of leaching Dechang bastnaesite with sulfuric acid solution.
2 Experimental
2.1 Materials
The raw ore in this study was obtained from a Dechang bastnaesite deposit which is associated with celestite. The concentrate containing rare earth was obtained by mineral processing. The raw ore was roasted in a muffle furnace at 550 °C for 2 h in air atmosphere. The chemical composition and X-ray diffraction pattern are given in Table 1 and Fig. 1, respectively.
Table 1 Main chemical compositions of roasted celestite- associated rare earth ore used in this study (mass fraction, %)
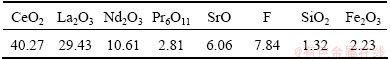
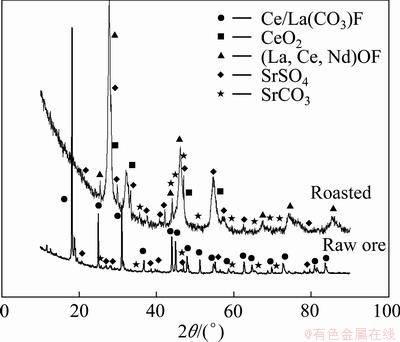
Fig. 1 XRD patterns of ore
The results of X-ray diffraction show that the main phases present in the roasted ore of Dechang bastnaesite were RE2O3, REOF, CeO2, CeOF and SrCO3 after a process of oxidation roasting at 550 °C for 2 h. The SrCO3 will be dissolved into the leaching solution if hydrochloric acid was chosen as the lixiviant.
2.2 Methods
The roasted ore of celestite-associated rare earth concentrate was leached in a glassware-flask (multiple neck, 500 mL) in a water bath with temperature controller. The accuracy of leaching temperature was ±1 °C. In each experiment, 50 g celestite-associated rare earth roasted ore was added to the agitated sulfuric acid solution (400 mL) of a certain concentration (1.50 mol/L) at a required temperature. An acid resistance mechanical agitator with stirring speed of 500 r/min was applied to agitating the reaction content. The mass ratio of liquid to solid was 8. At predetermined time intervals, 5 mL slurry was sampled with a pipette and immediately filtered after being diluted. The filtrate of the sample was analyzed for RE content, and the fraction of RE extraction was calculated for examining the leaching rate of the roasted ore.
3 Results and discussion
3.1 Leaching process and controlling models for reaction rate
During the leaching process, the main reactions of the roasted ore and sulfuric acid can be written as follows:
REOF+H2SO4→RE2(SO4)3+H2F+H2O (1)
RE2O3+H2SO4→RE2(SO4)3+H2O (2)
CeOF+H2SO4→Ce(SO4)2F2+Ce(SO4)2+H2O (3)
CeO2+H2SO4→Ce(SO4)2+H2O (4)
SrCO3+H2SO4→SrSO4+CO2+H2O (5)
No reaction happened between sulfuric acid and the celestite of the roasted ore in the leaching process, and SrSO4-product layer occurred when strontianite in the roasting ore reacted with sulfuric acid. When the ore particle is regarded as spherical type, the liquid-solid leaching process of RE from the roasting celestite-associated rare earth ore can be described by the model shown in Fig. 2.
The reaction includes four steps as follows: 1) outer diffusion of leaching agent (H2SO4) through the liquid boundary layer; 2) inner diffusion of leaching agent (H2SO4) or liquid products (Ce(SO4)2F2/ Ce(SO4)2) through the product/ash layer; 3) interfacial chemical reaction; 4) diffusion of liquid products (Ce(SO4)2F2/Ce(SO4)2) to liquid of leaching system.
