
A green process for recovering nickel from nickeliferous laterite ores
ZHAI Yu-chun(翟玉春)1, MU Wen-ning (牟文宁)2, LIU Yan (刘 岩)1, XU Qian(许 茜)1
1. Department of Metallurgical Physical Chemistry, Northeastern University, Shenyang 110004, China;
2. Department of Materials Science and Engineering, Northeastern University at Qinhuangdao Branch,Qinhuangdao 066004, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract:
A novel process was proposed for recovering nickel from nickeliferous laterite ores. First of all, silicon and magnesium were removed from lateritic ore by high concentration sodium hydroxide leaching and carbonation respectively, so as to enrich nickel. Then the method of ammonia carbonate leaching was adopted to recover nickel from the carbonized slag, and the remaining residue was used as a raw material for recovering iron. The effects of temperature, ammonia carbonate concentration, liquid/solid ratio and stirring speed on the recovery of nickel were examined. When the leached residue reacted with ammonia carbonate (6 mol·L-1 ) in a ratio of liquid-to-solid of 5?1 at 60 ℃ for 150 min at the stirring speed of 300 r·min-1, approximate more than 95% nickel was recovered. During the whole process, there was no contamination produced and the chemical raw materials were recycled, thus the process was a green technology that having good social benefit.
Key words:
nickeliferous laterite ore; kinetics; silicon; magnesium; nickel;
____________________________________________________________________________________________
1 IntroductionIn recent years, there is an increasing focus on the utilization of low-grade nickeliferous laterite ores, along with the growing demand for stainless steel and the declining of sulphide ores and high-grade nickeliferous laterite ores[1-4]. Nickeliferous laterite ore deposits are formed by the chemical weathering of nickeliferous peridotite rock under humid climates[5-6]. Through the weathering processes, the nickel is finally concentrated in different secondary minerals. The complex mineralogy and low nickel content of the nickeliferous laterite cause the difficulties of extracting nickel directly[7-9].
At present, the typical metallurgical methods for processing nickeliferous laterite ores include reduction roasting followed by ammonia leaching[10-11] and high pressure leaching with sulfuric acid[12-16]. These methods, however, are mainly used for the recovery of nickel, generate large amounts of environmental contamination. Therefore, in the long term it will be necessary to develop new and more economical processing technologies, so as to achieve the optimum resource utilization.
In this work, a novel process for treating nickeliferous laterite ore was established. First, silicon was leached from the laterite-nickel ore by high concentration sodium hydroxide for preparing silica, and then magnesium was extracted from desiliconization slag by carbonation for preparing magnesium carbonate, at last, nickel was recovered from carbonized slag by ammonia carbonate leaching for preparing nickel oxide, the remaining residue was used for raw material of recovering iron. The present work mainly focuses on recovering nickel from the carbonized slag and preparing of the nickel oxide product. The operation parameters examined during the ammonium carbonate leaching process include stirring speed, temperature, liquid/solid ratio and ammonium carbonate concentration.
2 ExperimentalAll the chemical reagents employed were analytical grade and deionized water was used throughout the experiments whenever needed. The lateritic ore sample used was got from Shanxi, China. The chemical composition was examined by ICP-OED, and the analytical results are listed in Table 1.
Table 1 Chemical compositions of lateritic ore expressed as oxides (mass fraction, %)

The X-ray diffraction (XRD) analysis of the sample was performed using Cu Kα radiation (λ=1.540 6) at 40 kV and 30 mA. Fig.1 shows that serpentine is the major mineral phase, and silicon dioxide and hematite are minor phases.
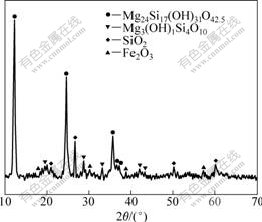
Fig.1 XRD pattern of nickeliferous laterite ores
2.1 Procedure of removing silicon
The ground lateritic ore sample was mixed with a certain sodium hydroxide solution at mass ratios of 4?1-5?1. Then the reactor was heated to 100-300 ℃ under the condition of stirring at the speed of 600 r/min. When reactor arrived at the temperature, the reaction began. The main chemical reactions taking place between ore and sodium hydroxide are as follows:
Mg3Si2O5(OH)4+4NaOH=3Mg(OH)2+2Na2SiO3+H2O (1)
Mg24Si17(OH)31O42.5+34NaOH=24Mg(OH)2+17Na2SiO3+8.5H2O (2)
SiO2+2NaOH=Na2SiO3+H2O (3)
After reaction for 60-90 min, the slurry was diluted with deionized water, and then filtered. The leached liquid is sodium silicate solution, which can be used for preparing silica by carbonation[17]. The desiliconization slag obtained was washed and dried for analyzing the content of nickel, magnesium and iron by ICP-OED.
