J. Cent. South Univ. Technol. (2009) 16: 0236-0241
DOI: 10.1007/s11771-009-0040-4
![]()
Effect of doping Bi on oxygen evolution potential and corrosion behavior of Pb-based anode in zinc electrowinning
LAI Yan-qing(赖延清), ZHONG Shui-ping(衷水平), JIANG Liang-xing(蒋良兴),
L? Xiao-jun(吕晓军), CHEN Pei-ru(陈佩如), LI Jie(李 劼), LIU Ye-xiang(刘业翔)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract:
A new anodic material of ternary Pb-0.8%Ag-(0-5.0%)Bi alloy for zinc electrowinning was obtained by doping Bi. The anodic oxygen evolution potential, corrosion rate, surface products after polarization, and microstructures before and after polarization were studied and compared with those of Pb-0.8%Ag anode used in industry. The results show the anodic overpotential decreases with the increase of Bi content in the alloys. When the content of Bi is 1.0% (mass fraction), the anodic overpotential is 40-50 mV lower than that of Pb-0.8%Ag anode. While the corrosion rate decreases and then increases with the increase of Bi content. The Pb-0.8%Ag-0.1%Bi anode has the lowest corrosion rate (0.090 6 mg/(h?cm2). Doping Bi influences the structure of the anodic layer, but does not change the phase. The Pb-0.8%Ag-1.0%Bi anode layer is of a more fine-grained structure compared with Pb-0.8%Ag anode.
Key words:
Pb-Ag anode; doping Bi; zinc electrowinning; oxygen evolution potential; corrosion rate;
1 Introduction
The energy consumption of the zinc electrowinning process represents about 80% of the entire electric power used in the extraction of zinc by hydrometallurgical methods [1-2]. Anode is the key equipment of the electrowinning process, which influences energy consumption, anode length of life and cathodic product purity. Because of high positive potential and acid content of electrolyte, only several kinds of inert metals can stabilize under that circumstance. Fewer kinds of metals can be selected taking cost into consideration. Regardless of the wide variety of compositions available, the anode composed of Pb-0.8%Ag (mass fraction) alloy is practically the only one widely used in the hydrometallurgical production of zinc. The major disadvantage is the high overvoltage (nearly 1 V) for oxygen evolution on the anode surface, which can increase the energy consumption about 1 000 kW?h/t, accounting for about 30% of the total energy consumption (3 200 kW?h/t) in Zn electrowinning [3]. For this reason, one of the important objectives of the experimental research is to minimize the energy consumption of the electrowinning process. Many studies focused on Pb-based anodes [4-5] and dimensionally stable anodes (coated titanium anodes) [6-8] with respect to its electrical conductivity, corrosion resistance, electrocatalytical capabilities, mechanical strength and handling characteristics. At the same time, new techniques such as combination electrolysis [9], methanol oxidation [10] and gas diffusion [11-12] electrowinning were also studied in detail. The most promising one among these tried methods is the addition of some metal elements to anode for reducing anodic polarization, because this method does not need to change anode production process and the existing plant.
Bi has attracted the most investigations on the performance of lead-acid batteries (above 70% Pb was used in lead-acid batteries), because it is costly to separate Bi from Pb to lower than 250×10-6 due to their similarity in physical and chemical performances. Pb industry hopes that Bi has favorable but not detrimental effects on lead-acid battery, so that Bi does not need to be removed from Pb. Much work has been done on the effects of Bi on oxygen evolution reaction on Pb grids. The results showed that Bi in Pb alloys could catalyze the oxygen evolution on positive plate [13-14], which is disadvantageous for the performance of lead-acid batteries. But it is one of the pursuing characteristics in Zn electrowinning. In addition, as a kind of electron- doped functional material, Bi is extensively applied in gas sensing device and electrolyte material [15]. However, the effect of Bi on the performance of Pb-Ag alloy anode in Zn electrowinning has not been reported previously. In this work, the electrochemical behavior of Pb-0.8%Ag-(0-5.0%)Bi alloys was studied by galvanostatic and potentiostatic measurements. Corrosion rate, surface products after polarization and microstructures before and after polarization of the new anode were investigated and compared with those of Pb-0.8 %Ag anode used in industry.