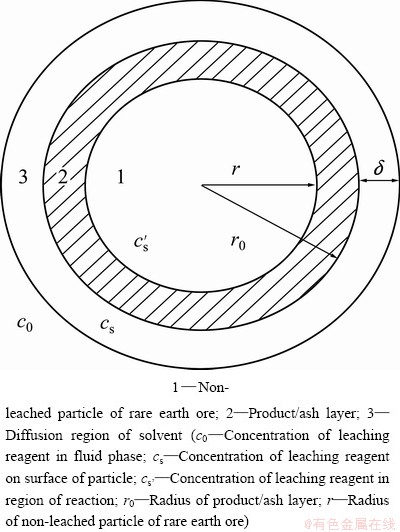
Fig. 2 Illustrative diagram of leaching process
Let D1 be the effective diffusion coefficient of leaching agent (H2SO4) through the liquid boundary layer, D2 be the effective diffusion coefficient of leaching agent (H2SO4) or liquid products (Ce(SO4)2F2/Ce(SO4)2) through the product/ash layer, δ be the thickness of leaching reagent on surface of particle and kr be the reaction rate constants. Suppose that J is the mole flux of the reaction through the product/ash layer per unit time. Then, the rate equation of reaction which was controlled by diffusion procedures, solid film diffusion and interfacial chemical reaction mixed controlling can be described as
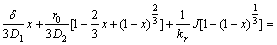
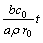 (6)
(6)
The overall reaction rate is determined by the slowest controlling step in the practical leaching process. When the leaching process is under chemical reaction control, the kinetic equation may be expressed as
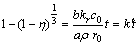 (7)
(7)
When the leaching process is under mixed control, the kinetic equation may be expressed as
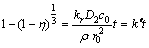 (8)
(8)
When the leaching process is under internal diffusion control, the kinetic equation may be expressed as
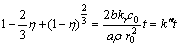 (9)
(9)
where k′, k′′ and k′′′ are the linear rate constants; η is the leaching rate of rare earth.
3.2 Effect of particle size
The roasted ore with three particle sizes was leached under the typical conditions: 30 °C and stirring speed 500 r/min and mass ratio of liquid to solid 8, acid concentration 1.5 mol/L. The effect of particle size of the roasted ore on the kinetics of RE extraction is shown in Fig. 3. The leaching rate of RE decreases with increasing the particle size. The faster rate of RE extraction observed with the finer particle size may be attributed to the larger surface area and thinner ash layer presented on the finer particles.
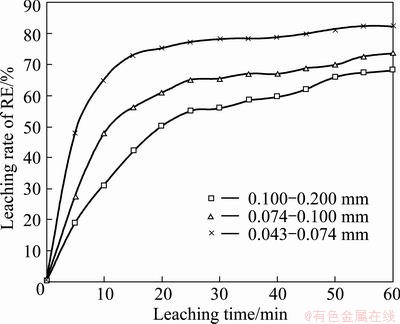
Fig. 3 Effect of particle size on RE extraction efficiency
3.3 Effect of stirring speed
Experiments with various stirring speeds were carried out under typical test conditions: leaching temperature 30 °C, mass ratio of liquid to solid 8 for roasted ore of 0.074-0.100 mm particle size, and acid concentration of 1.5 mol/L. It is found that the leaching rate of RE has little dependence on the stirring speed. A leaching system controlled by transport through an product/ash layer indicates that agitation has a slight effect on the leaching rate. And the test results agreed with the characteristics of ash layer controlled leaching process well. The results are concluded as shown in Fig. 4.
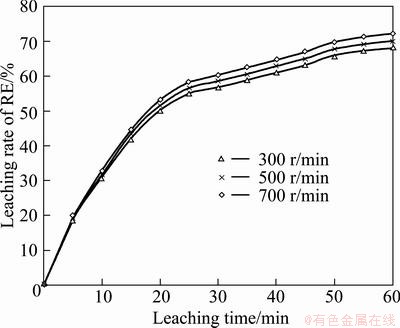
Fig. 4 Effect of stirring speed on RE extraction efficiency
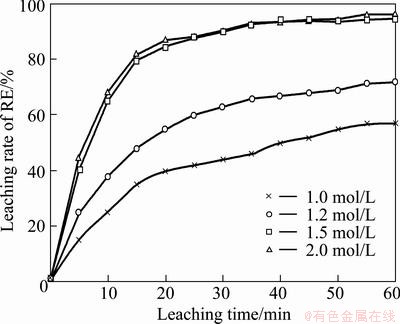
Fig. 5 Effect of sulfuric acid concentration on RE extraction efficiency
3.4 Effect of sulfuric acid concentration
The effect of sulfuric acid concentration on the RE leaching rate was investigated at 30 °C, a stirring speed of 500 r/min and a mass ratio of liquid to solid of 8 for roasted ore of 0.074-0.100 mm particle size when the acid concentration was varied in the range of 1.0-2.0 mol/L. As seen from Fig. 5. It is found that the leaching rate of RE increases with increasing the sulfuric acid concentration. For 1.0 mol/L concentration, there is virtually an acid deficiency effect. And there is no beneficia1 effect of increasing acid concentration more than 1.5 mol/L. However, after elapsing a certain time, namely, 30 min, the nickel extraction efficiency practically remains constant. The leaching rate of RE after 60 min leaching with 1.0, 1.2, 1.5 and 2.0 mol/L sulfuric acid are found to be 57.2%, 72.00%, 95.00% and 96.50%, respectively.
