J. Cent. South Univ. (2016) 23: 39-43
DOI: 10.1007/s11771-016-3046-8
Kinetic study on leaching of nickel from Turkish lateritic ore in nitric acid solution
Tevfik AGACAYAK, Veysel ZEDEF, Ali ARAS
Department of Mining Engineering, Faculty of Engineering,  University, 42075 Konya, Turkey
University, 42075 Konya, Turkey
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
dissolution kinetics of nickel from lateritic ore in nitric acid solution was investigated. Experimental parameters used were stirring speed (100-600 r/min), temperature (40-96 °C), nitric acid concentration (0.1-2 mol/L) and particle size (<106 μm). The shrinking core model was applied to the results of experiments investigating the effects of leaching temperature in the range of 40-90 °C and nitric acid concentration in range of 0.1-2 mol/L on nickel dissolution rate. The kinetic analysis shows that the nickel dissolution from lateritic ore could be described by diffusion model. The activation energy (Ea) for the dissolution reaction is calculated as 79.52 kJ/mol.
Key words:
leaching; lateritic ore; nickel; kinetic analysis; diffusion; nitric acid;
1 Introduction
Nickel is an important metal and mainly used in production of stainless steel and alloys because of its resistance to corrosion and high temperature [1-2]. Nickel occurs in nature as sulfides and oxides. Laterites are oxide ores widely distributed in the equatorial regions. Nickel laterites and other oxide ores constitute an important part of the world reserves of nickel. Laterites formed by weathering of ultramafic rocks. Lateritic deposits usually consist of three layers, namely, the limonitic, saprolitic and garnieritic layer [3-6]. Approximately 70% of the world’s nickel reserves is contained within laterite ores, with the remaining nickel associated with sulphide deposits [7-9]. However, 60% of world’s nickel production is provided by sulfidic nickel ores [6-7, 10-11]. The production of nickel from lateritic ores includes pyro-metallurgical and hydro- metallurgical processes [12]. Thus, there is almost twice as much laterite resource that is amenable to hydrometallurgical processing (limonite, nontronite/ smectite) as that amenable to pyrometallurgical processing (saprolite, garnierite) [11].
Several leaching studies about lateritic nickel extraction have been performed using different solutions, such as sulphuric acid [4, 13-22], hydrochloric acid [2, 16, 23-27], nitric acid [16, 28], ammonia [29-31], citric acid, oxalic acid and acetic acid [32-34].
In the present work, dissolution kinetics of nickel from lateritic ore in nitric acid solutions was investigated. For this purpose, the effects of parameters such as stirring speed, temperature, acid concentration and particle size on the nickel extraction were examined.
2 Experimental
2.1 Material and method
The lateritic ore came from the  region,
region,  , Turkey. The raw material used in this work was a typical limonitic laterite ore with high iron content. It was ground to size less than 106 μm for acid leaching. The elemental composition of the sample is given in Table 1.
, Turkey. The raw material used in this work was a typical limonitic laterite ore with high iron content. It was ground to size less than 106 μm for acid leaching. The elemental composition of the sample is given in Table 1.
Table 1 Elemental analysis of lateritic nickel ore sample [35]
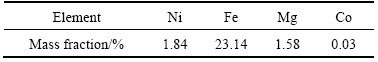
XRD pattern of the ore sample is given in Fig. 1. According to the mineralogical analysis of lateritic nickel ore sample, the main minerals are goethite, hematite and wustite. The gangue minerals of the ore are determined as retgersite, gaspeite, quartz and clay type minerals.
Experiments were carried out by agitation leaching using 1/50 g/mL (solid-liquid ratio) in a covered 1 L pyrex beaker in a temperature-controlled water bath. The leach solution was stirred by Heidolph mark RZR 2021 model mechanical stirrer with teflon lined impeller. Nickel in the solution was determined using flame atomic absorption spectrophotometer (GBC Scientific Equipment, SensAA Model, Australia). Distilled water and reagent grade HNO3 (Merck brand) were used to adjust the concentration of the leach solutions.