2 Experimental
2.1 Anode preparation
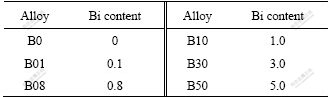
Pb (99.99%), Ag (99.9%) and Bi (99.99%) were used to prepare alloys. Different Bi contents of alloys cast in this experiment are listed in Table 1. Alloy electrodes were prepared by the following methods: metals were melted at 700 ℃, mixed by stirring, cast into cubes, cut into small pieces of 10 mm×10 mm×5 mm, welded with a plastic isolated copper wire and sealed with epoxy resin. Before each experiment, the working electrodes were polished with a silicon carbide paper of 600 grits, washed with water, degreased with ethanol and washed with double-distilled water. The working surface area was always 1 cm2.
Table 1 Bi contents of Pb-0.8%Ag-(0-5.0%)Bi alloys (mass fraction, %)
2.2 Performance test and characterization
The experiments were carried out in a glass three-electrode cell. The potential was measured with respect to Hg/Hg2Cl2 electrode (SCE). A platinum plate of 4 cm2 was served as counter electrode. The temperature was kept a constant of (37±1) ℃ by means of an HH-1 thermostat. The electrolyte was prepared by analytically pure grade chemicals and double-distilled water. The composition of the basic electrolyte (BE) for zinc electrowinning was as follows: 60 g/L Zn2+ (0.92 mol/L ZnSO4·7H2O) and 160 g/L (1.63 mol/L) H2SO4. Galvanostatic measurements were performed at an anodic current density of 50 mA/cm2 for 120 h. A multimeter (UNI-T 70D) with a high ohmic input (5×1012 Ω) indicated the potential during the prolonged polarization, and the data were collected by Personal Computer automatically every 1 min. Potentiostatic measurements were performed with model 273 potentionstat/galvanostat supplied by EG&G Princeton. The behavior of the electrode was influenced by the oxides on its surface, so before every experiment, the tested electrodes were held at a potential of -1.0 V for 10 min to reduce the oxides. Potentiostatic measurements were performed at an anodic potential of 1.80 V. The duration of the experiments was 18 h and the anodic currents were registered every 1 min.
The corrosion rate was determined by weighing the anode polarizing at a current density of 50 mA/cm2 for 96 h. The anode was washed with distilled water, dried and weighed. The anode was then treated with a boiling solution of NaOH and glucose in order to dissolve the anodic layer. The anode was again washed with distilled water, dried and weighed. The corrosion rate of the anode was determined by
vk=![]() (1)
(1)
where vk is the corrosion rate,m1 is the mass of anode before polarization, m2 is the mass of anode after polarization, S is the surface area of anode, and t is the time of anodic polarization.
Microscopic structure was analyzed with metallographic microscope (XJP-6A) after anodic samples were prepared according to Section 2.1. After anodic polarization at 50 mA/cm2 for 120 h, the samples were rinsed, dried immediately and analyzed. The compositions and morphologies of the surface layers were obtained by X-ray diffraction(XRD) (Rigaku 3014) and SEM(JSM-6360LV) analysis, respectively.
3 Results and discussion
3.1 Galvanostatic characteristics
The potential—time curves of various anodes under galvanostatic condition are presented in Fig.1, showing that the potentials significantly decrease in the first 10 h and are almost unalterable after that. It can be seen that the anodic overpotential decreases with the increase of Bi contents in the alloys. When the contents of Bi are 0.1%, 1.0% and 5.0%, the anodic overpotential re 20-30, 40-50 and 60-80 mV lower than those of the Pb-0.8%Ag alloy, respectively. From the above results it can be inferred that Bi in the Pb-Ag alloys can catalyze the oxygen evolution on anodes used in electrowinning of zinc. It can be expected that if a constant potential is imposed on the anodes, the corresponding current of the anodes with Bi will be higher than that of anodes without Bi.