2.2 Procedure of removing magnesium
The desiliconization slag and water were mixed at a certain proportion, and then the mixture was heated to the temperature of 20-60 ℃ under stirring. When the temperature reached and remained stable, the carbon dioxide gas was blown into the reactor at 0.5 L/min and the mixture was well stirred at 600 r/min under atmospheric pressure. The chemical reaction taking place between leaching residue and carbon dioxide is as follows:
Mg(OH)2+2CO2=Mg(HCO3)2 (4)
After 30-36 h, the mixture was filtered, and the leached liquid was magnesium bicarbonate solution, which can be used for preparing magnesium carbonate by pyrogenation[18]. The leached residue was washed with deionized water and dried for analyzing the content of nickel and iron by ICP-OED.
2.3 Procedure of recovering nickel
2.3.1 Ammonia leaching
The carbonized slag was mixed with ammonium carbonate solution, and then heated to 30-70 ℃ under stirring. The reaction between leached residue and ammonium carbonate is as follows:
Ni2++6NH3?H2O=[Ni(NH3)6]2++6H2O (5)
After a certain time, the mixture was filtrated, then nickel-ammonia complex compound solution and leached residue were obtained. The main component of leached residue was ferric oxide, which can be directly used as raw materials for recovering iron.
2.3.2 Evaporation of ammonia
The nickel-ammonia complex compound solution was heated to 80-100 ℃ to evaporate ammonia. The basic nickel carbonate was obtained after filtration and separation. Ammonia and carbon dioxide generated during this process were absorbed by the dilute ammonia to produce ammonium carbonate, which can be used as raw materials of ammonia leaching process. The chemical reactions occurred are as follows:
2Ni(NH3)6CO3+2H2O=Ni(OH)2?NiCO3?H2O↓+12NH3↑+CO2↑ (6)
(NH4)2CO3=2NH3↑+CO2↑+H2O↑ (7)
2.3.3 Calcination
The basic nickel carbonate was calcined at 400-500 ℃ to prepare nickel oxide products. The carbon dioxide generated during calcination could be absorbed by ammonia to produce ammonium carbonate. The chemical reaction occurred is as follows:
3Ni(OH)2?2NiCO3=5NiO+3H2O+2CO2↑ (8)
The whole technological flow of recovering nickel from nickeliferous laterite ores is shown in Fig.2.
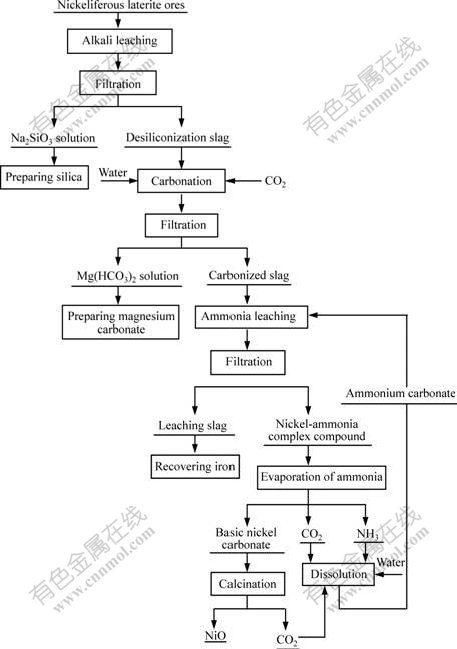
Fig.2 Flow diagram of recovering nickel from nickeliferous laterite ores
3 Results and discussion3.1 Effect of stirring speed
The influence of stirring speed on the nickel recovery was studied in experiment conducted at 60 ℃, reaction time 150 min, liquid/solid ratio 5?1 and 6 mol·L-1 ammonium carbonate. The results are shown in Fig.3.

Fig.3 Effect of stirring speed on nickel recovery
It is evident from Fig.3 that nickel recovery increases sharply with stirring speed from 150 to 300 r/min. When the stirring speed is up to 300 r/min and above, the recovery of nickel has no obvious change, which shows that there is an adequate suspension of the solid particles. Thus the stirring speed is determined as 300 r/min.
3.2 Effect of liquid/solid ratio
The influence of liquid-to-solid ratio on nickel recovery was carried out at 60 ℃, with a stirring speed of 300 r/min, reaction time 150 min and 6 mol·L-1 ammonium carbonate.