3.5 Effect of leaching temperature
The effect of leaching temperature was examined in the range of 20-70 °C under the conditions of sulfuric acid concentration of 1.5 mol/L, stirring speed of 300 r/min and mass ratio of liquid to solid of 8. The results are plotted in Fig. 6. It is found that leaching temperature only mildly affects the leaching rate of RE, which shows that the dissolution process of RE does not seem to be controlled by chemical reaction. When the leaching temperature increases from 20 °C to 70 °C, the leaching rate of RE after 60 min improves from 68% to 93.0%. It seems that the higher the leaching temperature, the higher the RE leaching rate. A leaching temperature of 30 °C is usually chosen in actual production to avoid the RE ion hydrolysis.
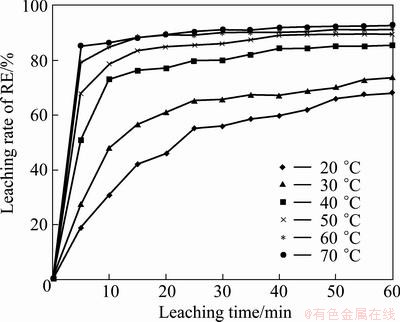
Fig. 6 Effect of leaching temperature on RE extraction efficiency
3.6 Leaching kinetics analysis
In order to determine the kinetic parameters and rate-controlling step for leaching RE from roasted ore of celestite-associated rare earth concentrate in sulfuric acid solution, the experimental data presented in Fig.6 were analyzed on the basis of the shrinking-core model [20].
The kinetics equation can be obtained through fitting all experimental data with different kinetic models and various rate-controlling mechanisms.
By fitting all the experimental data in Fig. 6 and Eq. (7) or Eq. (8), the plots of 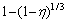 vs time at various temperatures are shown in Fig. 7. There are neither straight lines nor zero point intercepts. The results show that the data derived from Eq. (7) or Eq. (8) cannot be correlated by this model.
vs time at various temperatures are shown in Fig. 7. There are neither straight lines nor zero point intercepts. The results show that the data derived from Eq. (7) or Eq. (8) cannot be correlated by this model.
According to the experimental data in Fig. 6 and Eq. (9), the plots of 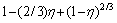 vs time are presented in Fig. 8.
vs time are presented in Fig. 8.
The linear relationship between 1-(2/3)η+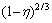 and leaching time is significant. It is found that the leaching process is controlled by diffusion through the product/ash layer in the temperature range of 20-70 °C.
and leaching time is significant. It is found that the leaching process is controlled by diffusion through the product/ash layer in the temperature range of 20-70 °C.
3.7 Calculation of activation energy
The activation energy of a diffusion controlled process is less than 13 kJ/mo1. While for a chemical reaction controlled process, it is usually greater than 43 kJ/mol. And for a mixed control process, it is in a range of 13-43 kJ/mol.
According to Arrhenius equation, it is represented as
k=Aexp[-E/(RT)] (10)
where k is the reaction rate constant; A is the pre- exponential factor; R is the mole gas constant; T is the thermodynamic temperature; E is the apparent activation energy.
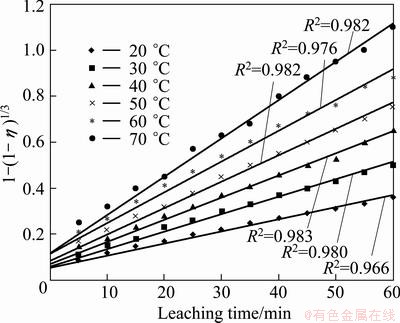
Fig. 7 Plots of 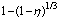 vs time at various temperatures
vs time at various temperatures
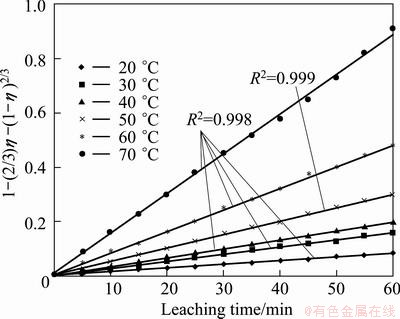
Fig. 8 Plots of 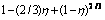 vs time at various temperatures
vs time at various temperatures
To calculate the activation energy, a plot of ln k vs 1/T should be a straight line with a slope of –E/R and an intercept of ln k with Arrhenius equation:
ln k=ln A-E/(RT) (11)
From the slopes of these plots in Fig. 8, the rate constants (k, min-1) were determined and plotted against 1/T×10-3 (Arrhenius plot) as presented in Fig. 9.