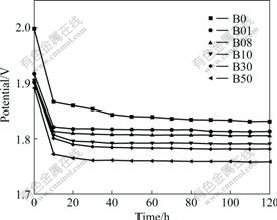
Fig.1 Relationship between anodic potential of Pb-alloy electrodes and time in basic electrolyte at 50 mA/cm2 and 37 ℃
3.2 Potentiostatic characteristics
Fig.2 shows the current density—time curves of Pb-0.8%Ag and Pb-0.8%Ag-1.0%Bi anodes under potentiostatic conditions. The results show that the current density significantly increases within 0-3 h and slightly decreases after 4 h. It is almost unalterable the after 10 h. It is true that the current density on Pb-0.8%Ag anode is 12 mA/cm2 lower than that on Pb-0.8%Ag-1.0%Bi anode. And this observation confirms the above expectation.
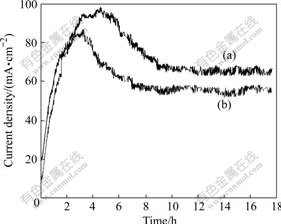
Fig.2 Potentiostatic polarization curves of Pb-alloy electrodes in basic electrolyte at 1.80 V (vs SCE) and 37 ℃: (a) Pb-0.8%Ag-1.0%Bi; (b) Pb-0.8%Ag
The mechanism of Bi effect is as follows: in the process of zinc electrowinning from sulphuric acid electrolytes, the main anodic reaction on the anode is the oxygen evolution. Porous PbO2 is formed on the surface of Pb alloys due to oxygen evolution and Bi in the alloys is dissolved into solution at high potentials [14]. The dissolved Bi ions in the solution are adsorbed on PbO2. The PbO2 with the adsorbed Bi has a quicker kinetics than that without the adsorbed Bi. It can be inferred that the oxygen evolution reaction becomes faster as the Bi content increases in the alloys.
3.3 Corrosion rate and microstructure of anodic polarization
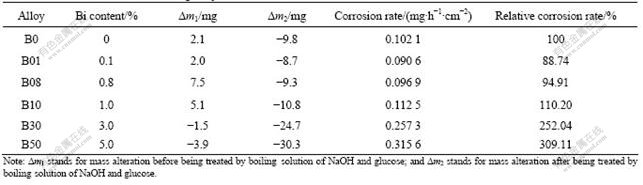
From Table 2 it can be seen that doping Bi is beneficial to anode Pb-0.8%Ag-Bi to increase the corrosion resistance when Bi content is not greater than 0.8% and the corrosion resistance reduces a little with the increase of Bi content. When Bi content is greater than 1.0%, the corrosion of anode will be accelerated. The reason is that the excessive amount of Bi will change the microstructure of the anode and the structure of the surface layer, which can be seen from Fig.3. At the same time, the anode mass alteration after being treated by boiling solution of NaOH and glucose is also a very good description of the impact of Bi on the anode corrosion rate. The reason is that the anode mass alteration before removing the surface oxide film depends on the mass increase because of Pb converting into PbSO4, PbO2 by oxidation and the mass reduction because of Pb going into the solution by corrosion. When the content of Bi is not more than 1.0%, the mass increase of Pb converting into PbSO4 and PbO2 will be greater than the mass reduction of Pb going into the solution by corrosion, which leads to the mass increase of anode before removing the surface oxide film. When the content of Bi is greater than 1.0%, the anode mass increases as a result of Pb converting into PbSO4 and the mass of PbO2 will be smaller than that of Pb going into the solution by corrosion, so the anode mass before removing the surface oxide film will be reduced.
Table 2 Corrosion rates of Pb-0.8 %Ag alloys with different Bi contents
3.3.1 Effect of alloy composition and microstructure on corrosion rate
Fig.3 indicates the metallographies of Pb-0.8%Ag- Bi alloy with different Bi contents. It can be seen that the metallography changes obviously with the increase of Bi content. The microstructure of the alloy in original state distributes very unevenly, although some grain boundaries are narrow and most impure elements in grain boundary aggregate badly (as shown by the arrow in Fig.3(a)). The corrosion resistance of the alloy is relatively poor. With the increase of Bi content to 0.1%, the microstructure is obviously improved. Perhaps the addition of Bi will make Ag elements in the basal body more even and the grain more refined. So at this time the best corrosion resistance is obtained. However, with further increase of Bi content to 1.0%, the grain sizes and the intergranular width increase with seriously partial aggregation, and the atom bond makes corrosion happen at the grain boundary firstly, which declines the corrosion resistance of alloy ultimately.