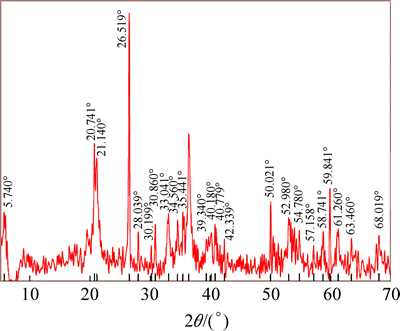
Fig. 1 XRD pattern of lateritic nickel ore sample [35]
3 Results and discussion
3.1 Effect of parameters
The effect of stirring speed on the nickel extraction from lateritic nickel ore was investigated in 0.5 mol/L HNO3 solution at 60 °C in the range of 100-600 r/min stirring speed. It was determined that nickel extraction values presented in Fig. 2 are independent from stirring speed. Therefore, 200 r/min was selected as optimum operating stirring speed in order to provide the stability of leaching solution during subsequent experiments by investigating the effects of other parameters on dissolution of nickel.
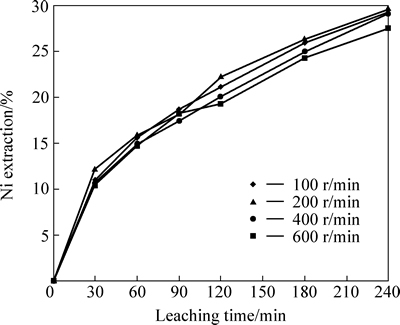
Fig. 2 Effect of stirring speed on nickel extraction from lateritic ore
The effect of temperature on nickel extraction was examined in 40-96 °C with conditions of particle size <106 μm and 0.5 mol/L HNO3 concentration over a period of 240 min leaching time. The results, shown in Fig. 3, indicate that temperature has significant effect on leaching and overall extraction efficiency of nickel.
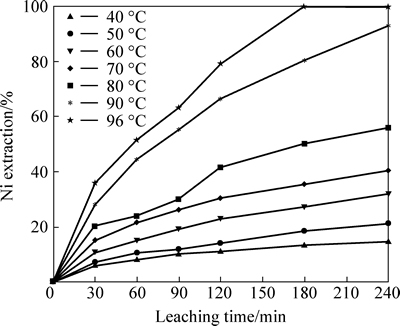
Fig. 3 Effect of temperature on nickel extraction from lateritic ore
The effect of acid concentration on nickel extraction was investigated by using different concentration of HNO3 solutions (0.1, 0.25, 0.5, 1.0, 1.5 and 2.0 mol/L). In these experiments, the leaching temperature was kept constant as 80 °C, particle size as <106 μm and stirring speed as 200 r/min. The results presented in Fig. 4 show that Ni extraction increases as acid concentration increases.
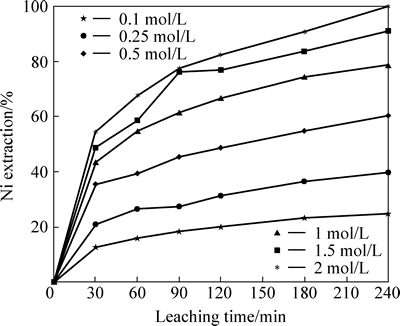
Fig. 4 Effect of acid concentration on nickel extraction from lateritic ore
The leaching experiments were carried out using different particle size fractions (75-106, 45-75, 38-45 and <38 μm) at condition of effect of acid concentration tests. The results presented in Fig. 5 show that Ni extraction increases as particle size decreases. The higher the specific surface area of the ore is, the higher the Ni extraction is.
3.2 Dissolution kinetics
The dissolution kinetics of the solid-liquid interactions involved three established shrinking core models such as chemical reaction model, diffusion model and combination of both chemical control and diffusion model [36-39].
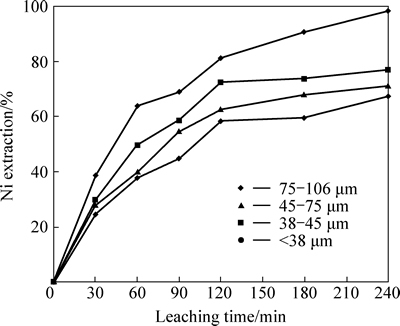
Fig. 5 Effect of particle size on nickel extraction from lateritic ore
The dissolution of nickel from lateritic ore can be explained by the shrinking core model. Therefore, diffusion and surface reaction control models were investigated in this work.