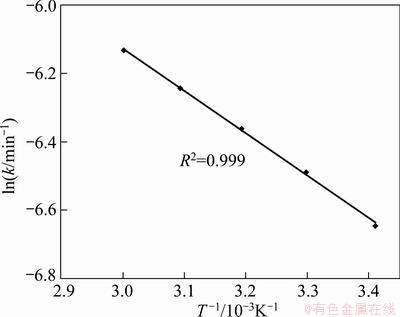
Fig. 9 Arrhenius plot for leaching RE from roasting ore
The apparent rate constant k can be calculated. The activation energy of the overall reaction was calculated as 9.977 kJ/mol. The value of the activation energy clearly indicates that the leaching of RE from the roasting ore in sulfuric acid liquid system is most likely controlled by diffusion through the product/ash layer.
4 Conclusions
1) In the sulfuric acid leaching of roasting ore of Dechang bastnaesite, the effect of leaching temperature is mild, the effect of particle size and sulfuric acid concentration on the leaching rate of RE are remarkable, while the effect of stirring speed is negligible.
2) Leaching kinetics analysis of RE by different models indicates that the RE leaching process is controlled by diffusion through the product/ash layer.
3) The activation energy is 9.977kJ/mol, which matches with the range of reported for diffusion controlled processes.
References
[1] BIAN Xue, YIN Shao-hua, LUO Yao, WU Wen-yuan. Leaching kinetics of bastnaesite concentrate in HC1 solution [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(10): 2306-2310.
[2] TIAN Jun, CHI Ru-an, YIN Jing-Qun. Leaching process of rare earths from weathered crust elution-deposited rare earth ore [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(10): 892-896.
[3] GE Fang, CHI Ru-an. Analysis of kinetics control on leaching rare earth from weathered with hydrochloric acid [J]. Chinese Rare Earths, 2003, 23(6): 16-19.(in Chinese)
[4] WANG Sheng-dong, JIANG Kai-xi, JIANG Xun-xiong, FENG Ling-yong, FAN Yan-qing, JIANG Wei, ZHANG Deng-gao. Kinetic study on leaching of RE in phosphorite [J]. Nonferrous Metals: Extractive Metallurgy, 2011, 10(9): 28-31. (in Chinese)
[5] TIAN Jun, YIN Jing-qun. A Kinetic analysis of leaching a south China heavier rare earth ore [J]. Jiangxi Science, 1996, 14(2): 81-86. (in Chinese)
[6] ISREA (Investigation Group of Sichuan Rare Earth Industry Association). Winter travaling in Liangshan, Sichuan province— Report of Mianning and Dechang country’s rare earth mining enterprises [J]. Sichuan Rare Earth, 2001, 10(4): 18-21.(in Chinese)
[7] LI Xiao-yu. Characteristics and the significance of the exploitation of associated strontium in rear earth deposit of Daluchao, Dechang Country, Sichuan Province [J]. Sichuan Rare Earth, 2006, 20(2): 26-28. (in Chinese)
[8] LI Fang-ji, ZENG Xing-lan. Beneficiation technology for bastnaesite in Panxi area [J]. Journal of Shanghai Second Polytechnic University, 1999, 12(1): 2-7. (in Chinese)
[9] YU Yong-fu, CHE Li-ping. Dressing technology of RE ore of China and its development [J]. Chinese Rare Earths, 2006, 27(1): 95-102. (in Chinese)
[10] HUANG Xiao-wei, LONG Zhi-qi, LI Hong-wei, YING Wei-juan, ZHANG Guo-cheng, XUE Xiang-xin. Development of rare earth hydeometallurgy technology in China [J]. Journal of Rare Earths, 2005, 23(1): 1-4.