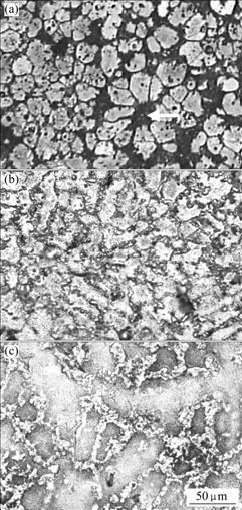
Fig.3 Metallographies of Pb-0.8%Ag-Bi alloys with different Bi contents: (a) Pb-0.8%Ag; (b) Pb-0.8%Ag-0.1%Bi; (c) Pb- 0.8%Ag-1.0%Bi
3.3.2 Structures and compositions of anode surface layer
Figs.4-6 show SEM images of Pb-0.8%Ag, Pb-0.8%Ag-1.0%Bi and Pb-0.8%Ag-3.0%Bi anodes, respectively, indicating that the surface morphology of oxide film has a clear relationship with the content of Bi in the anodes. The film on Pb-0.8%Ag is loose and is of crimple structure. Pb-0.8%Ag-1.0%Bi is of similar structure, which is clearly seen on the images with small magnification (Figs.4(a) and 5(a). But different structures of the surface layer are observed on Pb-0.8%Ag-3.0%Bi anode, which are porous and rough with an amorphous tumour-like pattern. (Fig.6(a)). In contrast to the images with high magnification (Figs.4(b), 5(b) and 6(b)), the surface layers of the Pb-0.8%Ag and Pb-0.8%Ag-1.0%Bi anodes have different structures. The latter is of a more fine-grained structure compared with the former. The reason may be that a low content of Bi can modify the crystal structure of alloys by decreasing its grain size. The film on Pb-0.8%Ag- 3.0%Bi alloy is rather friable, cracked and not tightly attached to the anode. It is evident that increasing Bi content from 1.0% to 3.0% leads to a significant modification of the anodic structure, because the higher the Bi content, the more the Bi dissolves into the solution and the larger the pores are produced.
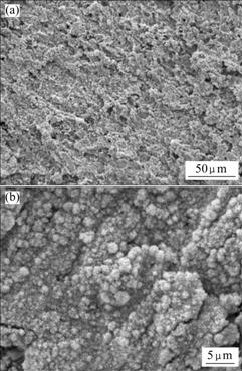
Fig.4 SEM images of Pb-0.8%Ag anode after 120 h anodic polarization: (a) Lower magnification; (b) Higher magnification
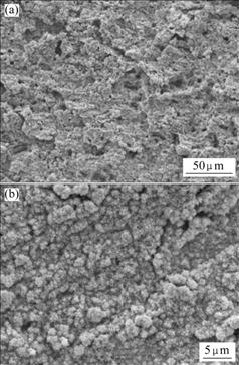
Fig.5 SEM images of Pb-0.8%Ag-1.0%Bi anode after 120 h anodic polarization: (a) Lower magnification; (b) Higher magnification
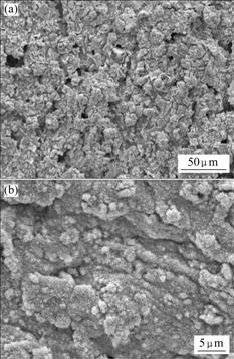
Fig.6 SEM images of Pb-0.8%Ag-3.0%Bi anode after 120 h anodic polarization: (a) Lower magnification; (b) Higher magnification
XRD patterns (Fig.7) show that the surface layers on Pb-0.8%Ag, Pb-0.8%Ag-1.0%Bi and Pb-0.8%Ag- 3.0%Bi anodes are mainly composed of α-PbO2 with a small amount of β-PbO2. The content of Bi does not change the phases in the film, but changes their relative abundances. In the cases of Pb-0.8%Ag-1.0%Bi and Pb-0.8%Ag-3.0%Bi (Figs.7(b) and 7(c), the presence of Bi leads to the formation of larger amounts of α-PbO2 than absence of Bi, but it does not noticeably affect the amount of β-PbO2. The results are different from some other studies [16]. The reason is that the electrochemical behavior of Pb electrodes in aqueous sulphuric acid solutions is complex, for example, the complex composition and morphology of anodic film become unstable when it is taken off from the electrochemical environment. By XRD analysis, LAITINEN et al [17] found that if the Pb anodes with steady PbO2 film formed in aqueous sulphuric acid solutions were taken out from electrochemical environment without any treatment, the mainly composition was PbSO4 instead of PbO2 because the rudimental sulphuric acid reacted with PbO2: PbO2+H2SO4=PbSO4+0.5O2↑+H2O.