If the reaction is controlled by diffusion, the following equation can be used [38, 40].
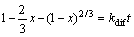 (1)
(1)
If the reaction is controlled by surface reaction [38, 40],
 (2)
(2)
where x is the fraction reacted, t is the reaction time, kdif and ks are the rate constants. Equations (1) and (2) were applied to the experimental results and the correlation coefficients for each temperature were calculated and given in Table 2.
Table 2 values of kdif, ks and correlation coefficients for different temperatures
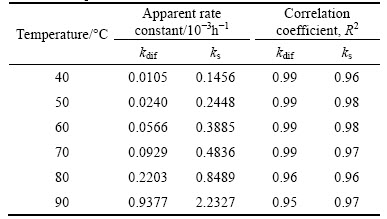
These results indicate that the dissolution of nickel is diffusion controlled. The application of the diffusion model is shown in Fig. 6. Equation (1) is applied to the data obtained at each temperature to determine the rate constants used in the Arrhenius plot (Fig. 7). The activation energy was calculated as 79.52 kJ/mol. The activation energies for lateritic nickel ore leaching reported by various authors are summarized in Table 3.
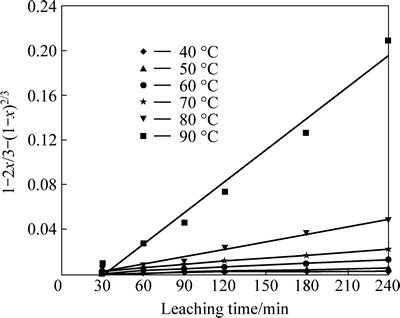
Fig. 6 Plot of Eq. (1) vs leaching time for different temperatures
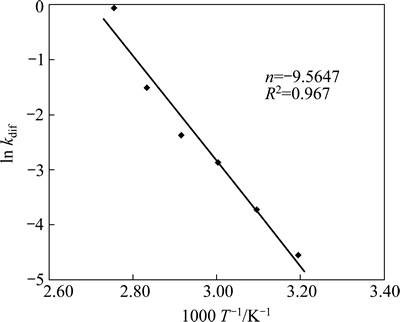
Fig. 7 Arrhenius plot of data presented in Fig. 6.
Table 3 Reported activation energies for leaching of nickel from lateritic ore with different leaching reagents

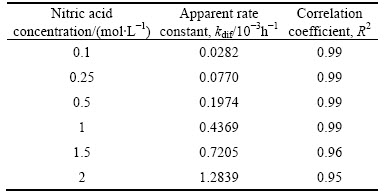
Equation (1) was applied to the obtained results for acid concentration values. The kdif values for each acid concentration are listed in Table 4.
Table 4 values of kdif and correlation coefficients for each acid concentration
The application of the diffusion model for different acid concentrations is shown in Fig. 8. From the corresponding kdif and nitric acid concentration values, plots of lnkdif versus ln[HNO3] were obtained. As seen from Fig. 9, the order of the reaction was found proportional to the power of 1.251 of HNO3 concentration ([HNO3]1.251).
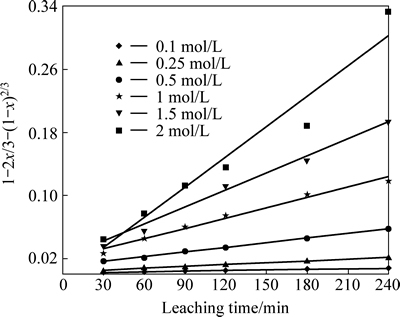
Fig. 8 Plot of Eq. (1) vs leaching time for different HNO3 concentrations
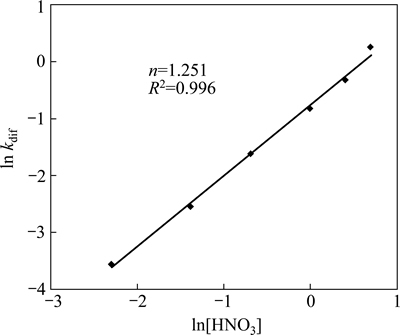
Fig. 9 Dependence of nickel dissolution to HNO3 concentration
4 Conclusions
1) Dissolution kinetics of nickel from lateritic ore in nitric acid solution is investigated. It is determined that the reaction rate increases with increasing nitric acid concentration and temperature, but decreasing with particle size. However, stirring speed did not significantly affect the dissolution process.