The results are presented in Fig.4. It can be seen
that the recovery of nickel improves with the increase of liquid-to-solid ratio. When the liquid-to-solid ratio increases, the amount of solid in the reaction mixture decreases, the solid pulp density decreases, therefore the mass transfer resistance decreases in liquid-solid interface, and finally the reaction between ammonium carbonate and nickel is accelerated. However, if the ratio of liquid to solid is too large, the energy consumption of evaporating ammonia will increase, therefore liquid-to- solid ratio of 5:1 is chosen.

Fig.4 Effect of liquid-to-solid ratio on nickel recovery
3.3 Effect of temperature
The influence of reaction temperature on nickel recovery, under the following conditions: stirring speed 300 r/min, ammonium carbonate 6 mol/L, liquid-to-solid ratio 5?1 and reaction time 150 min, is shown in Fig.5.
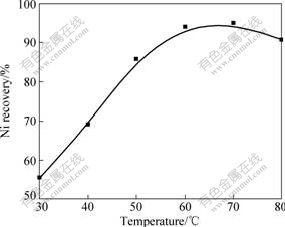
Fig.5 Effect of temperature on nickel recovery
From Fig.5, it can be seen that nickel recovery increases sharply with the increase of temperature. The maximum recovery obtained is approximately 95% when the temperature is 60 ℃. This is because the increasing of temperature not only increases the rate of chemical reaction, but also improves the rate of diffusion. Furthermore, a significant slowing down of the reaction rate in the final stage of reaction is noted. The reason is that high temperature will lead to the decomposition of ammonium carbonate, and decrease the concentration of ammonia ion in the mixture, and thereby decrease the recovery of nickel finally. Thus the temperature is chosen as 60 ℃.
3.4 Effect of ammonium carbonate concentration
The influence of ammonium carbonate concentration on the recovery of nickel was carried out at 60 ℃, solid-to-liquid ratio 5?1, stirring speed 300 r/min and reaction time 150 min.
From the results in Fig.6, it can be seen that the concentration of ammonium carbonate has a large influence on the recovery of nickel. The nickel recovery increases rapidly with the increase of ammoniumcarbonate concentration, and over 95% of nickel can be recovered when the concentration of ammonium carbonate is more than 6 mol/L. This may be attributed to the fact that increasing ammonium carbonate concentration improves the activity of ammonia ion, thereby increases the rate of reaction. However, the excess ammonium carbonate could significantly affect the economy of the whole process, so the concentration of ammonium carbonate is chosen as 6 mol/L.

Fig.6 Effect of ammonium carbonate concentration on nickel recovery
3.5 Analysis and characterization
3.5.1 Chemical analysis of solid residuals
The chemical composition of solid residuals is shown in Table 2. Compared with Table 1, it can be seen that the concentration of silica in the desiliconization slag is low, and the content of magnesia, iron oxide and nickel oxide is improved to 39.78%, 23.87% and 1.41%, respectively. The composition of carbonized slag presents that the elements of iron and nickel have been further enriched after removing magnesium from desiliconization slag, and the content of nickel oxide is up to 2.96%. From the composition of the remaining residue, the concentration of nickel is very low, and the content of iron oxide is improved to 59.72%, which indicates that the remaining residue is an excellent material containing iron.
Table 2 Chemical composition of solid residuals expressed as oxides (mass fraction, %)

3.5.2 Analysis of nickel oxide product
The X-ray diffraction pattern of products obtained by calcining basic nickel carbonate is shown in Fig.7. It can be seen that the diffraction peaks of the product match with the standard diffraction peaks of NiO, which presents that the product is NiO. The SEM image of NiO product (Fig.8) shows that the particle size of products is less than 5 μm. The component of NiO product is presented in Table 3. The Ni content of the products is 75.47%, and the NiO product is up to the technical indicators of GB8633-88 standard.
Table 3 Chemical compositions of nickel oxide products (mass fraction, %)
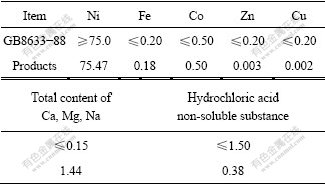
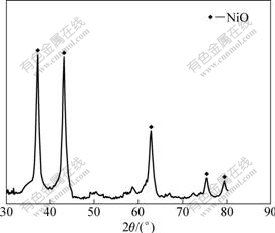
Fig.7 XRD pattern of nickel oxide products

Fig.8 SEM image of nickel oxide products
4 Conclusions1) A novel green technology of the comprehensive utilization of nickeliferous laterite ores is established, which provides new routes for exploitation and utilization of nickeliferous laterite ores.
2) It is estimated that more than 95% nickel can be recovered from carbonized slag reacted with ammonium carbonate solution (6 mol/L) at liquid-to- solid ratio of 5?1, 60 ℃ for 120 min under the stirring speed of 300 r/min.