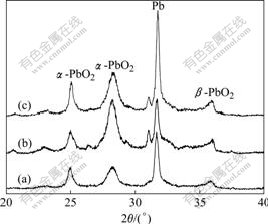
Fig.7 XRD patterns of anodic film formed after polarization for 120 h in basic electrolyte at 50 mA/cm2 and 37 ℃: (a) Pb- 0.8%Ag; (b) Pb-0.8%Ag-1.0%Bi; (c) Pb-0.8%Ag-3.0%Bi
4 Conclusions
(1) At the anodic current density of 50 mA/cm2, the result of galvanostatic measurements shows that Bi doping Pb-based anode displays lower anodic polarization than Pb-0.8%Ag anode. This observation is confirmed by potentiostatic measurement studies. It can be inferred that Bi can catalyze the oxygen evolution in the Pb-Ag alloys and be hopeful to reduce the energy consumption of the zinc electrowinning process.
(2) Doping Bi can increase the corrosion resistance of Pb-0.8%Ag-Bi anodes when Bi content is not greater than 0.8%. The corrosion rates of Pb-0.8%Ag, Pb-0.8%Ag-0.1%Bi and Pb-0.8%Ag-0.8%Bi are 0.102 1, 0.090 6 and 0.096 9 mg/(h·cm2), respectively.
(3) SEM observations show that the oxide surface morphology has a clear relationship with the content of Bi in the anode. Doping a low content of Bi (1.0%) to Pb-0.8%Ag alloy leads to a more fine-grained structure compared with Pb-0.8%Ag alloy without doping Bi. XRD analysis shows that doping Bi does not change the phases in the film, which is mainly composed of α-PbO2 and β-PbO2.
References[1] WANG Yan-jun, XIE Gang, YANG Da-jin, LI Yong-jia, XIAO Ting-man. Analysis on decreasing of direct current power consumption in zinc electrowinning [J]. Hydrometallurgy of China, 2005, 24(4): 208-211. (in Chinese)
[2] PFNG Gen-fang. Analyses and optimization exploration of D.C. consumption in zinc electrowinning [J]. Energy Saving of Non-ferrous Metallurgy, 2003, 20(2): 17-20. (in Chinese)
[3] IVANOVI, STEFANOVY, NONCHEVAZ, PETROVAM, DOBREVT, MIRKOVAL, VERMEERSCHR, DEMAERELJ P. Insoluble anodes used in hydrometallurgy. Part II. Anodic behaviour of Pb and Pb-alloy anodes [J]. Hydrometallurgy, 2000, 57(2): 125-139.
[4] IVANOVI, STEFANOVY, NONCHEVAZ, PETROVAM, DOBREVT, MIRKOVAL, VERMEERSCHR, DEMAERELJ P. Insoluble anodes used in hydrometallurgy. Part I. Corrosion resistance of Pb and Pb alloy anodes [J]. Hydrometallurgy, 2000, 57(2): 109-124.
[5] STEFANOVY, DOBREVT. Potentiodynamic and electronmicroscopy investigations of Pb-cobalt alloy coated Pb composite anodes for zinc electrowinning [J]. Transactions of the Institute of Metal Finishing, 2005, 83(6): 296-299.