2) The optimum leaching conditions are determined: stirring speed of 200 r/min, acid concentration of 2 mol/L HNO3, temperature of 80 °C and particle size of <38 μm. Under this condition, 98% of nickel extraction is obtained for 240 min leaching period.
3) Shrinking core model is used to describe the dissolution kinetics of nickel from lateritic ore in nitric acid solution at 40-90 °C. The kinetic analysis shows that the nickel dissolution from lateritic ore occurred by diffusion controlled. The activation energy for the leaching process is calculated as 79.52 kJ/mol and the Arrhenius constant is found to be 9.5647 s-1.
Acknowledgement
This study was produced from the PhD thesis of Tevfik Agacayak and supported by The Research Foundation of the Selcuk University under the Project No: 06101021.
References
[1] LI Jin-hui, XIONG Dao-ling, CHEN Hao, WANG Rui-xiang, LIANG Yong. Physicochemical factors affecting leaching of laterite ore in hydrochloric acid [J]. Hydrometallurgy, 2012, 129/130: 14-18.
[2] PARK K H, NAM C W. Status and prospect of nickel resources and processing [J]. Trending Metals Mater. Eng, 2008, 21: 1-9.
[3] GOLIGHTLY J P. Nickeliferous laterite deposits [J]. Econ Geol, 1981, 75(1): 710-735.
[4] GEORGIOU D, PAPANGELAKIS G V. Sulphuric acid pressure leaching of a limonitic laterite: chemistry and kinetics [J]. Hydrometallurgy, 1998, 49: 23-46.
[5] SAGAPOA C V, IMAI A, WATANABE K. Laterization process of ultramafic rocks in siruka, solomon islands [J]. Journal of Novel Carbon Resource Sciences, 2011, 3: 32-39.
[6] GLEESON S A, BUTT C R, WLIAS M. Nickel laterites: a review [J]. SEG Newsletter, Society of Economic Geologists, 2003, 54: 12-18.
[7] ELIAS M. Nickel laterite deposits—geological overview, resources and exploitation [M]// Giant ore deposits: Characteristics, genesis and exploration. Hobant: University of Tasmania, 2002.
[8] SOLER J M, CAMA J,  S, MELENDEZ W, RAMIREZ A, ESTANGA J. Composition and dissolution kinetics of garnierite from the Loma de Hierro Ni-laterite deposit, Venezuela [J]. Chemical Geology, 2008, 249(1/2): 191-202.
S, MELENDEZ W, RAMIREZ A, ESTANGA J. Composition and dissolution kinetics of garnierite from the Loma de Hierro Ni-laterite deposit, Venezuela [J]. Chemical Geology, 2008, 249(1/2): 191-202.
[9] LANDERS M, GILKES R J, WELLS M. Dissolution kinetics of dehydroxylated nickeliferous goethite from limonitic lateritic nickel ore [J]. Applied Clay Science, 2009, 42: 615-624.
[10] BRAND N W, BUTT C R M, ELIAS M. Nickel laterites: classification and features [J]. Journal of Australian Geology and Geophysic, 1998, 17: 81-88.
[11] DALVI A D, BACON W, OSBORNE RC. The past and the future of nickel laterites [C]// PDAC 2004 International Conference Trade Show and Investors Exchange. Toronto: 2004.
[12] DEEPATANA A, TANG J A, VALIX M. Comparative study of chelating ion exchange resins for metal recovery from bioleaching of nickel laterite ores [J]. Minerals Engineering, 2006, 19: 1280-1289.
[13] AGACAYAK T, ZEDEF V. Dissolution kinetics of a lateritic nickel ore in sulphuric acid medium [J]. Acta Montanistica Slovaca, 2012, 17(1): 33-41.