3) The product of NiO is prepared, which is characterized by the methods of chemical analysis, XRD analysis and SEM analysis. The quality of NiO product is up to the national standard.
4) The whole process achieves the recycling of chemical raw materials without waste residue, water and gas.
References[1] PICKLES C A. Microwave heating behaviour of nickeliferous limonitic laterite ores [J]. Minerals Engineering, 2004, 17(6): 775-784.
[2] LEE H Y, KIM S G, OH J K. Electrochemical leaching of nickel from low-grade laterites [J]. Hydrometallurgy, 2005, 77(3/4): 263-268.
[3] MCDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review (Part I). Sulphuric acid technologies [J]. Hydrometallurgy, 2008, 91(1/4): 35-55.
[4] PICKLES C A. Drying kinetics of nickeliferous limonitic laterite ores [J]. Minerals Engineering, 2003, 16(12): 1327-1338.
[5] SOLER J M, CAMA J, GAL? S, MEL?NDEZ W, RAM?REZ A, ESTANGA J. Composition and dissolution kinetics of garnierite from the Loma de Hierro Ni-laterite deposit, Venezuela [J]. Chemical Geology, 2008, 249(1/2): 191-202.
[6] BRAND N W, BUTT C R M, ELIAS M. Nickel laterites: classification and features [J]. AGSO Journal of Australian Geology and Geophysics, 1998, 17(4): 81-88.
[7] CHANDRA D, WANG G X, FUERSTENAU M C, SIEMENS R E. Alkali roasting of low-grade chromite [J]. Transactions of the Institution of Mining and Metallurgy, Section C. Mineral processing and Extractive Metallurgy, 1996: C105-C112.
[8] GLEESON S A, HERRINGTON R J, DURANGO J, VEL?ZQUEZ C A, KOLL G. The mineralogy and geochemistry of de Cerro Matoso S.A. Ni laterite deposit,Montelíbano, Colombia [J]. Economic Geology, 2004, 99(6): 1197-1213.
[9] SWAMY Y V, KAR B B, MOHANTY J K. Physico-chemical characterization and sulphatization roasting of low-grade nickeliferous laterites [J]. Hydrometallurgy, 2003, 69(1/3): 89-98.
[10] PANDA S C, SUKLA L B, RAO P K, JENA P K. Extraction of nickel through reduction roasting and ammoniacal leaching of lateritic nickel ores [J]. Trans Indian Inst Met, 1980, 33(22): 161-165.
[11] CHANDER S, SHARMA V N. Reduction roasting/ammonia leaching of nickeliferous laterites [J]. Hydrometallurgy, 1981, 7(4): 315-327.
[12] WHITTINGTON B, MUIR D M. Pressure acid leaching of nickel laterites: a review [J]. Mineral Processing and Extractive Metallurgy Review, 2000, 21(6): 527-600.
[13] WHITTINGTON B I, MCDONALD R G, JOHNSON J A, MUIR D M. Pressure acid leaching of Arid-region nickel laterite ore: (Part I). Effect of water quality [J]. Hydrometallurgy, 2003, 70(1/3): 31-46.
[14] RUBISOV D H, PAPANGELAKIS V G. Sulphuric acid pressure leaching of laterites—A comprehensive model of a continuous autoclave [J]. Hydrometallurgy, 2000, 58(2): 89-101.
[15] RUBISOV D H, KROWINKEL J M, PAPANGELAKIS V G. Sulphuric acid pressure leaching of laterites—Universal kinetics of nickel dissolution for limonites and limonitic/saprolitic blends [J]. Hydrometallurgy, 2000, 58(1): 1-11.
[16] KAR B B, SWAMY Y V, MURTHY B V R. Design of experiments to study the extraction of nickel from lateriticore by sulphatization using sulphuric acid [J]. Hydrometallurgy, 2000, 56(3): 387-394.
[17] MU W N, LIU Y, ZHAI Y C. Preparation of SiO2 from nickeliferous laterite ore through leaching by high concentration alkali [J]. Journal of Northeastern University: Natural Science, 2009, 30(S2): 132-136. (in Chinese)
[18] MU W N, ZHAI Y C. Preparation of magnesium carbonate from desiliconization slag of laterite nickel ore by carbonation [J]. Journal of Chemical Industry and Engineering, 2009, 60(5): 1332-1336. (in Chinese)
____________________________________
Foundation item: Project(2007CB613603) supported by the National Basic Research Program of China
Corresponding author: MU Wen-ning; Tel: +86-335-8056635; E-mail: danae2007@yahoo.cn