[6] LI Bao-song, LIN An, GAN Fu-xing. Preparation and electrocatalytic properties of Ti/IrO2-Ta2O5 anodes for oxygen evolution [J]. Trans Nonferrous Met Soc China, 2006, 16(5): 1193-1199.
[7] HU Ji-ming, ZHANG Jian-qing, CAO Chu-nan. Oxygen evolution reaction on IrO2-based DSA type electrodes: Kinetics analysis of Tafel lines and EIS [J]. International Journal of Hydrogen Energy, 2004, 29(8): 791-797.
[8] STEFANOVY, DOBREVT. Developing and studying the properties of Pb-TiO2 alloy coated Pb composite anodes for zinc electrowinning [J]. Transactions of the Institute of Metal Finishing, 2005, 83(6): 291-295.
[9] ZHONG Shui-ping, LAI Yan-qing, JIANG Liang-xing. Research development of new anode and techniques for zinc electrowinning [J]. Materials Review, 2008, 22(2): 86-89. (in Chinese)
[10] WESSELMARK M, LAGERGREN C, LINDERGH G. Methanol oxidation as anode reaction in zinc electrowinning [J]. Journal of the Electrochemical Society, 2005, 152(11): D201-D207.
[11] JIN Bing-Jie, YANG Xian-wan. Application of gas diffusion anode for zinc hydrometallurgy [J]. Yunnan Metallurgy, 2007, 36(3): 37-39. (in Chinese)
[12] SU Yi, JIN Zuo-mei, DAI Zu-yuan. SO2 anodic reaction kinetics in zinc electrowinning [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(3): 495-498. (in Chinese)
[13] RICE D M. Effects of bismuth on the electrochemical performance of Pb/acid batteries [J]. Journal of Power Sources, 1989, 28(1/2): 69-83.
[14] LI Wei-shan, CHEN Hong-yu, LONG Xue-mei, WU Fan-hua, YAN Jun-hua, ZHANG Cheng-ru. Oxygen evolution reaction on Pb-bismuth alloys in sulfuric acid solution [J]. Journal of Power Sources, 2006, 158(2): 902-907.
[15] HE Qiu-xing TU Wei-ping, HU Jian-qing. Synthesis and characterization of bismuth-doped tin dioxide nanometer powders [J]. Journal of Central South University of Technology, 2006, 13(5): 519-524.
[16] HRUSSANOVA A, MIRKOVA L, DOBREVT. Electrochemical properties of Pb-Sb, Pb-Ca-Sn and Pb-Co3O4 anodes in copper electrowinning [J]. Journal of Applied Electrochemistry, 2002, 32(5): 505-512.
[17] LAITINEN T, SUNDHOLM G, VILHUNEN J K. Comments on sample treatment in the X-ray diffraction analysis of the oxidation products of Pb [J]. Journal of Power Sources, 1990, 32(1): 71-80.
Foundation item: Project(2007SK2009) supported by the Science and Technology Research Project of Hunan Province, China
Received date: 2008-10-15; Accepted date: 2008-12-10
Corresponding author: LAI Yan-qing, Professor; Tel: +86-731-8876454; E-mail: zspcsu@163.com
(Edited by CHEN Wei-ping)
Abstract: A new anodic material of ternary Pb-0.8%Ag-(0-5.0%)Bi alloy for zinc electrowinning was obtained by doping Bi. The anodic oxygen evolution potential, corrosion rate, surface products after polarization, and microstructures before and after polarization were studied and compared with those of Pb-0.8%Ag anode used in industry. The results show the anodic overpotential decreases with the increase of Bi content in the alloys. When the content of Bi is 1.0% (mass fraction), the anodic overpotential is 40-50 mV lower than that of Pb-0.8%Ag anode. While the corrosion rate decreases and then increases with the increase of Bi content. The Pb-0.8%Ag-0.1%Bi anode has the lowest corrosion rate (0.090 6 mg/(h?cm2). Doping Bi influences the structure of the anodic layer, but does not change the phase. The Pb-0.8%Ag-1.0%Bi anode layer is of a more fine-grained structure compared with Pb-0.8%Ag anode.