[14] LUO Wei, FENG Qi-ming, OU Leming, ZHANG Guo-fan, CHEN Yun. Kinetics of saprolitic laterite leaching by sulphuric acid at atmospheric pressure [J]. Minerals Engineering, 2010, 23(6): 458-462.
[15] STOPIC S, FRIEDRICH B, FUCH R. Kinetics of sulphuric acid leaching of the Serbian nickel laterite ore under atmospheric pressure [J]. Metalurgica Journal of Metallurgy, 2002, 8(3): 235-244.
[16] AYANDA O S, ADEKOLA F A, BABA A A, FATOKI O S, XIMBA B J. Comparative study of the kinetics of dissolution of laterite in some acidic media [J]. Journal of Minerals & Materials Characterization & Engineering, 2011, 10(15): 1457-1472.
[17] RUBISOV D H, KROWINKEL J M, PAPANGELAKIS V G. Sulphuric acid pressure leaching of laterites universal kinetics of nickel dissolution for limonites and limonitic/saprolitic blends [J]. Hydrometallurgy, 2000, 58(1): 1-11.
[18] AGATZINI-LEONARDOU S, ZAFIRATOS I G. Beneficiation of a Greek serpentinic nickeliferous ore. Part II. Sulfuric acid heap and agitation leaching [J]. Hydrometallurgy, 2004, 74: 267-275.
[19] MCDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review (Part I). Sulphuric acid technologies [J]. Hydrometallurgy, 2008, 91(1/4): 35-55.
[20] THUBAKGALE C K, MBAYA R K K, KABONGO K. A study of atmospheric acid leaching of a South African nickel laterite [J]. Minerals Engineering, 2013, 54: 79-81.
[21] LANDERS M, GILKES R J, WELLS M. Dissolution kinetics of dehydroxylated nickeliferous goethite from limonitic lateritic nickel ore [J]. Applied Clay Science, 2009, 42: 615-624.
[22] MOHAMMADREZA F, MOHAMMAD N, ZIAEDDIN S S. Nickel extraction from low grade laterite by agitation leaching at atmospheric pressure [J]. International Journal of Mining Science and Technology, 2014, 24: 543-548.
[23] AGACAYAK T, ZEDEF V, AYDOGAN S. Leaching of lateritic nickel ores of Karacam (Eskisehir-Turkey) with hydrochloric acid [C]// 11th International Multidisciplinary Scientific Geo- Conference&EXPO SGEM, Modern Management of Mine Producing, Geology and Environmental Protection. Albena-Varna/ Bulgaria, 2011, 1: 1155-1162.
[24] OLANIPEKUN E O. Kinetics of leaching laterite [J]. International Journal of Mineral Processing, 2000, 60: 9-14.
[25] WANG Bao-quan, GUO Qiang, WEI Guang-ye, ZHANG Pei-yu, QU Jing-kui, QI Tao. Characterization and atmospheric hydrochloric acid leaching of a limonitic laterite from Indonesia [J]. Hydrometallurgy, 2012, 129/130: 7-13.
[26] GUO Qiang, QU Jing-kui, HAN Bing-bing, ZHANG Pei-yu, SONG Yun-xia, QI Tao. Innovative technology for processing saprolitic laterite ores by hydrochloric acid atmospheric pressure leaching [J]. Minerals Engineering, 2015, 71: 1-6.
[27] MCDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review. Part II. Chloride and bio-technologies [J]. Hydrometallurgy, 2008, 91: 56-69.
[28] MA Bao-zhong, WANG Cheng-yan, YANG Wei-jiao, YANG Bo, ZHANG Yong-lu. Selective pressure leaching of Fe (II)-rich limonitic laterite ores from Indonesia using nitric acid [J]. Minerals Engineering, 2013, 45: 151-158.
[29] ZHAI Yu-chun, MU Wen-ning, LIU Yan, XU Qian. A green process for recovering nickel from nickeliferous laterite ores [J]. Trans Nonferrous Met Soc China, 2010, 20: 65-70.