[11] ZHOU Jing, XU Yan, CHEN Long-ming. Preparation of RE carbonate from Dechang bastnasite concentrate by oxidation baking decomposition [J]. Chinese Rare Earths, 2000, 21(2): 19-25.(in Chinese)
[12] CHENG Jian-zhong, CHE Li-ping. Current mining situation and potential development of rare earth in China [J]. Chinese Rare Earths, 2010, 31(2): 66-69, 85. (in Chinese)
[13] XU Yan-hui. Patents reviews of hydrometallurgy in the field of rare earth of China [J]. Rare Earth Information, 2009, 12(5): 28-30. (in Chinese)
[14] ZHU Chang-luo, XIONG Shu-Qing. Comprehensive utilization of a associated ore with bastnaesite and celestite: China: CN101157992 [P]. 2008-04-09. (in Chinese)
[15] HUANG Xiao-wei, LI Hong-wei, WANG Cai-feng, WANG Guo-zhen, XUE Xiang-xin, ZHANG Guo-cheng. Development status and research progress in rare earth industry in China [J]. Chinese Journal of Rare Metals, 2007, 31(3): 279-288.(in Chinese)
[16] LI Liang-cai, GE Xing-fang, LI Lin-shen. Development of hydrometallurgial process for Panxi bastnaesite [J]. Chinese Rare Earths, 1999, 20(4): 51-56.(in Chinese)
[17] LIU Hong, AO Bo, TAO Ming, LUO Qian. Development status and research progress in rare earth hydrometallurgy for Sichuan bastnaesite [J]. China Nonferrous Metallurgy, 2009, 6(1): 33-37. (in Chinese)
[18] WANG Wei-sheng, WANG Song-ling, JIA Jiang-tao, ZHANG Zhao-bing. Economical and technical indices of decomposition processes of typical rare earth minerals in China [J]. Journal of the Chinese Society of Rare Earths, 2006, 24(4): 385-390.(in Chinese)
[19] HUANG Xiao-wei, LI Hong-wei, LONNG Zhi-qi, PENG Xin-lin, LI Jian-ning, ZHANG Yong-qi, CUI Da-li, YANG Gui-lin. Development of new progress of green-metallurgy and separation technology for rare earth [J]. Sichuan Rare Earth, 2008, 4(4): 15-19.(in Chinese)
[20] ZHU Bing-chen. Chemical reaction engineering [M]. Beijing: Chemical Industry Press, 1993: 353-357. (in Chinese).
焙烧氟碳铈矿硫酸浸出稀土的动力学
冯兴亮1,2,龙志奇1,2,崔大立1,2,王良士1,2,黄小卫1,2,张国成1,2
1. 北京有色金属研究总院 稀土材料国家工程研究中心,北京 100088;
2. 有研稀土新材料股份有限公司,北京 100088
摘 要:研究了硫酸浸出德昌稀土与天青石共伴生矿的焙烧矿过程。考查粒度、搅拌速度、硫酸浓度和温度对稀土浸出率的影响,并对稀土的浸出动力学进行分析。在选定的浸出条件下:粒径0.074~0.100 mm、硫酸浓度1.5 mol/L、液固比8:1、搅拌速度500 r/min,稀土浸出反应受内扩散控制,表观活化能为9.977 kJ/mol。
关键词:氟碳铈矿;稀土;焙烧矿;浸出;动力学
(Edited by Hua YANG)
Foundation item: Project (NDRC high-tech No. 606, 2009) supported by the Major Industries Technological Development Special Fund of Development and Reform Commission, China; Project (50934004) supported by the National Natural Science Foundation of China
Corresponding author: Xiao-wei HUANG; Tel: +86-10-82241180; E-mail: hxw0129@126.com
DOI: 10.1016/S1003-6326(13)62538-8
Abstract: Sulfuric acid leaching process was applied to extracting rare earth (RE) from roasted ore of Dechang bastnaesite in Sichuan, China. The effect of particle size, stirring speed, sulfuric acid concentration and leaching temperature on RE extraction efficiency was investigated, and the leaching kinetics of RE was analyzed. Under selected leaching conditions, including particle size (0.074-0.100 mm), sulfuric acid concentration 1.50 mol/L, mass ratio of liquid to solid 8 and stirring speed 500 r/min, the leaching kinetics analysis shows that the reaction rate of leaching process is controlled by diffusion through the product/ash layer which can be described by the shrinking-core model, and the calculated activation energy of 9.977 kJ/mol is characteristic for a diffusion-controlled process.