[30] CHEN Sheng-li, GUO Xue-yi, SHI Wen-tang, LI Dong. Extraction of valuable metals from low-grade nickeliferous laterite ore by reduction roasting-ammonia leaching method [J]. Journal of Central South University of Technology, 2010, 17: 765-769.
[31] ZUNIGA M, PARADA F, ASSELIN E. Leaching of a limonitic laterite in ammoniacal solutions with metallic iron [J]. Hydrometallurgy, 2010, 104: 260-267.
[32] SAHU S, KAVURI N C, KUNDU M. Dissolution kinetics of nickel laterite ore using different secondary metabolic acids [J]. Brazilian Journal of Chemical Engineering, 2011, 28(2): 251-258.
[33] BEHERA S K, SUKLA L B, MISHRA B K. Leaching of nickel laterite using fungus mediated organic acid and synthetic organic acid: a comparative study [C]// Proceedings of the XI International Seminar on Mineral Processing Technology. 2010: 946-954.
[34] SUKLA L B, PANCHANADIKAR V. Bioleaching of lateritic nickel ore using a heterotrophic micro-organism [J]. Hydrometallurgy, 1993, 32: 373-379.
[35] AGACAYAK T. Beneficiation of Karacam (Eskisehir) lateritic nickel ore by physical and chemical methods [D]. Turkey: Selcuk University, 2008.
[36] BEHERA S K, PANDA S K, PRADHAN N, SUKLA L B, MISHRA B K. Extraction of nickel by microbial reduction of lateritic chromite overburden of Sukinda, India [J]. Bioresource Technology, 2012, 125: 17-22.
[37] GHARABAGHI M, IRANNAJAD M, NOAPARAST M. A review of the beneficiation of calcareous phosphate ores using organic acid leaching [J]. Hydrometallurgy, 2010, 103: 96-107.
[38] LEVENSPIEL O. Chemical reaction engineering [D]. New York: John Wiley & Sons, 1999.
[39] ZAFAR I Z. Determination of semi empirical kinetic model for dissolution of bauxite ore with sulfuric acid: Parametric cumulative effect on the Arrhenius parameters [J]. Chemical Eng J, 2008, 141(1/2/3): 233-241.
[40] HABASHI F. Principles of extractive metallurgy [D]. New York: Gordon and Breach, 1969.
[41] AGACAYAK T, AYDOGAN S, ZEDEF V. Dissolution of lateritic nickel ore in sulphuric acid medium with potassium dichromate [C]// 8th International Scientific Conference, Modern Management of Mine Producing, Geology and Environmental Protection SGEM 2008. Bulgaria, 2008: 51-58.
[42] AGACAYAK T, ZEDEF V. Determination of leach conditions of  lateritic nickel ores with sulfuric acid under effect of sodium fluoride [C]// 11th International Multidisciplinary Scientific Geo-Conference & EXPO SGEM, Modern Management of Mine Producing, Geology and Environmental Protection. Albena-Varna/ Bulgaria. 2011, 1: 1109-1116.
lateritic nickel ores with sulfuric acid under effect of sodium fluoride [C]// 11th International Multidisciplinary Scientific Geo-Conference & EXPO SGEM, Modern Management of Mine Producing, Geology and Environmental Protection. Albena-Varna/ Bulgaria. 2011, 1: 1109-1116.
(Edited by YANG Hua)
Received date: 2015-02-06; Accepted date: 2015-04-16
Corresponding author: Tevfik AGACAYAK, PhD; Tel: +90-3322232028; E-mail: tevfik@selcuk.edu.tr
Abstract: dissolution kinetics of nickel from lateritic ore in nitric acid solution was investigated. Experimental parameters used were stirring speed (100-600 r/min), temperature (40-96 °C), nitric acid concentration (0.1-2 mol/L) and particle size (<106 μm). The shrinking core model was applied to the results of experiments investigating the effects of leaching temperature in the range of 40-90 °C and nitric acid concentration in range of 0.1-2 mol/L on nickel dissolution rate. The kinetic analysis shows that the nickel dissolution from lateritic ore could be described by diffusion model. The activation energy (Ea) for the dissolution reaction is calculated as 79.52 kJ/mol.


